High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
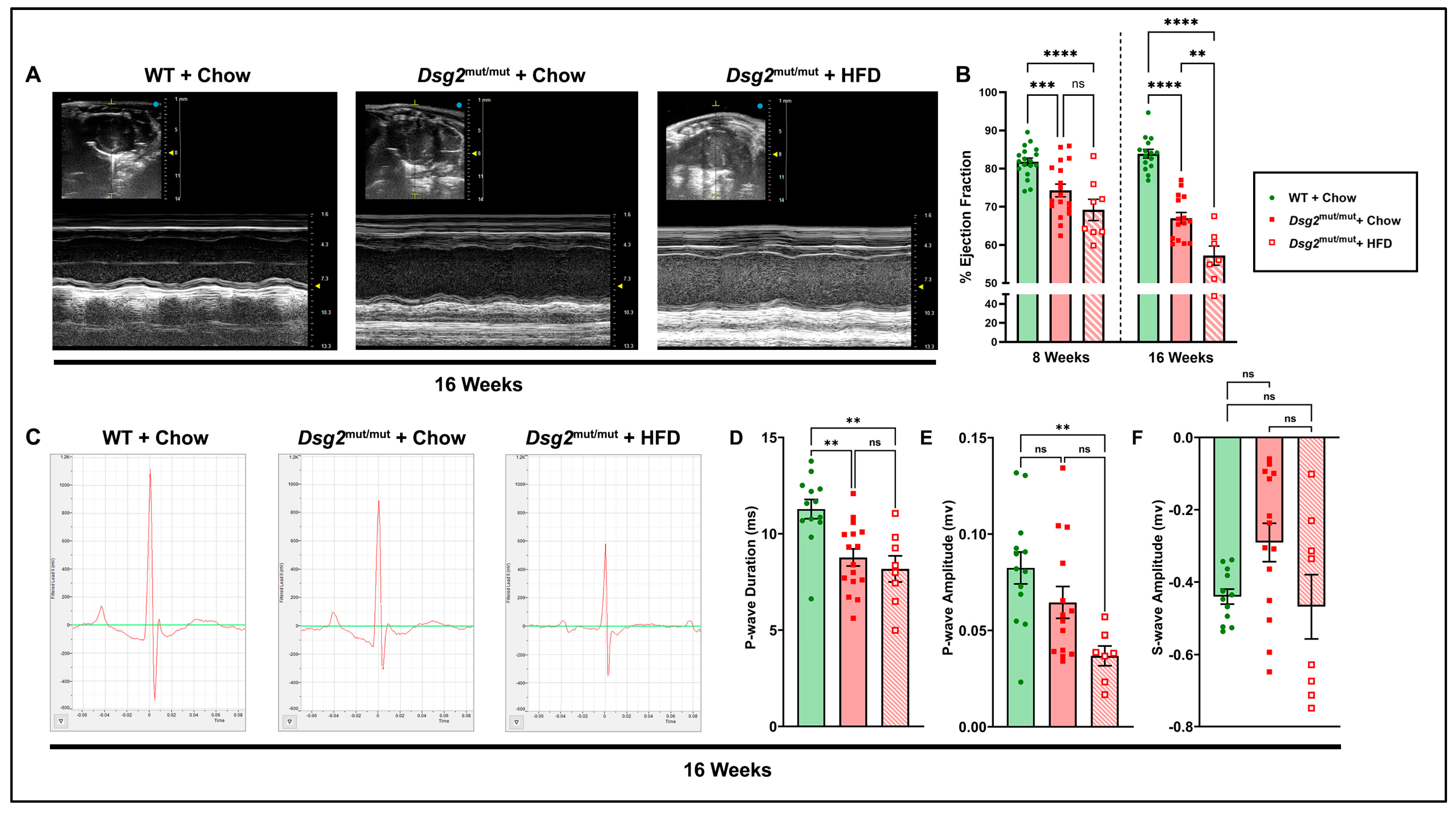
2.2. Mouse Echo- and Electrocardiography
2.3. Plasma Marker Assessment
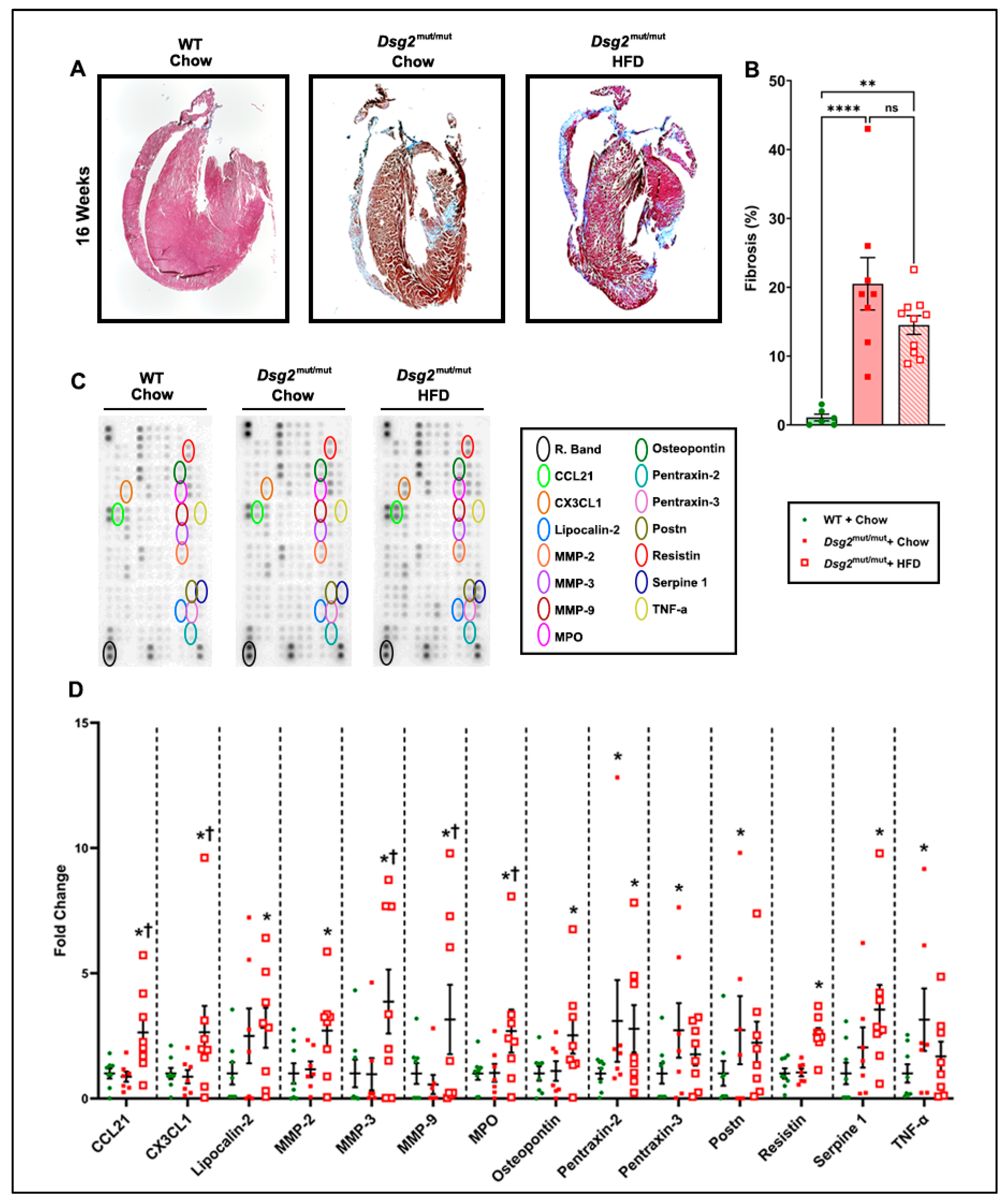
2.4. Cardiac Fibrosis
2.5. Cardiac Cytokine Arrays
2.6. Statistical Analysis
3. Results
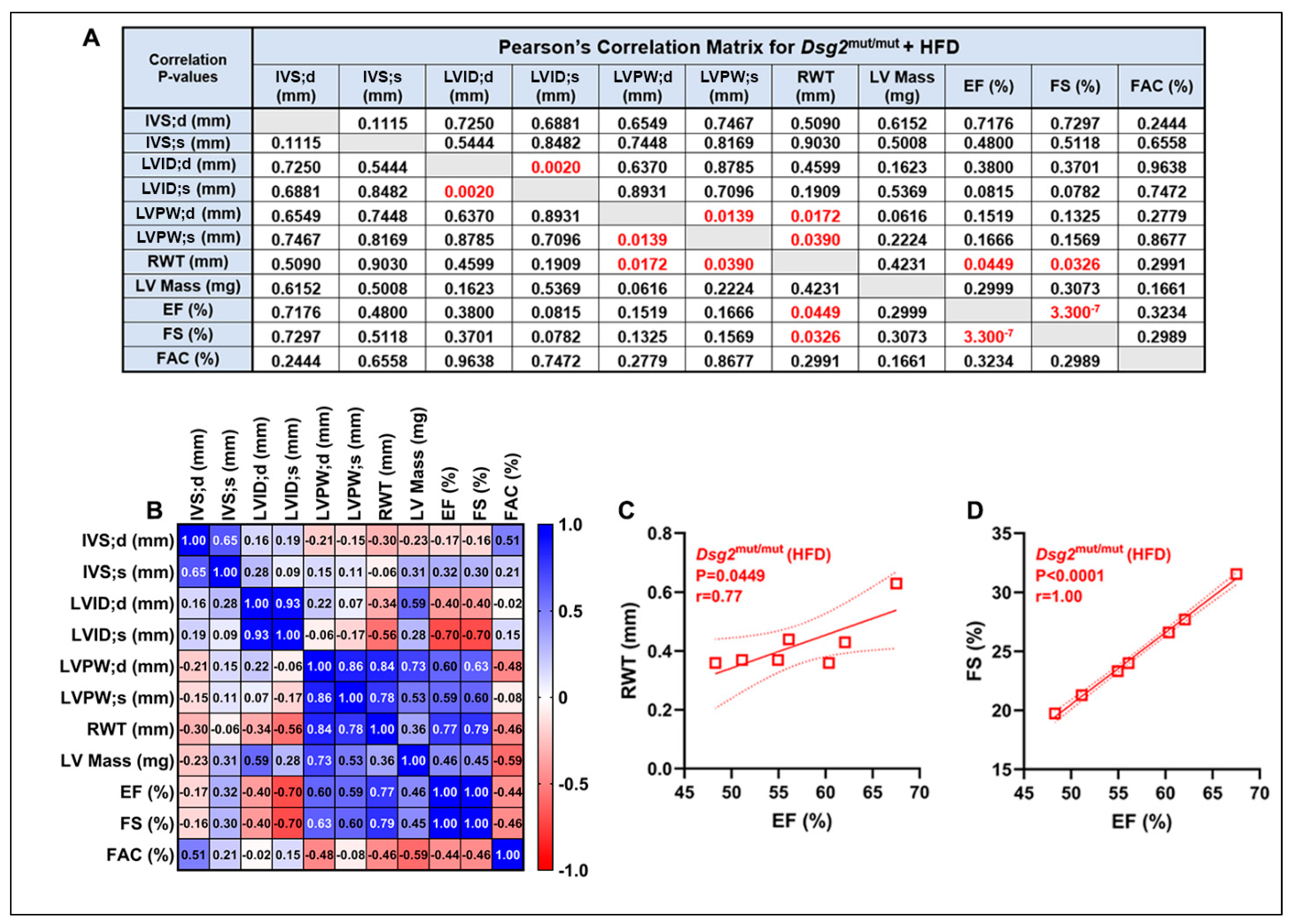
3.1. Impaired Cardiac Function and ECG Anomalies Following High-Fat-Diet Exposure in Dsg2mut/mut Mice
3.2. HFD-Induced Elevations in Plasma Lipids in Dsg2mut/mut Mice
3.3. HFD Consuption Elevated Myocardial Inflammatory Cytokines in Dsg2mut/mut Mice
4. Discussion
4.1. HFD and Cholesterol: Their Role in Myocardial Inflammation
4.2. Inflammation-Induced Cardiac Remodeling
4.3. HFD-Induced ECG Alterations and Cardiac Dysfunction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; Fontaine, G.H.; Guiraudon, G.; Frank, R.; Laurenceau, J.L.; Malergue, C.; Grosgogeat, Y. Right ventricular dysplasia: A report of 24 adult cases. Circulation 1982, 65, 384–398. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Thiene, G.; Nava, A.; Fontaliran, F.; Blomstrom-Lundqvist, C.; Fontaine, G.; Camerini, F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 1994, 71, 215–218. [Google Scholar] [CrossRef]
- Kaddoura, R.; Al-Tamimi, H. Physical Exercise and Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An Overview. Heart Views 2022, 23, 215–220. [Google Scholar] [CrossRef]
- Basso, C.; Pilichou, K.; Bauce, B.; Corrado, D.; Thiene, G. Diagnostic Criteria, Genetics, and Molecular Basis of Arrhythmogenic Cardiomyopathy. Heart Fail. Clin. 2018, 14, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Mattesi, G.; Cipriani, A.; Bauce, B.; Rigato, I.; Zorzi, A.; Corrado, D. Arrhythmogenic Left Ventricular Cardiomyopathy: Genotype-Phenotype Correlations and New Diagnostic Criteria. J. Clin. Med. 2021, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Zorzi, A.; Cipriani, A.; Bauce, B.; Bariani, R.; Beffagna, G.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; et al. Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021987. [Google Scholar] [CrossRef]
- Day, S.M. Anxiety in patients with arrhythmogenic right ventricular cardiomyopathy and implantable cardioverter defibrillators. Circ. Cardiovasc. Genet. 2012, 5, 2–4. [Google Scholar] [CrossRef]
- James, C.A.; Tichnell, C.; Murray, B.; Daly, A.; Sears, S.F.; Calkins, H. General and disease-specific psychosocial adjustment in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy with implantable cardioverter defibrillators: A large cohort study. Circ. Cardiovasc. Genet. 2012, 5, 18–24. [Google Scholar] [CrossRef]
- Agrimi, J.; Scalco, A.; Agafonova, J.; Williams Iii, L.; Pansari, N.; Keceli, G.; Jun, S.; Wang, N.; Mastorci, F.; Tichnell, C.; et al. Psychosocial Stress Hastens Disease Progression and Sudden Death in Mice with Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2020, 9, 3804. [Google Scholar] [CrossRef]
- Cerrone, M.; Marrón-Liñares, G.M.; van Opbergen, C.J.M.; Costa, S.; Bourfiss, M.; Pérez-Hernández, M.; Schlamp, F.; Sanchis-Gomar, F.; Malkani, K.; Drenkova, K.; et al. Role of plakophilin-2 expression on exercise-related progression of arrhythmogenic right ventricular cardiomyopathy: A translational study. Eur. Heart J. 2022, 43, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Chelko, S.P.; Keceli, G.; Carpi, A.; Doti, N.; Agrimi, J.; Asimaki, A.; Beti, C.B.; Miyamoto, M.; Amat-Codina, N.; Bedja, D.; et al. Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2021, 13, eabf0891. [Google Scholar] [CrossRef]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality among US Adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.T.; Kelemen, L.E.; de Koning, L.; Lonn, E.; Vuksan, V.; Jacobs, R.; Davis, B.; Teo, K.K.; Yusuf, S.; Anand, S.S.; et al. Interrelation of saturated fat, trans fat, alcohol intake, and subclinical atherosclerosis. Am. J. Clin. Nutr. 2008, 87, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G.; Corpas, E.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Hofland, J.; et al. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Mahdy Ali, K.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- USDA. Dietary Guidelines for Americans, 2020–2025; USDA: Washington, DC, USA, 2020; Volume 9.
- Mean Macronutrient Intake among Adults Aged 20 and Over, by Sex and Age: United States, 2015–2018. 2021. Available online: https://www.cdc.gov/nchs/hus/data-finder.htm (accessed on 2 August 2023).
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; van der Velden, S.; Ríos-Morales, M.; van Faassen, M.J.R.; Loreti, M.G.; de Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Chelko, S.P.; Asimaki, A.; Lowenthal, J.; Bueno-Beti, C.; Bedja, D.; Scalco, A.; Amat-Alarcon, N.; Andersen, P.; Judge, D.P.; Tung, L.; et al. Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation 2019, 140, 1491–1505. [Google Scholar] [CrossRef]
- Chelko, S.P.; Penna, V.R.; Engel, M.; Shiel, E.A.; Centner, A.M.; Farra, W.; Cannon, E.N.; Landim-Vieira, M.; Schaible, N.; Lavine, K.; et al. NFĸB signaling drives myocardial injury via CCR2+ macrophages in a preclinical model of arrhythmogenic cardiomyopathy. J. Clin. Investig. 2024, 134, e172014. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, G.; Fontaliran, F.; Andrade, F.R.; Velasquez, E.; Tonet, J.; Jouven, X.; Fujioka, Y.; Frank, R. The arrhythmogenic right ventricle. Dysplasia versus cardiomyopathy. Heart Vessel. 1995, 10, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Thiene, G.; McKenna, W.J.; Davies, M.J.; Fontaliran, F.; Nava, A.; Silvestri, F.; Blomstrom-Lundqvist, C.; Wlodarska, E.K.; et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: A multicenter study. J. Am. Coll. Cardiol. 1997, 30, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Stadiotti, I.; Casella, M.; Catto, V.; Dello Russo, A.; Carbucicchio, C.; Arnaboldi, L.; De Metrio, S.; Milano, G.; Scopece, A.; et al. Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol. Med. 2021, 13, e14365. [Google Scholar] [CrossRef] [PubMed]
- Chelko, S.P.; Asimaki, A.; Andersen, P.; Bedja, D.; Amat-Alarcon, N.; DeMazumder, D.; Jasti, R.; MacRae, C.A.; Leber, R.; Kleber, A.G.; et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016, 1, e85923. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Diwan, A.; Hahn, H.S. Murine echocardiography: A practical approach for phenotyping genetically manipulated and surgically modeled mice. J. Am. Soc. Echocardiogr. 2005, 18, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Bosman, L.P.; Cadrin-Tourigny, J.; Bourfiss, M.; Aliyari Ghasabeh, M.; Sharma, A.; Tichnell, C.; Roudijk, R.W.; Murray, B.; Tandri, H.; Khairy, P.; et al. Diagnosing arrhythmogenic right ventricular cardiomyopathy by 2010 Task Force Criteria: Clinical performance and simplified practical implementation. Europace 2020, 22, 787–796. [Google Scholar] [CrossRef]
- Eisinger, K.; Liebisch, G.; Schmitz, G.; Aslanidis, C.; Krautbauer, S.; Buechler, C. Lipidomic analysis of serum from high fat diet induced obese mice. Int. J. Mol. Sci. 2014, 15, 2991–3002. [Google Scholar] [CrossRef]
- McBride, P. Triglycerides and risk for coronary artery disease. Curr. Atheroscler. Rep. 2008, 10, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, R.N.; Blazeski, A.; Lowenthal, J.; Kannan, S.; Teuben, R.; DiSilvestre, D.; Morrissette-McAlmon, J.; Saffitz, J.E.; Boheler, K.R.; James, C.A.; et al. Altered Electrical, Biomolecular, and Immunologic Phenotypes in a Novel Patient-Derived Stem Cell Model of Desmoglein-2 Mutant ARVC. J. Clin. Med. 2021, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Inflammatory cytokines and cardiac arrhythmias: The lesson from COVID-19. Nat. Rev. Immunol. 2022, 22, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Abbate, A.; Boutjdir, M.; Capecchi, P.L. Fir(e)ing the Rhythm: Inflammatory Cytokines and Cardiac Arrhythmias. JACC Basic Transl. Sci. 2023, 8, 728–750. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Gordon, S.M.; Li, H.; Zhu, X.; Shah, A.S.; Lu, L.J.; Davidson, W.S. A comparison of the mouse and human lipoproteome: Suitability of the mouse model for studies of human lipoproteins. J. Proteome Res. 2015, 14, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Stanciulescu, L.A.; Scafa-Udriste, A.; Dorobantu, M. Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes-A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 6669. [Google Scholar] [CrossRef] [PubMed]
- Landim-Vieira, M.; Kahmini, A.R.; Engel, M.; Cannon, E.N.; Amat-Alarcon, N.; Judge, D.P.; Pinto, J.R.; Chelko, S.P. Efficacy and Safety of Angiotensin Receptor Blockers in a Pre-Clinical Model of Arrhythmogenic Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 13909. [Google Scholar] [CrossRef] [PubMed]
- Pagonas, N.; Mueller, R.; Weiland, L.; Jaensch, M.; Dammermann, W.; Seibert, F.S.; Hillmeister, P.; Buschmann, I.; Christ, M.; Ritter, O.; et al. Oxidized high-density lipoprotein associates with atrial fibrillation. Heart Rhythm. 2024, 21, 362–369. [Google Scholar] [CrossRef]
- Hussain, S.M.; Robb, C.; Tonkin, A.M.; Lacaze, P.; Chong, T.T.; Beilin, L.J.; Yu, C.; Watts, G.F.; Ryan, J.; Ernst, M.E.; et al. Association of plasma high-density lipoprotein cholesterol level with risk of incident dementia: A cohort study of healthy older adults. Lancet Reg. Health West Pac. 2024, 43, 100963. [Google Scholar] [CrossRef]
- Trimarco, V.; Izzo, R.; Morisco, C.; Mone, P.; Virginia Manzi, M.; Falco, A.; Pacella, D.; Gallo, P.; Lembo, M.; Santulli, G.; et al. High HDL (High-Density Lipoprotein) Cholesterol Increases Cardiovascular Risk in Hypertensive Patients. Hypertension 2022, 79, 2355–2363. [Google Scholar] [CrossRef]
- Franczyk, B.; Rysz, J.; Ławiński, J.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Sugiyama, D.; Watanabe, M.; Tamakoshi, A.; Iso, H.; Kotani, K.; Kiyama, M.; Yamada, M.; Ishikawa, S.; Murakami, Y.; et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J. Clin. Lipidol. 2018, 12, 674–684.e5. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in Health and Disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef]
- Alehagen, U.; Shamoun, L.; Wågsäter, D. Genetic variance and plasma concentration of CD93 is associated with cardiovascular mortality: Results from a 6.7-year follow-up of a healthy community-living elderly population. Mol. Med. Rep. 2020, 22, 4629–4636. [Google Scholar] [CrossRef]
- Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Agnoletti, D.; Marzioni, D.; Borghi, C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules 2023, 13, 910. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84, 104802. [Google Scholar] [CrossRef]
- Salvador, A.M.; Nevers, T.; Velázquez, F.; Aronovitz, M.; Wang, B.; Abadía Molina, A.; Jaffe, I.Z.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Intercellular Adhesion Molecule 1 Regulates Left Ventricular Leukocyte Infiltration, Cardiac Remodeling, and Function in Pressure Overload-Induced Heart Failure. J. Am. Heart Assoc. 2016, 5, e003126. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Bertolozzi, I.; Morozzi, G.; Lorenzini, S.; Simpatico, A.; Selvi, E.; Bacarelli, M.R.; Finizola, F.; Vanni, F.; et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 2017, 103, 1821–1829. [Google Scholar] [CrossRef]
- Nakagawa, N.; Barron, L.; Gomez, I.G.; Johnson, B.G.; Roach, A.M.; Kameoka, S.; Jack, R.M.; Lupher, M.L.; Gharib, S.A.; Duffield, J.S. Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease. JCI Insight 2016, 1, e87446. [Google Scholar] [CrossRef] [PubMed]
- Shklyaev, S.S.; Melnichenko, G.A.; Volevodz, N.N.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D.; Mokrysheva, N.G. Adiponectin: A pleiotropic hormone with multifaceted roles. Probl. Endokrinol. 2021, 67, 98–112. [Google Scholar] [CrossRef]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Ukhanov, V.; Muller-Delp, J.; Salazar, G. The Duality of Adiponectin: The Role of Sex in Atherosclerosis. Cells 2023, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Çolak, Y.; Benn, M.; Mason, A.; Burgess, S.; Nordestgaard, B.G. Plasma adiponectin levels and risk of heart failure, atrial fibrillation, aortic valve stenosis, and myocardial infarction: Large-scale observational and Mendelian randomization evidence. Cardiovasc. Res. 2024, 120, 95–107. [Google Scholar] [CrossRef]
- Chen, F.; Ye, Y.; Wu, G.; Wu, M. Correlation of Plasma Adiponectin Levels and Adiponectin Gene Polymorphisms with Idiopathic Atrial Fibrillation. Cardiology 2024, 149, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Ismaeel, A.; Koutakis, P.; Muller-Delp, J.; Salazar, G. AKT Mediates Adiponectin-Dependent Regulation of VSMC Phenotype. Cells 2023, 12, 2493. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef]
- Cho, G.S.; Lee, D.I.; Tampakakis, E.; Murphy, S.; Andersen, P.; Uosaki, H.; Chelko, S.; Chakir, K.; Hong, I.; Seo, K.; et al. Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy. Cell Rep. 2017, 18, 571–582. [Google Scholar] [CrossRef]
- Tomoaia, R.; Beyer, R.; Zdrenghea, D.; Dădârlat-Pop, A.; Popescu, M.I.; Cismaru, G.; Gușetu, G.; Bodisz, G.; Chețan, M.I.; Pop, D. Can Fetuin A Be Utilized in the Evaluation of Elderly Patients with Acute Myocardial Infarction? Life 2021, 11, 968. [Google Scholar] [CrossRef]
- Griffiths, M.; Yang, J.; Everett, A.D.; Jennings, J.M.; Freire, G.; Williams, M.; Nies, M.; McGrath-Morrow, S.A.; Collaco, J.M. Endostatin and ST2 are predictors of pulmonary hypertension disease course in infants. J. Perinatol. 2020, 40, 1625–1633. [Google Scholar] [CrossRef]
- Tilly, M.J.; Geurts, S.; Pezzullo, A.M.; Bramer, W.M.; de Groot, N.M.S.; Kavousi, M.; de Maat, M.P.M. The association of coagulation and atrial fibrillation: A systematic review and meta-analysis. Europace 2023, 25, 28–39. [Google Scholar] [CrossRef]
- Meng, X.W.; Zhang, M.; Hu, J.K.; Chen, X.Y.; Long, Y.Q.; Liu, H.; Feng, X.M.; Ji, F.H.; Peng, K. Activation of CCL21-GPR174/CCR7 on cardiac fibroblasts underlies myocardial ischemia/reperfusion injury. Front. Genet. 2022, 13, 946524. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Nymo, S.H.; Latini, R.; McMurray, J.J.; Kjekshus, J.; Yndestad, A.; Fucili, A.; Grosu, A.; Masson, S.; Maggioni, A.P.; et al. CCL21 is associated with fatal outcomes in chronic heart failure: Data from CORONA and GISSI-HF trials. Eur. J. Heart Fail. 2013, 15, 747–755. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, H.S.; Song, T.; Chen, Y.T.; Wang, T.; Tang, Y.H.; Barajas-Martinez, H.; Huang, C.X.; Hu, D. Abrogation of CC Chemokine Receptor 9 Ameliorates Ventricular Electrical Remodeling in Mice after Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 716219. [Google Scholar] [CrossRef]
- Guo, Y.; Apostalakis, S.; Blann, A.D.; Lip, G.Y. Plasma CX3CL1 levels and long term outcomes of patients with atrial fibrillation: The West Birmingham Atrial Fibrillation Project. Cerebrovasc. Dis. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Hinkle, C.C.; Ferguson, J.F.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Zhang, S.; Lu, H.; Hong, X.; Qian, J.; Sun, A.; Zou, Y.; Ge, J. Changes in fractalkine in patients with ST-elevation myocardial infarction. Coron. Artery Dis. 2015, 26, 516–520. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef]
- Gillers, B.S.; Chiplunkar, A.; Aly, H.; Valenta, T.; Basler, K.; Christoffels, V.M.; Efimov, I.R.; Boukens, B.J.; Rentschler, S. Canonical wnt signaling regulates atrioventricular junction programming and electrophysiological properties. Circ. Res. 2015, 116, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Maring, J.A.; Trojanowska, M.; ten Dijke, P. Role of endoglin in fibrosis and scleroderma. Int. Rev. Cell. Mol. Biol. 2012, 297, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Wilson, S.; Yunis, A.A.; Qiao, X.; Mackey, E.; Paruchuri, V.; Baker, C.; Aronovitz, M.J.; Karumanchi, S.A.; Letarte, M.; et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 2012, 125, 2728–2738. [Google Scholar] [CrossRef]
- Schoonderwoerd, M.J.A.; Goumans, M.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Shen, Z.; Quan, J.; Lin, C.; Li, X.; Hu, G. Endostatin in fibrosis and as a potential candidate of anti-fibrotic therapy. Drug Deliv. 2021, 28, 2051–2061. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, W.; Chen, Y.; Ahokas, R.A.; Sun, Y. Acidic and basic fibroblast growth factors involved in cardiac angiogenesis following infarction. Int. J. Cardiol. 2011, 152, 307–313. [Google Scholar] [CrossRef]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 2005, 174, 5789–5795. [Google Scholar] [CrossRef]
- Mesquita, T.; Cingolani, E. Targeting arrhythmogenic macrophages: Lessons learned from arrhythmogenic cardiomyopathy. J. Clin. Investig. 2024, 134, e180482. [Google Scholar] [CrossRef] [PubMed]
- Trojanek, J.B. [Role of matrix metalloproteinases and tissue inhibitors of metalloproteinases in hypertension. Pathogenesis of hypertension and obesity]. Postepy Biochem. 2015, 61, 356–363. [Google Scholar]
- Bennett, R.G.; Haqqani, H.M.; Berruezo, A.; Della Bella, P.; Marchlinski, F.E.; Hsu, C.J.; Kumar, S. Arrhythmogenic Cardiomyopathy in 2018–2019: ARVC/ALVC or Both? Heart Lung Circ. 2019, 28, 164–177. [Google Scholar] [CrossRef]
- Border, W.L.; Sachdeva, R.; Stratton, K.L.; Armenian, S.H.; Bhat, A.; Cox, D.E.; Leger, K.J.; Leisenring, W.M.; Meacham, L.R.; Sadak, K.T.; et al. Longitudinal Changes in Echocardiographic Parameters of Cardiac Function in Pediatric Cancer Survivors. JACC CardioOncol. 2020, 2, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Long-term cardiac safety of dose-dense anthracycline therapy cannot be predicted from early ejection fraction data. J. Clin. Oncol. 2009, 27, 6073–6075. [Google Scholar] [CrossRef] [PubMed]


| Parameter | WT + Chow | Dsg2mut/mut + Chow | Dsg2mut/mut + HFD |
|---|---|---|---|
| Morphometric | |||
| BW (g) | 25.5 ± 1.1 | 28.6 ± 1.4 | 31.5 ± 2.5 * |
| Heart (mg) | 113.6 ± 4.6 | 127.6 ± 3.3 | 138.1 ± 6.2 * |
| Liver (mg) | 1062 ± 77.3 | 1404 ± 69.6 * | 1171.1 ± 84.6 |
| Spleen (mg) | 77.4 ± 5.5 | 88.0 ± 4.2 | 97.0 ± 13.3 |
| HW/BW (mg/g) | 4.46 ± 0.08 | 4.51 ± 0.2 | 4.52 ± 0.4 |
| LW/BW (mg/g) | 41.5 ± 2.1 | 49.1 ± 0.5 * | 37.5 ± 2.0 † |
| Spleen/BW (mg/g) | 3.12 ± 0.3 | 3.15 ± 0.3 | 3.30 ± 0.6 |
| n-values | 10 | 9 | 8 |
| Echocardiographic | |||
| IVSd (mm) | 0.95 ± 0.02 | 0.96 ± 0.04 | 0.85 ± 0.1 |
| IVSs (mm) | 1.54 ± 0.03 | 1.47 ± 0.05 | 0.80 ± 0.07 *† |
| LVIDd (mm) | 2.71 ± 0.1 | 3.15 ± 0.1* | 4.07 ± 0.2 *† |
| LVIDs (mm) | 1.12 ± 0.09 | 1.94 ± 0.1 * | 3.06 ± 0.2 *† |
| LVPWd (mm) | 0.86 ± 0.03 | 0.96 ± 0.05 | 0.86 ± 0.07 |
| LVPWs (mm) | 1.50 ± 0.4 | 1.33 ± 0.05 | 1.08 ± 0.09 |
| LVEF (%) | 83.9 ± 1.2 | 67.0 ± 1.5 * | 57.2 ± 2.7 *† |
| LVFS (%) | 59.3 ± 2.0 | 39.7 ± 1.4 * | 24.9 ± 1.7 *† |
| RWT (mm) | 0.64 ± 0.03 | 0.64 ± 0.04 | 0.42 ± 0.04 *† |
| LVM (mg) | 74.7 ± 5.1 | 98.3 ± 5.3 * | 159.6 ± 25.6 *† |
| n-values | 15 | 15 | 7 |
| Electrocardiographic | |||
| Heart rate (bpm) | 423 ± 16 | 468 ± 14 | 479 ± 19 |
| RR-I (ms) | 145.6 ± 5.7 | 130.0 ± 3.8 * | 126.5 ± 5.0 * |
| PR-I (ms) | 42.1 ± 1.3 | 39.0 ± 1.6 | 39.5 ± 5.9 |
| Pd (ms) | 11.3 ± 0.5 | 8.77 ± 0.5 * | 8.18 ± 0.7 * |
| QRSd (ms) | 10.6 ± 0.3 | 12.5 ± 0.8 | 11.7 ± 0.6 |
| P-Amp (mV) | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 * |
| Q-Amp (mV) | −0.08 ± 0.01 | −0.10 ± 0.04 | −0.03 ± 0.01 |
| R-Amp (mV) | 0.99 ± 0.08 | 0.59 ± 0.06 * | 0.54 ± 0.04 * |
| S-Amp (mV) | −0.49 ± 0.02 | −0.29 ± 0.06 | −0.47 ± 0.09 |
| J-wave depression (%) | 7.7 ± 0.08 (n = 1/13) | 18.8 ± 0.1 (n = 3/16) | 87.5 ± 0.1 *† (n = 7/8) |
| n-values | 13 | 14–16 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centner, A.M.; Shiel, E.A.; Farra, W.; Cannon, E.N.; Landim-Vieira, M.; Salazar, G.; Chelko, S.P. High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy. Nutrients 2024, 16, 2087. https://doi.org/10.3390/nu16132087
Centner AM, Shiel EA, Farra W, Cannon EN, Landim-Vieira M, Salazar G, Chelko SP. High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy. Nutrients. 2024; 16(13):2087. https://doi.org/10.3390/nu16132087
Chicago/Turabian StyleCentner, Ann M., Emily A. Shiel, Waleed Farra, Elisa N. Cannon, Maicon Landim-Vieira, Gloria Salazar, and Stephen P. Chelko. 2024. "High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy" Nutrients 16, no. 13: 2087. https://doi.org/10.3390/nu16132087
APA StyleCentner, A. M., Shiel, E. A., Farra, W., Cannon, E. N., Landim-Vieira, M., Salazar, G., & Chelko, S. P. (2024). High-Fat Diet Augments Myocardial Inflammation and Cardiac Dysfunction in Arrhythmogenic Cardiomyopathy. Nutrients, 16(13), 2087. https://doi.org/10.3390/nu16132087








