Milk Fat Globule Membrane-Containing Protein Powder Promotes Fitness in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. MFGM-Containing Protein Powder (MProPow)
2.2. C. elegans Maintenance
2.3. Lifespan Analyses
2.4. Motility Measurement
2.5. RNA Sequencing
2.6. Quantitative RT-PCR (qRT-PCR)
2.7. Pseudomonas aeruginosa Assay
2.8. RNA Interference (RNAi)
2.9. Statistical Analysis
3. Results
3.1. MFGM-Containing Protein Powder (MProPow) Improves C. elegans Motility
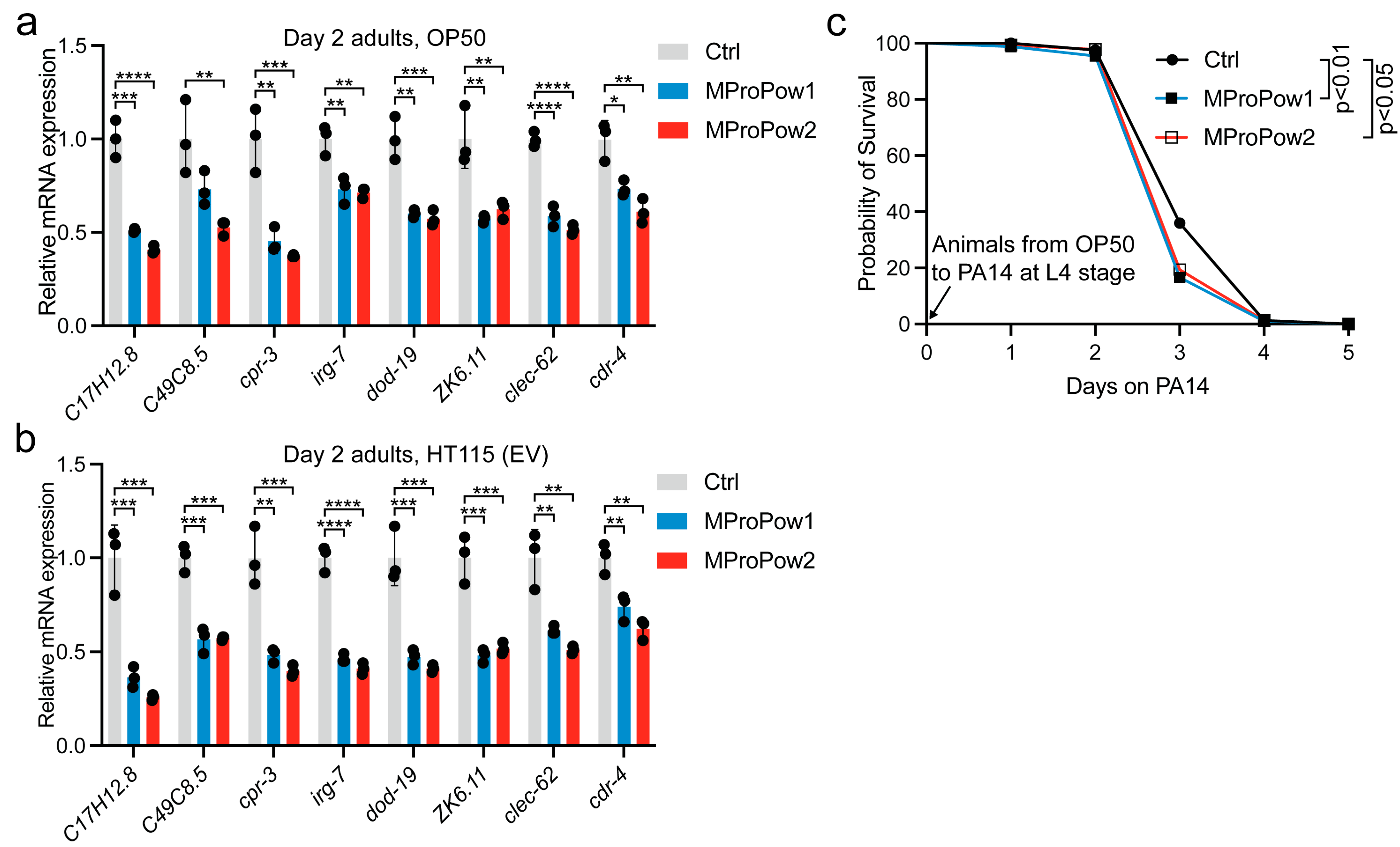
3.2. MProPow Decreases the Expression of Genes Related to Innate Immunity, and Reduces the Survival on Pathogenic Bacteria
3.3. Downregulation of cpr-3 Promotes Fitness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Zou, X.; Guo, Z.; Jin, Q.; Huang, J.; Cheong, L.; Xu, X.; Wang, X. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chem. 2015, 185, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jiang, B.; Zhou, L.; Ma, J.; Yang, L.; Wang, F.; Liu, H.; Zhang, N.; Li, X.; Petocz, P.; et al. Neurodevelopmental outcomes of healthy Chinese term infants fed infant formula enriched in bovine milk fat globule membrane for 12 months—A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2021, 30, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Calvo, M.V.; Kohen, V.L.; Díaz-Mardomingo, C.; García-Herranz, S.; Baliyan, S.; Tomé-Carneiro, J.; Colmenarejo, G.; Visioli, F.; Venero, C.; Fontecha, J. Milk fat globule membrane-enriched milk improves episodic memory: A randomized, parallel, double-blind, placebo-controlled trial in older adults. J. Funct. Foods 2023, 111, 105849. [Google Scholar] [CrossRef]
- Kim, H.; Won, C.W.; Kim, M.; Kojima, N.; Fujino, K.; Osuka, Y.; Hosoi, E.; Suzuki, T. The effects of exercise and milk-fat globule membrane (MFGM) on walking parameters in community-dwelling elderly Japanese women with declines in walking ability: A randomized placebo controlled trial. Arch. Gerontol. Geriatr. 2019, 83, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jyväkorpi, S.K.; Niskanen, R.T.; Markkanen, M.; Salminen, K.; Sibakov, T.; Lehtonen, K.-M.; Kunvik, S.; Pitkala, K.H.; Turpeinen, A.M.; Suominen, M.H. Effect of Milk Fat Globule Membrane- and Protein-Containing Snack Product on Physical Performance of Older Women-A Randomized Controlled Trial. Nutrients 2023, 15, 2922. [Google Scholar] [CrossRef]
- Lai, C.H.; Chou, C.Y.; Ch’ang, L.Y.; Liu, C.S.; Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, K.; Wang, Q.; Zhong, G.; Zhang, W.; Jiang, Y.; Mao, X.; Li, X.; Huang, Z. Caenorhabditis elegans as an emerging model in food and nutrition research: Importance of standardizing base diet. Crit. Rev. Food Sci. Nutr. 2024, 64, 3167–3185. [Google Scholar] [CrossRef]
- Carretero, M.; Solis, G.M.; Petrascheck, M. C. elegans as Model for Drug Discovery. Curr. Top. Med. Chem. 2017, 17, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Sibakov, T.; Tossavainen, O. Milk Product and Preparation Method. EP Patent 2632277B1, 4 September 2013. [Google Scholar]
- Laatikainen, R.; Salmenkari, H.; Sibakov, T.; Vapaatalo, H.; Turpeinen, A. Randomised Controlled Trial: Partial Hydrolysation of Casein Protein in Milk Decreases Gastrointestinal Symptoms in Subjects with Functional Gastrointestinal Disorders. Nutrients 2020, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Timmons, L.; Court, D.L.; Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263, 103–112. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Tan, M.W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 8. [Google Scholar] [CrossRef]
- Revtovich, A.V.; Lee, R.; Kirienko, N.V. Interplay between mitochondria and diet mediates pathogen and stress resistance in Caenorhabditis elegans. PLoS Genet. 2019, 15, e1008011. [Google Scholar] [CrossRef] [PubMed]
- Stuhr, N.L.; Curran, S.P. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Commun. Biol. 2020, 3, 653. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Hu, X.; Sykes, B.D.; Lemire, B.D. Caenorhabditis elegans diet significantly affects metabolic profile, mitochondrial DNA levels, lifespan and brood size. Mol. Genet. Metab. 2010, 100, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Tallila, M.; Matilainen, O. Folate receptor overexpression induces toxicity in a diet-dependent manner in C. elegans. Sci. Rep. 2024, 14, 1066. [Google Scholar] [CrossRef] [PubMed]
- Keith, S.A.; Amrit, F.R.G.; Ratnappan, R.; Ghazi, A. The C. elegans healthspan and stress-resistance assay toolkit. Methods 2014, 68, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, E277–E286. [Google Scholar] [CrossRef] [PubMed]
- Larminie, C.G.; Johnstone, I.L. Isolation and characterization of four developmentally regulated cathepsin B-like cysteine protease genes from the nematode Caenorhabditis elegans. DNA Cell Biol. 1996, 15, 75–82. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, M.; Yan, C.; Kong, W.; Lan, F.; Narengaowa; Zhao, S.; Yang, Q.; Bai, Z.; Qing, H.; et al. Cathepsin B in programmed cell death machinery: Mechanisms of execution and regulatory pathways. Cell Death Dis. 2023, 14, 255. [Google Scholar] [CrossRef]
- Hook, G.; Reinheckel, T.; Ni, J.; Wu, Z.; Kindy, M.; Peters, C.; Hook, V. Cathepsin B Gene Knockout Improves Behavioral Deficits and Reduces Pathology in Models of Neurologic Disorders. Pharmacol. Rev. 2022, 74, 600–629. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, M.; Yang, X.; Zhou, X.; Zhang, S. The Role of Cathepsin B in Pathophysiologies of Non-tumor and Tumor tissues: A Systematic Review. J. Cancer 2023, 14, 2344–2358. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhan, B.; Du, M.; Chang, R.; Li, T.; Mao, X. Dietary milk fat globule membrane regulates JNK and PI3K/Akt pathway and ameliorates type 2 diabetes in mice induced by a high-fat diet and streptozotocin. J. Funct. Foods 2019, 60, 103435. [Google Scholar] [CrossRef]
- Li, T.; Gao, J.; Du, M.; Song, J.; Mao, X. Milk Fat Globule Membrane Attenuates High-Fat Diet-Induced Obesity by Inhibiting Adipogenesis and Increasing Uncoupling Protein 1 Expression in White Adipose Tissue of Mice. Nutrients 2018, 10, 331. [Google Scholar] [CrossRef]
- Mckay, S.; Johnsen, R.; Khattra, J.; Asano, J.; Baillie, D.; Chan, S.; Dube, N.; Fang, L.; Goszczynski, B.; Ha, E.; et al. Gene Expression Profiling of Cells, Tissues, and Developmental Stages of the Nematode C. elegans. Cold Spring Harb. Symp. Quant. Biol. 2003, 68, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Alvarez-Llamas, G.; Szalowska, E.; de Vries, M.P.; Weening, D.; Landman, K.; Hoek, A.; Wolffenbuttel, B.H.R.; Roelofsen, H.; Vonk, R.J. Characterization of the Human Visceral Adipose Tissue Secretome. Mol. Cell. Proteom. 2007, 6, 589–600. [Google Scholar] [CrossRef]
- Mullaney, B.C.; Ashrafi, K. C. elegans fat storage and metabolic regulation. Biochim. Biophys. Acta 2009, 1791, 474–478. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, S.S.; Ellison, P.T.; Sugiyama, L.S.; Pontzer, H.; Eick, G.; Liebert, M.A.; Cepon-Robins, T.J.; Gildner, T.E.; Snodgrass, J.J. Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists. Proc. Natl. Acad. Sci. USA 2018, 115, E3914–E3921. [Google Scholar] [CrossRef]
- Garcia, A.R.; Blackwell, A.D.; Trumble, B.C.; Stieglitz, J.; Kaplan, H.; Gurven, M.D. Evidence for height and immune function trade-offs among preadolescents in a high pathogen population. Evol. Med. Public Health 2020, 2020, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Van Der Most, P.J.; De Jong, B.; Parmentier, H.K.; Verhulst, S. Trade-off between growth and immune function: A meta-analysis of selection experiments. Funct. Ecol. 2011, 25, 74–80. [Google Scholar] [CrossRef]
- Cheesman, H.K.; Feinbaum, R.L.; Thekkiniath, J.; Dowen, R.H.; Conery, A.L.; Pukkila-Worley, R. Aberrant Activation of p38 MAP Kinase-Dependent Innate Immune Responses Is Toxic to Caenorhabditis elegans. G3 2016, 6, 541–549. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, X.; Huang, S.; Li, T.; Zhang, X.; Pang, J.; Zhao, J.; Chen, L.; Zhang, B.; Wang, J.; et al. Milk Fat Globule Membrane Attenuates Acute Colitis and Secondary Liver Injury by Improving the Mucus Barrier and Regulating the Gut Microbiota. Front. Immunol. 2022, 13, 865273. [Google Scholar] [CrossRef]
- Zanabria, R.; Tellez, A.M.; Griffiths, M.; Sharif, S.; Corredig, M. Modulation of immune function by milk fat globule membrane isolates. J. Dairy Sci. 2014, 97, 2017–2026. [Google Scholar] [CrossRef]
- Marcone, S.; Haughton, K.; Simpson, P.J.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides inhibit human endothelial-monocyte interactions via PPAR-γ dependent regulation of NF-κB. J. Inflamm. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-T.; Lv, L.-L.; Pan, M.-M.; Wen, Y.; Wang, B.; Li, Z.-L.; Wu, M.; Wang, F.-M.; Crowley, S.D.; Liu, B.-C. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018, 9, 351. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitkänen, M.; Matilainen, O. Milk Fat Globule Membrane-Containing Protein Powder Promotes Fitness in Caenorhabditis elegans. Nutrients 2024, 16, 2290. https://doi.org/10.3390/nu16142290
Pitkänen M, Matilainen O. Milk Fat Globule Membrane-Containing Protein Powder Promotes Fitness in Caenorhabditis elegans. Nutrients. 2024; 16(14):2290. https://doi.org/10.3390/nu16142290
Chicago/Turabian StylePitkänen, Miina, and Olli Matilainen. 2024. "Milk Fat Globule Membrane-Containing Protein Powder Promotes Fitness in Caenorhabditis elegans" Nutrients 16, no. 14: 2290. https://doi.org/10.3390/nu16142290
APA StylePitkänen, M., & Matilainen, O. (2024). Milk Fat Globule Membrane-Containing Protein Powder Promotes Fitness in Caenorhabditis elegans. Nutrients, 16(14), 2290. https://doi.org/10.3390/nu16142290







