Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size
2.3. Measurements
2.3.1. Dietary Pattern
2.3.2. Fecal Microbiota
2.3.3. Covariates
2.4. Ethical Considerations
2.5. Sequence and Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Dietary Pattern Extraction
3.3. The Association between Dietary Patterns and GDM
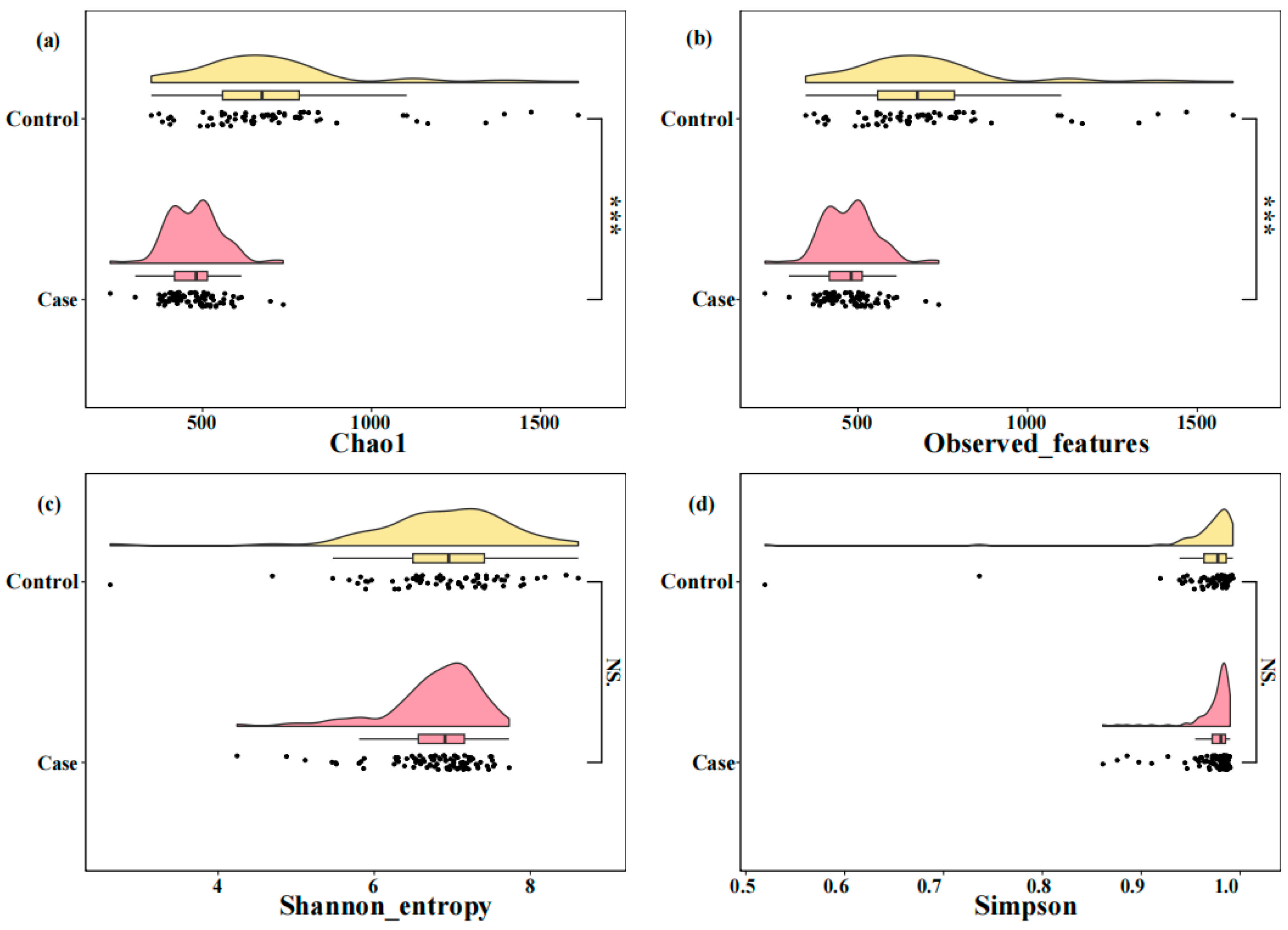
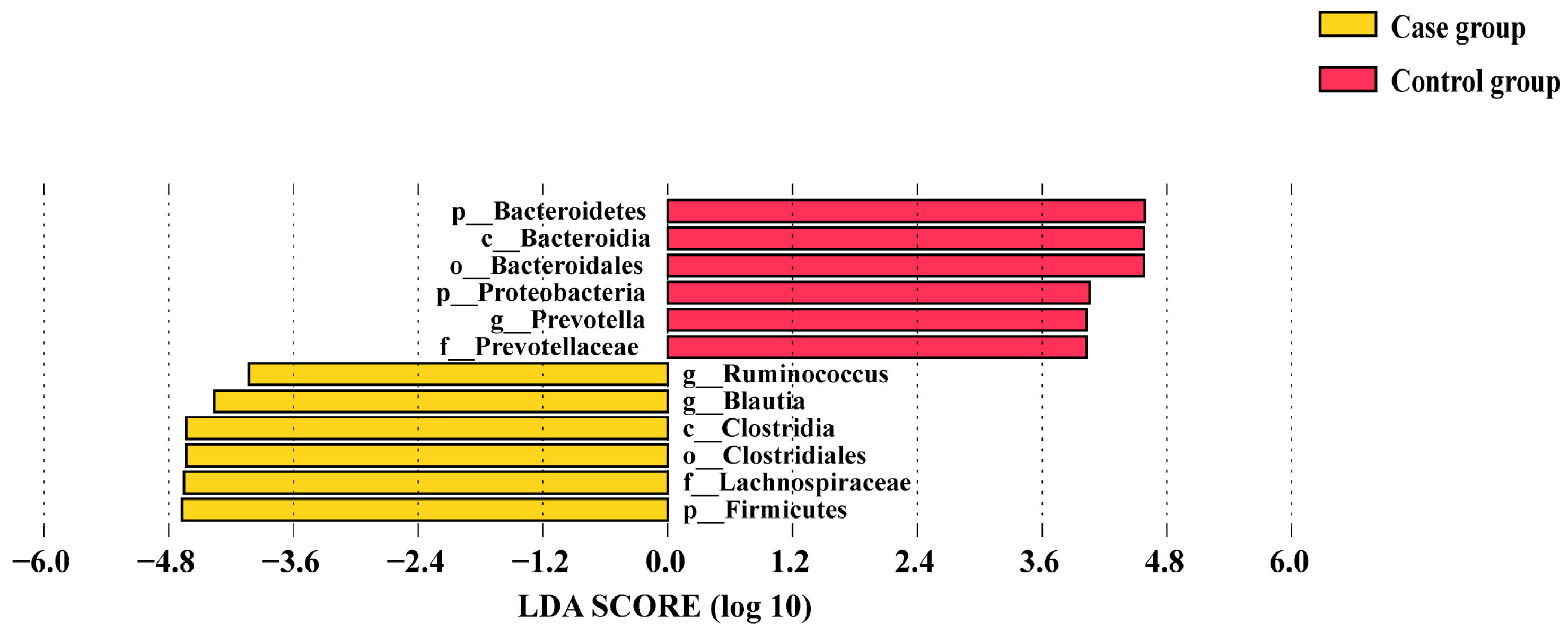
3.4. Comparison of Alpha Diversity and Differential Taxa in the Two Groups
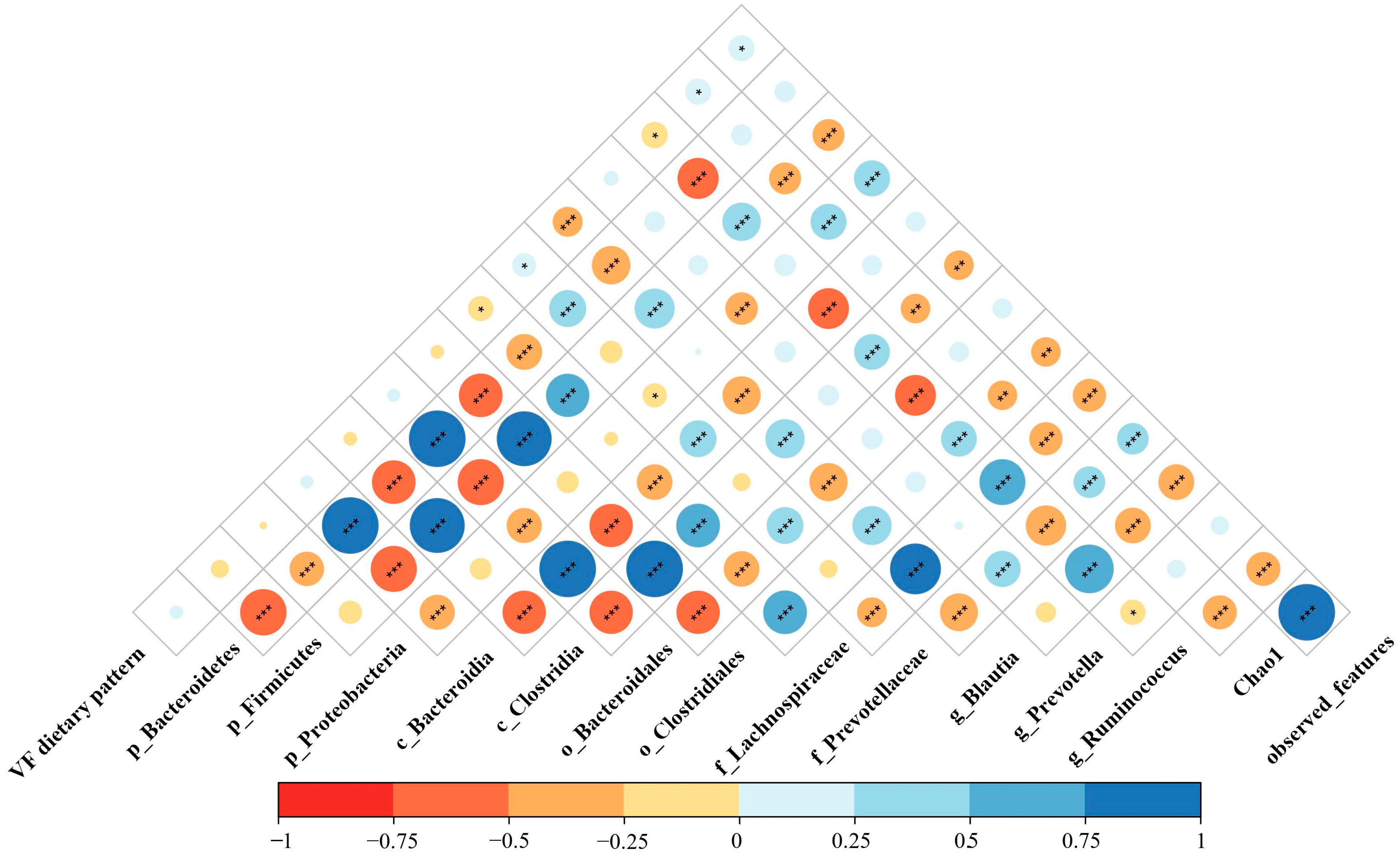
3.5. Association between Vegetables-Fruits Dietary Pattern and Gut Microbiota
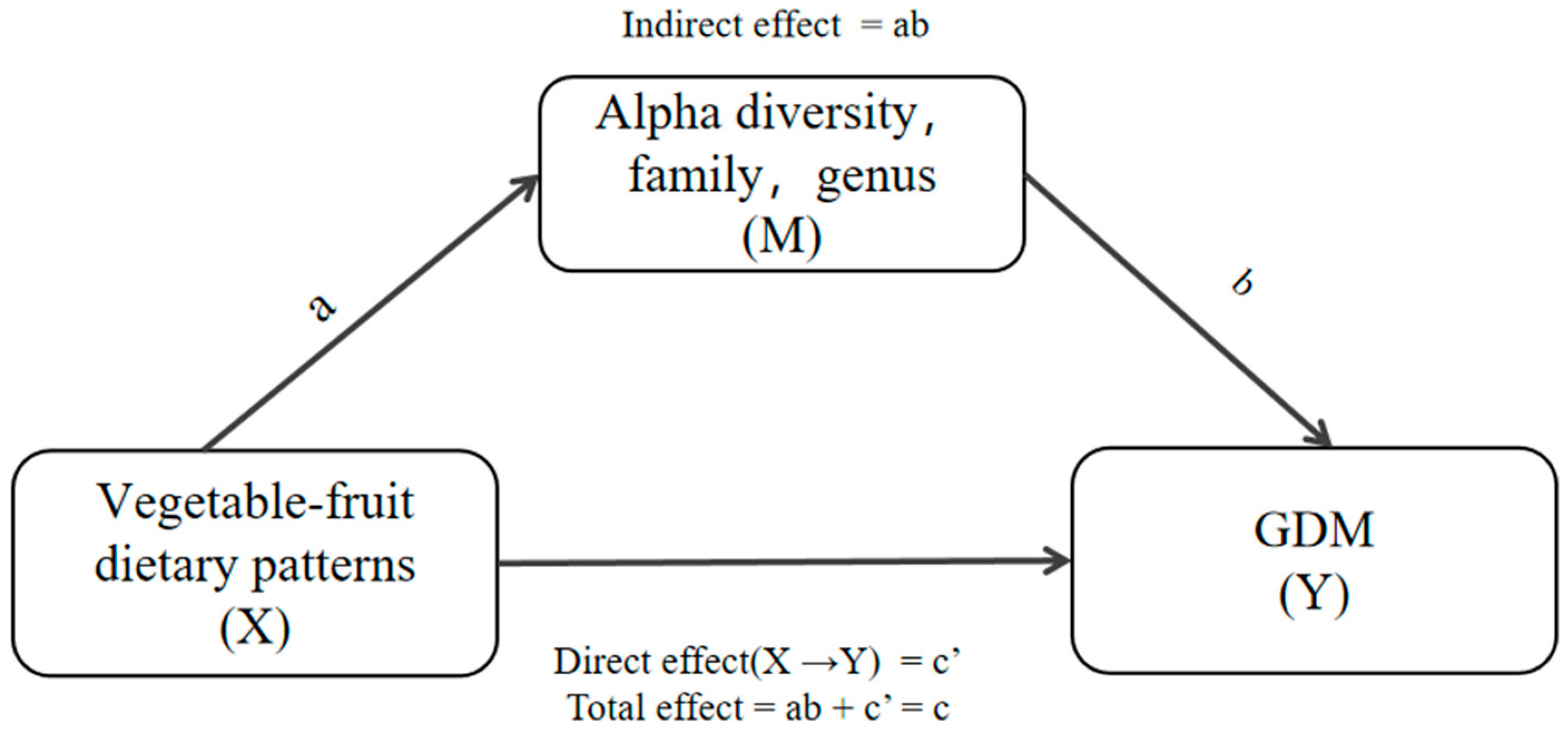
3.6. Mediation Effects of Differential Taxa on the Association between the Vegetables-Fruits Dietary Pattern and GDM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004, 27 (Suppl. S1), S88–S90. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, X.; Lu, L.; Liu, F.; Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2019, 10, 154–162. [Google Scholar] [CrossRef]
- Tieu, J.; McPhee, A.J.; Crowther, C.A.; Middleton, P.; Shepherd, E. Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochrane Database Syst. Rev. 2017, 2017, CD007222. [Google Scholar] [CrossRef]
- Xia, Y.-Y.; de Seymour, J.V.; Yang, X.-J.; Zhou, L.-W.; Liu, Y.; Yang, Y.; Beck, K.L.; Conlon, C.A.; Mansell, T.; Novakovic, B.; et al. Hair and cord blood element levels and their relationship with air pollution, dietary intake, gestational diabetes mellitus, and infant neurodevelopment. Clin. Nutr. 2023, 42, 1875–1888. [Google Scholar] [CrossRef]
- Bao, W.; Tobias, D.K.; Hu, F.B.; Chavarro, J.E.; Zhang, C. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: Prospective cohort study. BMJ—Br. Med. J. 2016, 352, h6898. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Olsen, S.F.; Zhang, C. Pre-pregnancy fried food consumption and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2014, 57, 2485–2491. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Martinez-Gonzalez, M.A.; Basterra-Gortari, F.J.; Gea, A.; Barbagallo, M.; Bes-Rastrollo, M. Fast Food Consumption and Gestational Diabetes Incidence in the SUN Project. PLoS ONE 2014, 9, e106627. [Google Scholar] [CrossRef]
- Quan, W.; Zeng, M.; Jiao, Y.; Li, Y.; Xue, C.; Liu, G.; Wang, Z.; Qin, F.; He, Z.; Chen, J. Western Dietary Patterns, Foods, and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2021, 12, 1353–1364. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.H.; Della Corte, K.; Yu, D.K.; Xue, H.M.; Shan, S.F.; Tian, G.; Liang, Y.; Zhang, J.Y.; He, F.; et al. Relevance of dietary glycemic index, glycemic load and fiber intake before and during pregnancy for the risk of gestational diabetes mellitus and maternal glucose homeostasis. Clin. Nutr. 2021, 40, 2791–2799. [Google Scholar] [CrossRef]
- Li, M.; Grewal, J.; Hinkle, S.N.; Yisahak, S.F.; Grobman, W.A.; Newman, R.B.; Skupski, D.W.; Chien, E.K.; Wing, D.A.; Grantz, K.L.; et al. Healthy dietary patterns and common pregnancy complications: A prospective and longitudinal study. Am. J. Clin. Nutr. 2021, 114, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Mohtashaminia, F.; Hosseini, F.; Jayedi, A.; Mirmohammadkhani, M.; Emadi, A.; Takfallah, L.; Shab-Bidar, S. Adherence to the Mediterranean diet and risk of gestational diabetes: A prospective cohort study. BMC Pregnancy Childbirth 2023, 23, 647. [Google Scholar] [CrossRef]
- Zhang, C.L.; Liu, S.M.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Roustazadeh, A.; Mir, H.; Jafarirad, S.; Mogharab, F.; Hosseini, S.A.; Abdoli, A.; Erfanian, S. A dietary pattern rich in fruits and dairy products is inversely associated to gestational diabetes: A case-control study in Iran. BMC Endocr. Disord. 2021, 21, 647. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef]
- Sedaghat, F.; Akhoondan, M.; Ehteshami, M.; Aghamohammadi, V.; Ghanei, N.; Mirmiran, P.; Rashidkhani, B. Maternal Dietary Patterns and Gestational Diabetes Risk: A Case-Control Study. J. Diabetes Res. 2017, 2017, 5173926. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.T.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.X.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef]
- Simpson, S.; Smith, L.; Bowe, J. Placental peptides regulating islet adaptation to pregnancy: Clinical potential in gestational diabetes mellitus. Curr. Opin. Pharmacol. 2018, 43, 59–65. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Xiao, X.H.; Zhang, Q.; Zheng, J.; Li, M.; Wang, X.J.; Deng, M.Q.; Zhai, X.; Liu, J.Y. Gut microbiota might be a crucial factor in deciphering the metabolic benefits of perinatal genistein consumption in dams and adult female offspring. Food Funct. 2019, 10, 4505–4521. [Google Scholar] [CrossRef]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Angelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, gix058. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.-L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus with Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, S.; Feng, Y.; Song, Y.; Lv, N.; Liu, F.; Zhang, X.; Wang, S.; Wei, Y.; Li, S.; et al. Perturbations of gut microbiota in gestational diabetes mellitus patients induce hyperglycemia in germ-free mice. J. Dev. Orig. Health Dis. 2020, 11, 580–588. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, B.; Hou, G.; Lei, L.; Mei, Z.; Jia, X.; Chen, X.; Zhu, W.; Li, J.; Kuang, Y.; et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Leo, E.E.M.; Campos, M.R.S. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition 2020, 71, 110609. [Google Scholar]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef]
- Ruiz-Saavedra, S.; Salazar, N.; Suarez, A.; de los Reyes-Gavilan, C.G.; Gueimonde, M.; Gonzalez, S. Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects. Nutrients 2020, 12, 3828. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zareei, S.; Homayounfar, R.; Naghizadeh, M.M.; Ehrampoush, E.; Rahimi, M. Dietary pattern in pregnancy and risk of gestational diabetes mellitus (GDM). Diabetes Metab. Syndr.-Clin. Res. Rev. 2018, 12, 399–404. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosomatics 1971, 12, 371–379. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.W.; Yang, H.L.; Zhang, P.; Feng, Y.L.; Wang, K.K.; Wang, Y.; Wang, S.P.; Zhang, Y.W. A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women. Diabetes Metab. J. 2020, 44, 887–896. [Google Scholar] [CrossRef]
- de Seymour, J.; Chia, A.R.; Colega, M.; Jones, B.; McKenzie, E.; Cai, S.R.; Godfrey, K.; Kwek, K.; Saw, S.M.; Conlon, C.; et al. Maternal Dietary Patterns and Gestational Diabetes Mellitus in a Multi-Ethnic Asian Cohort: The GUSTO Study. Nutrients 2016, 8, 574. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Merino, J.; Sun, Q.; Fito, M.; Salas-Salvado, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxidative Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Review Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S. [Google Scholar] [CrossRef]
- Oude Griep, L.M.; Wang, H.; Chan, Q. Empirically-derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr. Nutr. Rep. 2013, 2, 97–104. [Google Scholar] [CrossRef]
- Liang, Y.; Gong, Y.; Zhang, X.; Yang, D.; Zhao, D.; Quan, L.; Zhou, R.; Bao, W.; Cheng, G. Dietary Protein Intake, Meat Consumption, and Dairy Consumption in the Year Preceding Pregnancy and During Pregnancy and Their Associations with the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study in Southwest China. Front. Endocrinol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Mari-Sanchis, A.; Diaz-Jurado, G.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: The SUN project. Eur. J. Nutr. 2018, 57, 939–949. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.X.; Wang, Y.; Wang, F.; Huang, Y.C.; Chen, D.; Pan, X.F.; Pan, A. Associations between plasma n-3 polyunsaturated fatty acids and gestational diabetes mellitus in the second trimester. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2022, 56, 312–321. [Google Scholar]
- Ortega-Senovilla, H.; Schaefer-Graf, U.; Herrera, E. Pregnant women with gestational diabetes and with well controlled glucose levels have decreased concentrations of individual fatty acids in maternal and cord serum. Diabetologia 2020, 63, 864–874. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The Role of Energy, Nutrients, Foods, and Dietary Patterns in the Development of Gestational Diabetes Mellitus: A Systematic Review of Observational Studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Ma, S.J.; You, Y.P.; Huang, L.T.; Long, S.S.; Zhang, J.Y.; Guo, C.H.; Zhang, N.; Wu, X.R.; Xiao, Y.N.; Tan, H.Z. Alterations in Gut Microbiota of Gestational Diabetes Patients During the First Trimester of Pregnancy. Front. Cell. Infect. Microbiol. 2020, 10, 58. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.Q.; Zhang, Y.Y.; Shan, C.J.; Zhang, Y.Y.; Fang, K.; Xia, Y.K.; Shi, Z.H. Relationships between gut microbiota, plasma glucose and gestational diabetes mellitus. J. Diabetes Investig. 2021, 12, 641–650. [Google Scholar] [CrossRef]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Schade, J.; Weidenmaier, C. Cell wall glycopolymers of Firmicutes and their role as nonprotein adhesins. FEBS Lett. 2016, 590, 3758–3771. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhao, C.-N.; Xu, X.-Y.; Tang, G.-Y.; Corke, H.; Gan, R.-Y.; Li, H.-B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H.; et al. Article Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Grahnemo, L.; Nethander, M.; Coward, E.; Gabrielsen, M.E.; Sree, S.; Billod, J.M.; Engstrand, L.; Abrahamsson, S.; Langhammer, A.; Hveem, K.; et al. Cross-sectional associations between the gut microbe Ruminococcus gnavus and features of the metabolic syndrome. Lancet Diabetes Endocrinol. 2022, 10, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Ye, D.; Huang, J.; Wu, J.; Xie, K.; Gao, X.; Yan, K.; Zhang, P.; Tao, Y.; Li, Y.; Zang, S.; et al. Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microbes 2023, 15, 2154552. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xu, Q.; Huang, W.; Yan, Q.; Chen, Y.; Zhang, L.; Tian, Z.; Liu, T.; Yuan, X.; Liu, C.; et al. Gestational Diabetes Mellitus Is Associated with Reduced Dynamics of Gut Microbiota during the First Half of Pregnancy. Msystems 2020, 5, e00109-20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, M.; Zhang, J.; Sun, Z.; Ran, L.; Ban, Y.; Wang, B.; Hou, X.; Zhai, S.; Ren, L.; et al. Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E247–E253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, P.N.; Tong, C.; Zheng, P.; Qi, H. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 2020, 12, 1840765. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Oliveira, A.; Lopes, C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr. Res. 2013, 33, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gan, Y.; Li, Y.T.; He, W.Z.; Wu, W.Z.; Wang, K.J.; Li, Q. Association of gestational diabetes mellitus with changes in gut microbiota composition at the species level. BMC Microbiol. 2021, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Egshatyan, L.; Kashtanova, D.; Popenko, A.; Tkacheva, O.; Tyakht, A.; Alexeev, D.; Karamnova, N.; Kostryukova, E.; Babenko, V.; Vakhitova, M.; et al. Gut microbiota and diet in patients with different glucose tolerance. Endocr. Connect. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef]
| Control Group (n = 78) | Case Group (n = 107) | p | |
|---|---|---|---|
| Age, years, M (P25, P75) | 32 (29, 34) | 32 (29, 35) | 0.089 |
| Gestational weeks, weeks, M (P25, P75) | 24 (24, 25) | 25 (24, 26) | 0.052 |
| Pre-pregnancy BMI, kg/m2, Mean ± SD # | 20.91 ± 2.35 | 22.65 ± 3.25 | <0.001 |
| Weight gain during pregnancy, kg, M (P25, P75) # | 6.45 (4, 8) | 6.00 (5, 8) | 0.375 |
| Education level, n (%) | 0.687 | ||
| Senior middle school and below | 8 (10.3) | 13 (12.1) | |
| Associate degree | 21 (26.9) | 23 (21.5) | |
| Bachelor’s degree | 41 (52.5) | 55 (51.4) | |
| Master’s degree and above | 8 (10.3) | 16 (15.0) | |
| Employment status, n (%) | 0.245 | ||
| Unemployed | 32 (41.0) | 35 (32.7) | |
| Employed | 46 (59.0) | 72 (67.3) | |
| Household income status, RMB per month, n (%) | 0.291 | ||
| <5000 | 10 (12.8) | 8 (7.5) | |
| 5000~9999 | 19 (24.4) | 35 (32.7) | |
| ≥10,000 | 49 (62.8) | 64 (59.8) | |
| Parity, times, n (%) | 0.004 | ||
| 0 | 59 (75.6) | 59 (55.1) | |
| ≥1 | 19 (24.4) | 48 (44.9) | |
| Passive smoking, n (%) | 0.021 | ||
| No | 70 (89.7) | 82 (76.6) | |
| Yes | 8 (10.3) | 25 (23.4) | |
| Walking time, min/d, n (%) | 0.719 | ||
| ≤20 | 52 (66.7) | 74 (69.2) | |
| >20 | 26 (33.3) | 33 (30.8) | |
| PSQI score levels, n (%) | 0.715 | ||
| Good quality | 49 (62.8) | 70 (65.4) | |
| Bad quality | 29 (37.2) | 37 (34.6) | |
| Anxiety symptoms, n (%) | 0.719 | ||
| No | 76 (97.4) | 104 (97.2) | |
| Yes | 2 (2.6) | 3 (2.8) | |
| Depression symptoms, n (%) | 0.336 | ||
| No | 63 (80.8) | 80 (74.8) | |
| Yes | 15 (19.2) | 27 (25.2) |
| β | Wald χ2 | OR (95% CI) | p | |
|---|---|---|---|---|
| Crude Model | ||||
| low (Ref.) | ||||
| high | 0.98 | 10.10 | 0.38 (0.20, 0.68) | 0.001 |
| Model 1 | ||||
| low (Ref.) | ||||
| high | 1.08 | 10.14 | 0.34 (0.17, 0.65) | 0.001 |
| Model 2 | ||||
| low (Ref.) | ||||
| high | −1.10 | 7.12 | 0.33 (0.15, 0.74) | 0.008 |
| Mediators | Indirect Effect | Direct Effect | Proportion Mediated (%) | ||||
|---|---|---|---|---|---|---|---|
| β | SE | Boot 95% CI | β | SE | Boot 95% CI | ||
| Lachnospiraceae family | 0.094 | 0.040 | −0.175, −0.018 * | −0.140 | 0.064 | −0.265, −0.020 | 40.17 |
| Prevotellaceae family | −0.024 | 0.017 | −0.061, 0.001 | −0.210 | 0.078 | −0.359, −0.065 | 10.26 |
| Blautia genus | −0.092 | 0.037 | −0.177, −0.033 * | −0.143 | 0.073 | −0.283, −0.006 | 39.15 |
| Ruminococcus genus | −0.059 | 0.022 | −0.126, −0.022 * | −0.175 | 0.069 | −0.314, −0.041 | 25.21 |
| Chao1 | −0.075 | 0.035 | −0.148, −0.010 * | −0.160 | 0.065 | −0.292, −0.039 | 31.91 |
| Observed features | −0.075 | 0.035 | −0.142, −0.009 * | −0.159 | 0.063 | −0.280, −0.040 | 32.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Peng, C.; Zou, H.; Pan, Y.; Wu, M.; Xie, Q.; Lin, Q. Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota. Nutrients 2024, 16, 2300. https://doi.org/10.3390/nu16142300
Shan X, Peng C, Zou H, Pan Y, Wu M, Xie Q, Lin Q. Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota. Nutrients. 2024; 16(14):2300. https://doi.org/10.3390/nu16142300
Chicago/Turabian StyleShan, Xiaoxi, Caixia Peng, Hanshuang Zou, Yunfeng Pan, Minchan Wu, Qingqing Xie, and Qian Lin. 2024. "Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota" Nutrients 16, no. 14: 2300. https://doi.org/10.3390/nu16142300
APA StyleShan, X., Peng, C., Zou, H., Pan, Y., Wu, M., Xie, Q., & Lin, Q. (2024). Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota. Nutrients, 16(14), 2300. https://doi.org/10.3390/nu16142300







