Effects of Chronic Barley Consumption on Upper Respiratory Tract Symptoms in Japanese Healthy Adults: A Randomized, Parallel-Group, Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Test Foods
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Physical Conditions
3.2.1. Cumulative Days with URTS-Related Symptoms and Special Notes
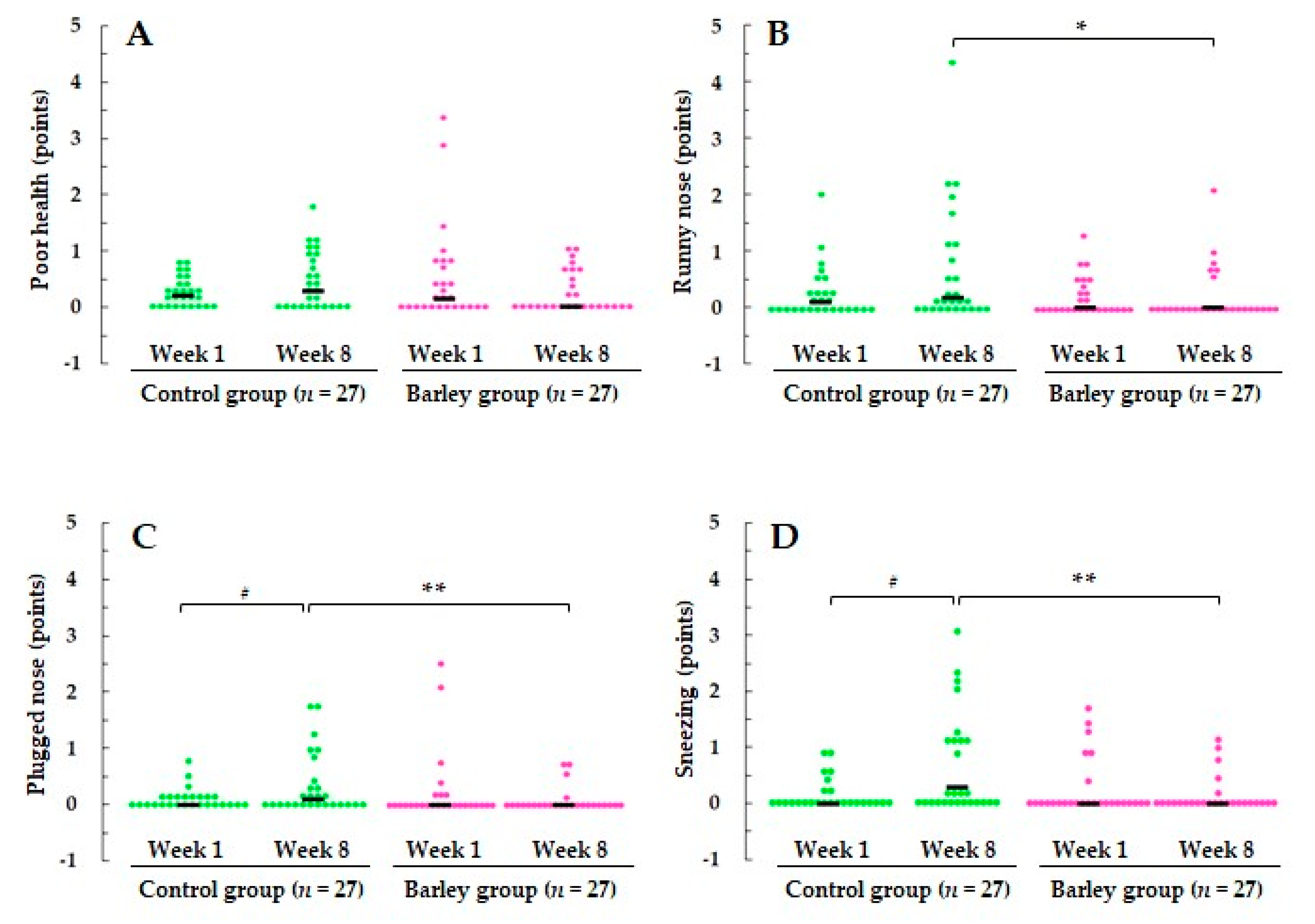
3.2.2. Severity of URTS-Related Symptoms
3.3. Mood Status
3.4. NK Cell Activity
4. Discussion
4.1. Scientific Significance of This Study
4.2. Mechanism Underlying the Immunomodulatory Effect of β-(1,3/1,4) Glucans
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Canazza, E.; Tessari, P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.G.; Granfeldt, Y.E.; Björck, I.M. Products based on a high fiber barley genotype, but not on common barley or oats, lower postprandial glucose and insulin responses in healthy humans. J. Nutr. 1996, 126, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.H.; Hudson, C.A.; Knuckles, B.E.; Chiu, M.-C.M.; Sayre, R.N.; Turnlund, J.R.; Schneeman, B.O. Effect of Barley β-Glucan in Durum Wheat Pasta on Human Glycemic Response. Cereal Chem. 1997, 74, 293–296. [Google Scholar] [CrossRef]
- McIntosh, G.H.; Whyte, J.; McArthur, R.; Nestel, P.J. Barley and wheat foods: Influence on plasma cholesterol concentrations in hypercholesterolemic men. Am. J. Clin. Nutr. 1991, 53, 1205–1209. [Google Scholar] [CrossRef]
- Bourdon, I.; Yokoyama, W.; Davis, P.; Hudson, C.; Backus, R.; Richter, D.; Knuckles, B.; Schneeman, B.O. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am. J. Clin. Nutr. 1999, 69, 55–63. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Food labeling: Health claims; soluble fiber from certain foods and risk of coronary heart disease. Interim final rule. Fed. Regist. 2008, 73, 9938–9947. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to beta-glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. ESFA J. 2009, 7, 1254. [Google Scholar]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhao, J.; Wang, J.; Song, Q.; Zhao, C. The phagocytic receptors of β-glucan. Int. J. Biol. Macromol. 2022, 205, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.F.; Chan, W.K.; Sze, D.M.Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper respiratory tract infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Talbott, J.A. Baker’s yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J. Am. Coll. Nutr. 2012, 31, 295–300. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef]
- Mah, E.; Kaden, V.N.; Kelley, K.M.; Liska, D.J. Beverage Containing Dispersible Yeast β-Glucan Decreases Cold/Flu Symptomatic Days After Intense Exercise: A Randomized Controlled Trial. J. Diet. Suppl. 2020, 17, 200–210. [Google Scholar] [CrossRef]
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects. J. Am. Coll. Nutr. 2019, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Falcone, P.H.; Crowley, D.C.; Sulley, A.M.; Campbell, M.; Zakaria, N.; Lasrado, J.A.; Fritz, E.P.; Herrlinger, K.A. Effect of a Euglena gracilis Fermentate on Immune Function in Healthy, Active Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Miura, A.; Naito, J.; Nishida, N.; Ishibashi, K.I.; Adachi, Y.; Ohno, N.; Naito, Y. High-parameter immune profiling and subjective health assessment of the immunomodulatory effects of paramylon-rich Euglena gracilis EOD-1: A randomized, double-blind, placebo-controlled, parallel-group study. J. Funct. Foods 2023, 109, 105804. [Google Scholar] [CrossRef]
- Lee, Y.J.; Paik, D.J.; Kwon, D.Y.; Yang, H.J.; Park, Y. Agrobacterium sp.-derived β-1,3-glucan enhances natural killer cell activity in healthy adults: A randomized, double-blind, placebo-controlled, parallel-group study. Nutr. Res. Pract. 2017, 11, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Nan, F.H.; Liu, M.W.; Yang, M.F.; Chang, Y.C.; Chen, S. Evaluation of Immune Modulation by β-1,3; 1,6 D-Glucan Derived from Ganoderma lucidum in Healthy Adult Volunteers, A Randomized Controlled Trial. Foods 2023, 312, 659. [Google Scholar] [CrossRef] [PubMed]
- Cortijo-Alfonso, M.E.; Romero, M.P.; Macià, A.; Yuste, S.; Moralejo, M.; Rubió-Piqué, L.; Piñol-Felis, C. Effect of Barley and Oat Consumption on Immune System, Inflammation and Gut Microbiota: A Systematic Review of Randomized Controlled Trials. Curr. Nutr. Rep. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Brown, R.; Mundt, M.; Safdar, N.; Dye, L.; Maberry, R.; Alt, J. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J. Clin. Epidemiol. 2005, 58, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Heuchert, J.; McNair, D.M.; Yokoyama, K.; Watanabe, K. Japanese Translation of POMS2: Profile of Mood States; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Gootjes, J.; Tel, R.M.; Bergkamp, F.J.M.; Gorgels, J.P.M.C. Laboratory evaluation of a novel capillary blood sampling device for measuring eight clinical chemistry parameters and HbA1c. Clin. Chim. Acta. 2009, 401, 152–157. [Google Scholar] [CrossRef]
- Doi, Y.; Ito, M.; Fujita, M.; Domon, E.; Ishikawa, N.; Katayama, T.; Kamio, M. Breeding of a new naked barley waxy cultivar “Daishimochi”. Bull. Shikoku Natl. Agric. Exp. Stn. 1999, 64, 21–36. [Google Scholar]
- Tonooka, T. Breeding of Waxy Barley Cultivars in the National Barley Breeding Program of Japan. JARQ-Jpn. Agr. Res. Q. 2023, 57, 251–259. [Google Scholar] [CrossRef]
- Lee, C.J.; Horsley, R.D.; Manthey, F.A.; Schwarz, P.B. Comparisons of β-Glucan Content of Barley and Oat. Cereal Chem. 1997, 74, 571–575. [Google Scholar] [CrossRef]
- Elsner, L.; Dressel, R. 51Cr-release to monitor NK cell cytotoxicity. Methods Enzymol. 2020, 631, 497–512. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Ullum, H. NK cell response to physical activity: Possible mechanisms of action. Med. Sci. Sports Exerc. 1994, 26, 140–146. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Smith, L.L.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Kaminsky, D.E.; Shute, M. Cytokine changes after a marathon race. J. Appl. Physiol. 2001, 91, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Theresine, M.; Patil, N.D.; Zimmer, J. Airway Natural Killer Cells and Bacteria in Health and Disease. Front. Immunol. 2020, 11, 585048. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carmichael, M.D.; Ghaffar, A.; Mayer, E.P. Effects of oat beta-glucan on innate immunity and infection after exercise stress. Med. Sci. Sports Exerc. 2004, 36, 1321–1327. [Google Scholar] [CrossRef]
- Estrada, A.; Yun, C.H.; Van Kessel, A.; Li, B.; Hauta, S.; Laarveld, B. Immunomodulatory activities of oat beta-glucan in vitro and in vivo. Microbiol. Immunol. 1997, 41, 991–998. [Google Scholar] [CrossRef]
- Derakhshan, A.; Khodadoost, M.; Ghanei, M.; Gachkar, L.; Hajimahdipour, H.; Taghipour, A.; Yousefi, J.; Khoshkhui, M.; Azad, J.F. Effects of a Novel Barley-Based Formulation on Allergic Rhinitis: A Randomized Controlled Trial. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1224–1231. [Google Scholar] [CrossRef]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of Flavonoids on Upper Respiratory Tract Infections and Immune Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Gorgori-González, A.; Massot-Cladera, M.; Castell, M.; Pérez-Cano, F.J. Does Flavonoid Consumption Improve Exercise Performance? Is It Related to Changes in the Immune System and Inflammatory Biomarkers? A Systematic Review of Clinical Studies since 2005. Nutrients 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, N.; Ono, H.; Yanagisawa, T. Changes in anthocyanins in the grains of purple waxy hull-less barley during seed maturation and after harvest. J. Agric. Food Chem. 2008, 56, 5770–5774. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Adachi, Y.; Ishibashi, K.; Tsubaki, K.; Ohno, N. Binding capacity of a barley beta-D-glucan to the beta-glucan recognition molecule dectin-1. J. Agric. Food Chem. 2008, 56, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]




| Packed Cooked White Rice | Packed Cooked Barley | |
|---|---|---|
| Energy (kcal) | 147 | 117 |
| Protein (g) | 2.2 | 2.8 |
| Fat (g) | 0.2 | 1.0 |
| Carbohydrate (g) | 34.1 | 27.2 |
| β-glucan (g) | 0.0 | 1.8 |
| Control Group (n = 27) | Barley Group (n = 27) | p b | |||
|---|---|---|---|---|---|
| Male/Female (n) | 10/17 | 9/18 | 0.776 c | ||
| Age (years) | 48.0 | (33.0–55.0) a | 45.0 | (30.0–52.0) | 0.436 |
| Body mass index (kg/m2) | 20.7 | (19.7–21.2) | 20.5 | (19.3–22.5) | 0.945 |
| The rate of completion of the test food (%) | 100.0 | (98.2–100) | 100.0 | (98.2–100) | 0.906 |
| Symptom | p a | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Fever | Control group (n = 27) | 1 | 1507 | 0.375 b |
| (days) | Barley group (n = 27) | 3 | 1504 | |
| Poor health | Control group (n = 27) | 8 | 1500 | 0.489 |
| (days) | Barley group (n = 27) | 11 | 1496 | |
| Runny nose | Control group (n = 27) | 14 | 1494 | 0.296 |
| (days) | Barley group (n = 27) | 9 | 1498 | |
| Plugged nose | Control group (n = 27) | 10 | 1498 | 0.179 |
| (days) | Barley group (n = 27) | 7 | 1500 | |
| Sneezing | Control group (n = 27) | 10 | 1498 | 0.038 b |
| (days) | Barley group (n = 27) | 2 | 1505 | |
| Sore throat | Control group (n = 27) | 7 | 1501 | 0.795 |
| (days) | Barley group (n = 27) | 8 | 1499 | |
| Scratchy throat | Control group (n = 27) | 4 | 1504 | 0.266 b |
| (days) | Barley group (n = 27) | 8 | 1499 | |
| Cough | Control group (n = 27) | 6 | 1502 | 0.315 |
| (days) | Barley group (n = 27) | 10 | 1497 | |
| Hoarseness | Control group (n = 27) | 4 | 1504 | 0.687 b |
| (days) | Barley group (n = 27) | 2 | 1505 | |
| Head congestion | Control group (n = 27) | 7 | 1501 | 0.795 |
| (days) | Barley group (n = 27) | 8 | 1499 | |
| Chest congestion | Control group (n = 27) | 2 | 1506 | 1.000 b |
| (days) | Barley group (n = 27) | 1 | 1506 | |
| Feeling tired | Control group (n = 27) | 52 | 1456 | <0.0001 |
| (days) | Barley group (n = 27) | 13 | 1494 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, R.; Ishikawa, C.; Kawasaki, T.; Kobori, T.; Shoji, T.; Takayama, Y. Effects of Chronic Barley Consumption on Upper Respiratory Tract Symptoms in Japanese Healthy Adults: A Randomized, Parallel-Group, Controlled Trial. Nutrients 2024, 16, 2298. https://doi.org/10.3390/nu16142298
Araki R, Ishikawa C, Kawasaki T, Kobori T, Shoji T, Takayama Y. Effects of Chronic Barley Consumption on Upper Respiratory Tract Symptoms in Japanese Healthy Adults: A Randomized, Parallel-Group, Controlled Trial. Nutrients. 2024; 16(14):2298. https://doi.org/10.3390/nu16142298
Chicago/Turabian StyleAraki, Risa, Chiaki Ishikawa, Tomomi Kawasaki, Toshiro Kobori, Toshihiko Shoji, and Yoshiharu Takayama. 2024. "Effects of Chronic Barley Consumption on Upper Respiratory Tract Symptoms in Japanese Healthy Adults: A Randomized, Parallel-Group, Controlled Trial" Nutrients 16, no. 14: 2298. https://doi.org/10.3390/nu16142298
APA StyleAraki, R., Ishikawa, C., Kawasaki, T., Kobori, T., Shoji, T., & Takayama, Y. (2024). Effects of Chronic Barley Consumption on Upper Respiratory Tract Symptoms in Japanese Healthy Adults: A Randomized, Parallel-Group, Controlled Trial. Nutrients, 16(14), 2298. https://doi.org/10.3390/nu16142298






