Self-Regulation in Eating Behaviors: The Role of Executive Function in Response to Food Stimuli
Abstract
1. Introduction
- (a)
- To test the association between classic and modified versions of executive tasks and to justify the adoption of tasks using food stimuli in the field of nutrition and eating habits.
- (b)
- (c)
- To verify the differences between normal weight and overweight conditions in executive performance toward food stimuli. We expect a greater difficulty in individuals who are overweight than those with normal weight condition in controlling the inhibitory responses toward food-related stimuli.
2. Materials and Methods
2.1. Participants
2.2. Outcomes
2.2.1. Demographic Information
2.2.2. Physiological Measures
2.2.3. Executive Functions
2.3. Classic Tasks for Executive Functions
2.4. Modified Version of the Tasks with Food Stimuli
2.5. General Procedure
2.6. Data Analysis
3. Results
3.1. General Information
3.2. The Relationship between Classical Task and Task Adopting Visual Food Stimuli
3.3. The Association between BMI and Executive Functions Associated with Food Stimuli
3.4. Analyses of Variance (Normal-Weight Group vs. Overweight Group)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, D.A.; Farley, T.A. Eating as an automatic behavior. Prev. Chron. Dis. 2008, 5, 1–7. [Google Scholar]
- Silventoinen, K.; Konttinen, H. Obesity and eating behavior from the perspective of twin and genetic research. Neurosci. Biobehav. Rev. 2020, 109, 150–165. [Google Scholar] [CrossRef]
- Konttinen, H. Emotional eating and obesity in adults: The role of depression, sleep and genes. Proc. Nutr. Soc. 2020, 79, 283–289. [Google Scholar] [CrossRef]
- Rosenqvist, E.; Kiviruusu, O.; Berg, N.; Konttinen, H. Stress-induced eating and drinking and their associations with weight among women and men during 30-year follow-up. Psych. Health 2023, 22, 1–16. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychology 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annual Rev. Psych. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Kanakam, N.; Treasure, J. A review of cognitive neuropsychiatry in the taxonomy of eating disorders: State, trait, or genetic? Cogn. Neuropsychiatry 2013, 18, 83–114. [Google Scholar] [CrossRef] [PubMed]
- van Elburg, A.; Treasure, J. Advances in the neurobiology of eating disorders. Curr. Opin. Psychiatry 2013, 26, 556–561. [Google Scholar] [CrossRef]
- Smith, K.E.; Mason, T.B.; Johnson, J.S.; Lavender, J.M.; Wonderlich, S.A. A systematic review of reviews of neurocognitive functioning in eating disorders: The state-of-the-literature and future directions. Int. J. Eat. Dis. 2018, 51, 798–821. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Pazzaglia, M.; Casagrande, M. Mental and body health: The association between psychological factors, overweight, and blood pressure in young adults. J. Clin. Med. 2022, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.; Franken, I.H.; Muris, P. Food-related Stroop interference in obese and normal-weight individuals: Behavioral and electrophysiological indices. Eat. Behav. 2010, 11, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Leehr, E.J.; Schag, K.; Dresler, T.; Grosse-Wentrup, M.; Hautzinger, M.; Fallgatter, A.J.; Ehlis, A.C. Food specific inhibitory control under negative mood in binge-eating disorder: Evidence from a multimethod approach. Int. J. Eat. Dis. 2018, 51, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Marini, A.; Casagrande, M. Emotional regulation and overeating behaviors in children and adolescents: A systematic review. Behav. Sci. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.T.; Kullmann, S.; Ketterer, C.; Heni, M.; Häring, H.U.; Fritsche, A.; Preissl, H. Neuronal correlates of reduced memory performance in overweight subjects. Neuroimage 2012, 60, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kulendran, M.; Borovoi, L.; Purkayastha, S.; Darzi, A.; Vlaev, I. Impulsivity predicts weight loss after obesity surgery. Surg. Obes. Relat. Dis. 2017, 13, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Lee, M.; Higgs, S. Food-specific response inhibition, dietary restraint and snack intake in lean and overweight/obese adults: A moderated-mediation model. Int. J. Obes. 2016, 40, 877–882. [Google Scholar] [CrossRef]
- Calitri, R.; Pothos, E.M.; Tapper, K.; Brunstrom, J.M.; Rogers, P.J. Cognitive biases to healthy and unhealthy food words predict change in BMI. Obesity 2010, 18, 2282–2287. [Google Scholar] [CrossRef]
- Bongers, P.; van de Giessen, E.; Roefs, A.; Nederkoorn, C.; Booij, J.; van den Brink, W.; Jansen, A. Being impulsive and obese increases susceptibility to speeded detection of high-calorie foods. Health Psychol. 2015, 34, 677. [Google Scholar] [CrossRef]
- Frank, S.; Wilms, B.; Veit, R.; Ernst, B.; Thurnheer, M.; Kullmann, S.; Schultes, B. Altered brain activity in severely obese women may recover after Roux-en Y gastric bypass surgery. Int. J. Obes. 2014, 38, 341. [Google Scholar] [CrossRef] [PubMed]
- Loeber, S.; Grosshans, M.; Korucuoglu, O.; Vollmert, C.; Vollstädt-Klein, S.; Schneider, S.; Kiefer, F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int. J. Obes. 2012, 36, 1334. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Marotta, A.; Casagrande, M. Food-Related Attentional Bias in Individuals with Normal Weight and Overweight: A Study with a Flicker Task. Nutrients 2020, 12, 492. [Google Scholar] [CrossRef]
- Brignell, C.; Griffiths, T.; Bradley, B.P.; Mogg, K. Attentional and approach biases for pictorial food cues. Influence of external eating. Appetite 2009, 52, 299–306. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Body Mass Index—BMI. Available online: http://www.euro.who.int/en/healthtopics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 2 May 2024).
- Maffeis, C.; Banzato, C.; Talamini, G.; Obesity Study Group of the Italian. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Watanabe, R.M. A better index of body adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey III. Available online: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/anthro.pdf (accessed on 10 January 2024).
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643. [Google Scholar] [CrossRef]
- Favieri, F.; Chen, E.; Casagrande, M. Executive functions and body weight at different ages: A preliminary study. Nutrients 2021, 13, 1174. [Google Scholar] [CrossRef]
- Simson, R.; Vaughan, H.G., Jr.; Ritter, W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Clin. Neurophysiol. 1977, 43, 864–875. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef]
- Hester, R.; Dixon, V.; Garavan, H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006, 81, 251–257. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings; NIMH Center for the Study of Emotion and Attention: Gainesville, FL, USA, 2008; Volume 1. [Google Scholar]
- Sutton, C.A.; L’Insalata, A.M.; Fazzino, T.L. Reward sensitivity, eating behavior, and obesity-related outcomes: A systematic review. Physiol. Behav. 2022, 252, 113843. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, X.; Yang, H.; Shi, J. Inhibitory Process of Collaborative Inhibition: Assessment Using an Emotional Stroop Task. Psychol. Rep. 2020, 123, 300–324. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Basso, G.; Poggi, P.; Canessa, N. Fronto-temporal brain activity and connectivity track implicit attention to positive and negative social words in a novel socio-emotional Stroop task. Neuroimage 2021, 226, 117580. [Google Scholar] [CrossRef]
- Hester, R.; Lubman, D.I.; Yücel, M. The role of executive control in human drug addiction. In Behavioral Neuroscience of Drug Addiction. Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2010; pp. 301–318. [Google Scholar]
- Fisk, G.D.; Haase, S.J. Classic Stroop Color Words Produce No Stroop Effect When the Display Characteristics Are Based Upon Emotional Stroop Studies with Subliminal Presentations. Psychol. Rep. 2020, 123, 1207–1225. [Google Scholar] [CrossRef]
- Rutters, F.; Kumar, S.; Higgs, S.; Humphreys, G.W. Electrophysiological evidence for enhanced representation of food stimuli in working memory. Exp. Brain Res. 2015, 233, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Hollitt, S.; Kemps, E.; Tiggemann, M.; Smeets, E.; Mills, J.S. Components of attentional bias for food cues among restrained eaters. Appetite 2010, 54, 309–313. [Google Scholar] [CrossRef]
- Biehl, S.C.; Keil, J.; Naumann, E.; Svaldi, J. ERP and oscillatory differences in overweight/obese and normal-weight adolescents in response to food stimuli. J. Eat Dis. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.J.; Cachia, R.L.; Kothe, E.J.; McPhie, S.; Skouteris, H.; Hayden, M.J. Attentional biases for food cues in overweight and individuals with obesity: A systematic review of the literature. Obes. Rev. 2015, 16, 424–432. [Google Scholar] [CrossRef]
- Hagan, K.E.; Alasmar, A.; Exum, A.; Chinn, B.; Forbush, K.T. A systematic review and meta-analysis of attentional bias toward food in individuals with overweight and obesity. Appetite 2020, 151, 104710. [Google Scholar] [CrossRef]
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and its risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef] [PubMed]
- Mekanna, A.N.; Panchal, S.K.; Li, L. Beyond lockdowns: A systematic review of the impacts of COVID-19 lockdowns on dietary pattern, physical activity, body weight, and food security. Nutr. Rev. 2023, 81, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Agostini, F.; Giovannoli, J.; Di Pace, E.; Langher, V.; Casagrande, M. The cognitive consequences of the COVID-19 pandemic on members of the general population in Italy: A preliminary study on executive inhibition. J. Clin. Med. 2020, 11, 170. [Google Scholar] [CrossRef]
- Marchitelli, S.; Ricci, E.; Mazza, C.; Roma, P.; Tambelli, R.; Casella, G.; Lenzi, A. Obesity and psychological factors associated with weight loss after bariatric surgery: A longitudinal study. Nutrients 2022, 14, 2690. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2020; Volume 894, pp. i–xii. [Google Scholar]

| Classic Version | Modified Version | ||
|---|---|---|---|
| Stroop Task | Stimuli Types | Words of color | Colored Pictures |
| Target Response | Color of the words | Color of the frame of the picture | |
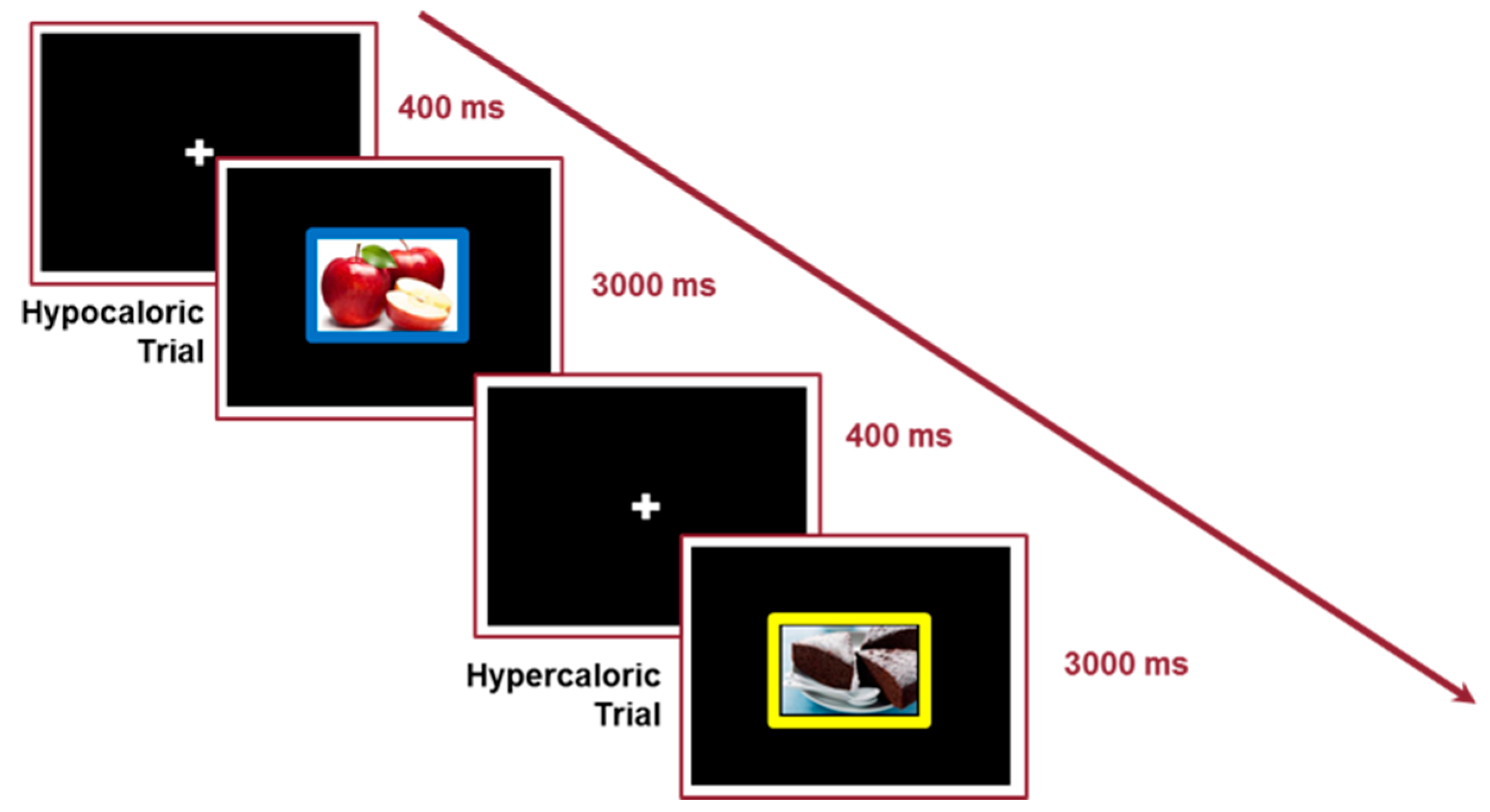
| Conditions | Congruent; Incongruent | Hypercaloric Food; Hypocaloric Food; Neutral No Food. | |
| Fixation cross time | 400 ms | 400 ms | |
| Target stimulus duration | 3000 ms | 3000 ms | |
| Number of Trials | 120 (60 incongruent; 60 congruent) | 288 (96 for each condition) | |
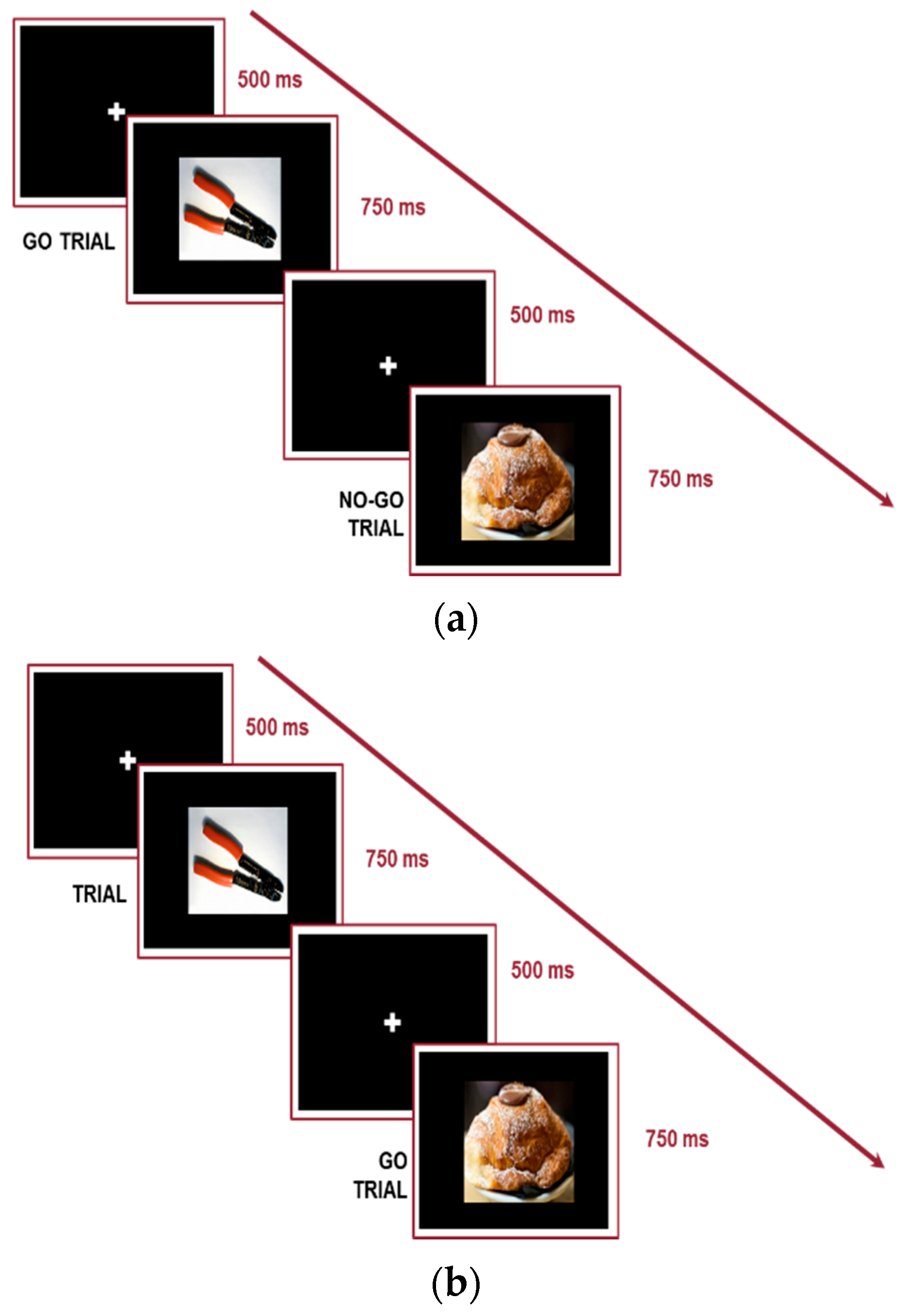
| Go/No-Go Task | Stimuli Types | Geometric shapes (green circle; green triangle) | Colored Pictures |
| Target Response | No-Go = no response; Go = response | No-Go = no response; Go = response | |
| Conditions | No-Go = green triangle | Version 1: No-Go = Food stimuli Version 2: No-Go = do-it-yourself stimuli | |
| Fixation cross time | 500 ms | 500 ms | |
| Target stimulus duration | 750 ms | 750 ms | |
| Number of Trials | 100 | Version 1: 100 Version 2: 100 | |
| N-Back Task | Stimuli Types | Letters | Colored Pictures |
| Target Response | 1-Back: Target stimulus is previous one 2-Back: Target stimulus is two previous one | 1-Back: Target stimulus is previous one 2-Back: Target stimulus is two previous one | |
| Conditions | Target (30% of the stimuli): Letter condition | Target (30% of the stimuli): Hypercaloric Trials; Hypocaloric Trials. | |
| Blank Screen | 2500 ms | 2500 ms | |
| Target stimulus duration | 500 ms | 500 ms | |
| Number of Trials | 1-Back: 40 2-Back: 40 | 1-Back: 40 2-Back: 40 | |
| N (%) | |
|---|---|
| Demographic Information | |
| Sex | |
| Males | 55 (38) |
| Females | 89 (62) |
| Lifestyles Habits | |
| Smoking Habits | |
| Yes | 55 (38) |
| No | 89 (62) |
| Caffeine Consumption | |
| Yes | 109 (76) |
| No | 35 (24) |
| Alcohol Consumption | |
| Yes | 81 (56) |
| No | 63 (44) |
| Physical Activity | |
| Yes | 70 (49) |
| No | 74 (51) |
| Health Risk Factors: Family diseases (yes) | |
| Dementia/Mild Cognitive Impairment | 28 (19) |
| Diabetes | 68 (47) |
| Obesity | 27 (19) |
| Cardiovascular Disorders | 70 (49) |
| Hypertension | 56 (39) |
| Normal Weight | Overweight | F | p | Pη2 | |
|---|---|---|---|---|---|
| N (m/f) | 40 (20/20) | 37 (18/19) | |||
| Age (mean, sd) | 24.33 (1.76) | 24.73 (2.68) | <1 | 0.43 | 0.01 |
| Years of education (mean, sd) | 17.10 (1.66) | 16.62 (1.85) | 1.43 | 0.24 | 0.02 |
| Physiological Measures (mean, sd) | |||||
| Weight (kg) | 65.12 (9.36) | 81.53 (14.79) | 34.10 | 0.0001 | 0.31 |
| Height (m) | 1.73 (0.10) | 1.73 (0.11) | <1 | 0.97 | 0.00001 |
| BMI | 21.93 (1.88) | 27.19 (2.86) | 103.54 | 0.0001 | 0.58 |
| Waist-to-height ratio | 0.45 (0.04) | 0.51 (0.05) | 35.75 | 0.0001 | 0.34 |
| Body adiposity index | 26.20 (4.71) | 32.09 (5.71) | 22.66 | 0.0001 | 0.25 |
| Systolic blood pressure | 119.58 (10.79) | 119.86 (10.77) | <1 | 0.91 | 0.00001 |
| Diastolic blood pressure | 71.68 (8.33) | 74.25 (7.28) | 2.04 | 0.16 | 0.03 |
| Heart rate | 78.05 (14.01) | 75.46 (11.03) | <1 | 0.38 | 0.01 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stroop Effect | r | 0.04 | 0.02 | 0.05 | 0.02 | −0.0002 | 0.04 | 0.07 | 0.12 | −0.17 | −0.17 |
| False Alarms (%) Go/No-Go | r | 0.14 | 0.08 | −0.17 | −0.21 | 0.56 *** | 0.51 *** | −0.24 | −0.20 | −0.11 | −0.12 |
| 1-Back Task | r | 0.09 | 0.13 | 0.001 | 0.10 | −0.17 | −0.09 | 0.11 | 0.36 *** | 0.001 | 0.27 *** |
| 2-Back Task | r | −0.25 ** | −0.28 *** | −0.09 | −0.21 * | −0.01 | −0.01 | 0.04 | 0.10 | 0.57 *** | 0.47 *** |
| Models | R2adj | F | B | SE | beta | p | Zero-Order Correlation |
|---|---|---|---|---|---|---|---|
| RT_Hypercaloric stimuli | 0.03 | 3.77 | −0.01 | 0.004 | −0.18 | 0.05 * | −0.18 |
| RT_Hypocaloric stimuli | 0.03 | 3.97 | −0.01 | 0.003 | −0.18 | 0.05 * | −0.18 |
| Food Go | −0.01 | <1 | −0.006 | 0.03 | −0.02 | 0.96 | −0.02 |
| Food No-Go | −0.003 | <1 | 0.02 | 0.03 | 0.08 | 0.40 | 0.08 |
| 1-Back hypercaloric | 0.02 | 3.83 | 4.87 | 2.49 | 0.18 | 0.05 * | 0.18 |
| 1-Back hypocaloric | 0.01 | 2.68 | 5.17 | 3.15 | 0.15 | 0.10 | 0.15 |
| 2-Back hypercaloric | 0.03 | 4.08 | 3.67 | 1.82 | 0.18 | 0.05 * | 0.18 |
| 2-Back hypocaloric | 0.03 | 4.13 | 3.60 | 1.77 | 0.19 | 0.04 * | 0.19 |
| Normal Weight | Overweight | F | p | Pη2 | |
|---|---|---|---|---|---|
| Classical Executive Tasks | |||||
| Stroop Task Reaction Times | |||||
| Congruent condition | 689.34 (73.94) | 682.74 (78.60) | <1 | 0.71 | 0.001 |
| Incongruent condition | 759.11 (87.01) | 760.29 (93.60) | <1 | 0.96 | 0.00001 |
| Stroop effect | 69.78 (50.25) | 77.55 (49.03) | <1 | 0.50 | 0.01 |
| Stroop Task % of accuracy | |||||
| Congruent condition | 96.40 (0.92) | 96.94 (0.97) | <1 | 0.68 | 0.002 |
| Incongruent condition | 95.25 (6.81) | 94.86 (5.67) | <1 | 0.79 | 0.001 |
| Go/No-Go Task | |||||
| % False Alarms | 9.62 (7.64) | 12.14 (7.62) | 2.02 | 0.16 | 0.03 |
| 1-Back and 2-Back | |||||
| 1-Back % Target Accuracy | 94.97 (8.87) | 93.86 (11.47) | <1 | 0.37 | 0.01 |
| 2-Back % Target Accuracy | 81.80 (17.61) | 85.44 (14.05) | <1 | 0.33 | 0.01 |
| Executive Tasks with Food Cue | |||||
| Picture Emotional Stroop Food Cue Reaction Times | |||||
| Neutral Cue | 712.23 (89.61) | 695.48 (84.89) | <1 | 0.43 | 0.01 |
| Color Cue | 688.36 (93.24) | 690.83 (87.79) | <1 | 0.91 | 0.00001 |
| Hypercaloric Cue | 709.18 (87.77) | 700.59 (76.61) | <1 | 0.67 | 0.003 |
| Hypocaloric Cue | 721.17 (102.19) | 693.22 (84.20) | 1.52 | 0.22 | 0.02 |
| Hypercaloric Effect | −8.93 (34.29) | −7.44 (48.71) | <1 | 0.88 | 0.0001 |
| Hypocaloric Effect | −20.93 (7.43) | 6.54 (7.42) | 4.45 | 0.04 | 0.06 |
| Picture Emotional Stroop Food Cue % of accuracy | |||||
| Neutral Cue | 96.43 (0.45) | 97.03 (0.50) | <1 | 0.37 | 0.01 |
| Color Cue | 96.73 (0.43) | 98.74 (0.49) | 2.39 | 0.13 | 0.03 |
| Hypercaloric Cue | 97.75 (0.60) | 96.29 (0.68) | <1 | 0.62 | 0.004 |
| Hypocaloric Cue | 97.25 (0.61) | 95.45 (0.70) | 1.89 | 0.17 | 0.03 |
| Hypercaloric Effect | −0.18 (3.40) | 1.11 (3.6) | 2.34 | 0.13 | 0.03 |
| Hypocaloric Effect | −0.15 (3.00) | 1.93 (3.81) | 6.49 | 0.01 | 0.09 |
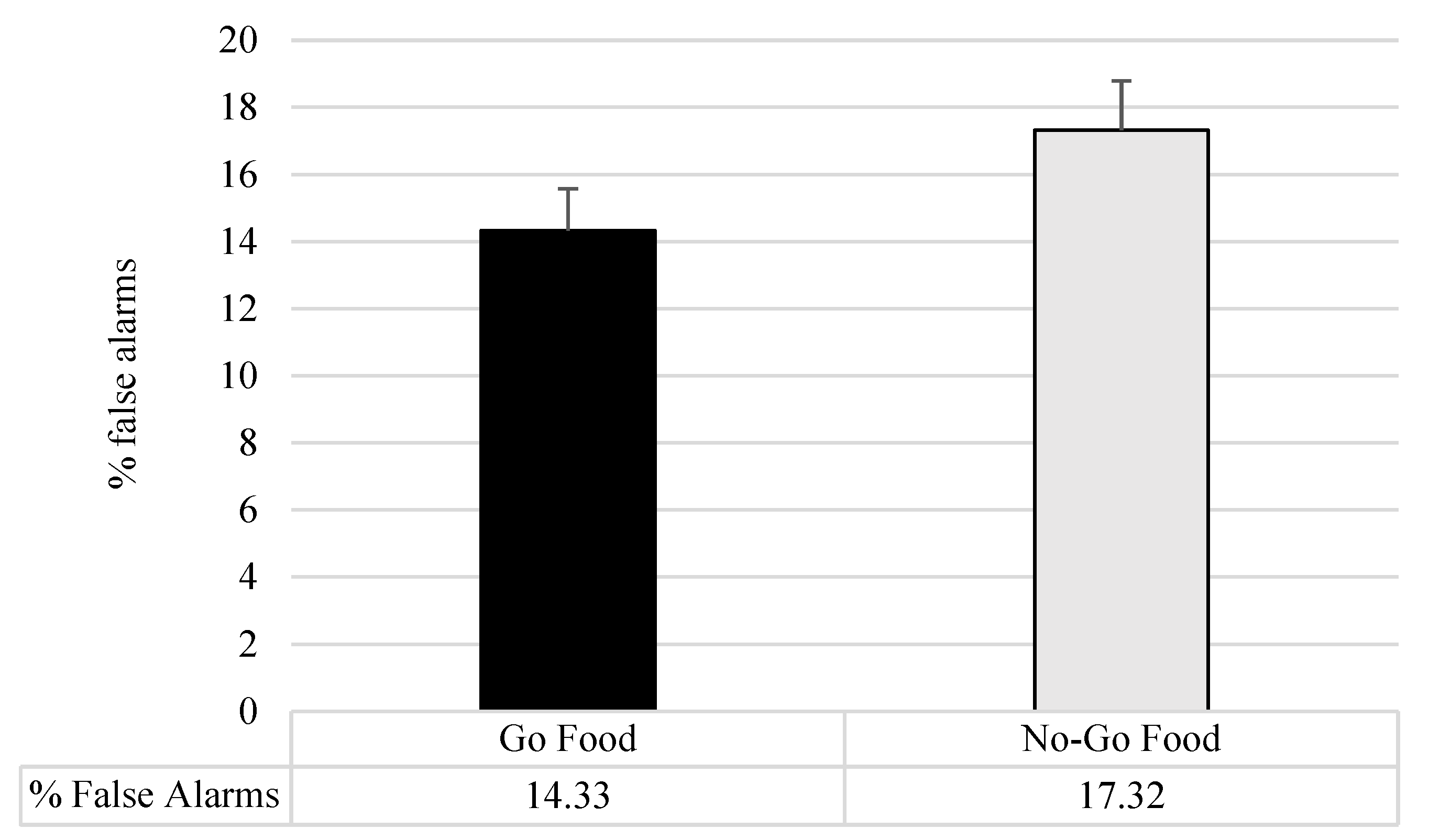
| Food Cue Go/No-Go Task % False Alarms | |||||
| Food Go Task | 13.65 (8.73) | 14.73 (10.92) | <1 | 0.66 | 0.003 |
| No-Go Task | 16.20 (11.28) | 16.94 (11.74) | <1 | 0.79 | 0.001 |
| Visual 1-Back and 2-Back Food Cue % of Target accuracy | |||||
| 1-Back Hypercaloric | 94.68 (15.95) | 96.19 (6.63) | <1 | 0.62 | 0.004 |
| 1-Back Hypocaloric | 95.32 (6.70) | 95.62 (6.70) | <1 | 0.85 | 0.001 |
| 2-Back Hypercaloric | 76.10 (21.72) | 82.09 (16.24) | 1.68 | 0.19 | 0.02 |
| 2-Back Hypocaloric | 78.20 (20.19) | 82.31 (15.21) | <1 | 0.34 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favieri, F.; Tambelli, R.; Chen, E.; Casagrande, M. Self-Regulation in Eating Behaviors: The Role of Executive Function in Response to Food Stimuli. Nutrients 2024, 16, 2318. https://doi.org/10.3390/nu16142318
Favieri F, Tambelli R, Chen E, Casagrande M. Self-Regulation in Eating Behaviors: The Role of Executive Function in Response to Food Stimuli. Nutrients. 2024; 16(14):2318. https://doi.org/10.3390/nu16142318
Chicago/Turabian StyleFavieri, Francesca, Renata Tambelli, Eunice Chen, and Maria Casagrande. 2024. "Self-Regulation in Eating Behaviors: The Role of Executive Function in Response to Food Stimuli" Nutrients 16, no. 14: 2318. https://doi.org/10.3390/nu16142318
APA StyleFavieri, F., Tambelli, R., Chen, E., & Casagrande, M. (2024). Self-Regulation in Eating Behaviors: The Role of Executive Function in Response to Food Stimuli. Nutrients, 16(14), 2318. https://doi.org/10.3390/nu16142318






