Oyster Peptides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating the Gut Microbiota and Inhibiting the TLR4/NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Analysis of Amino Acid Composition
2.3. OP Peptide Identification
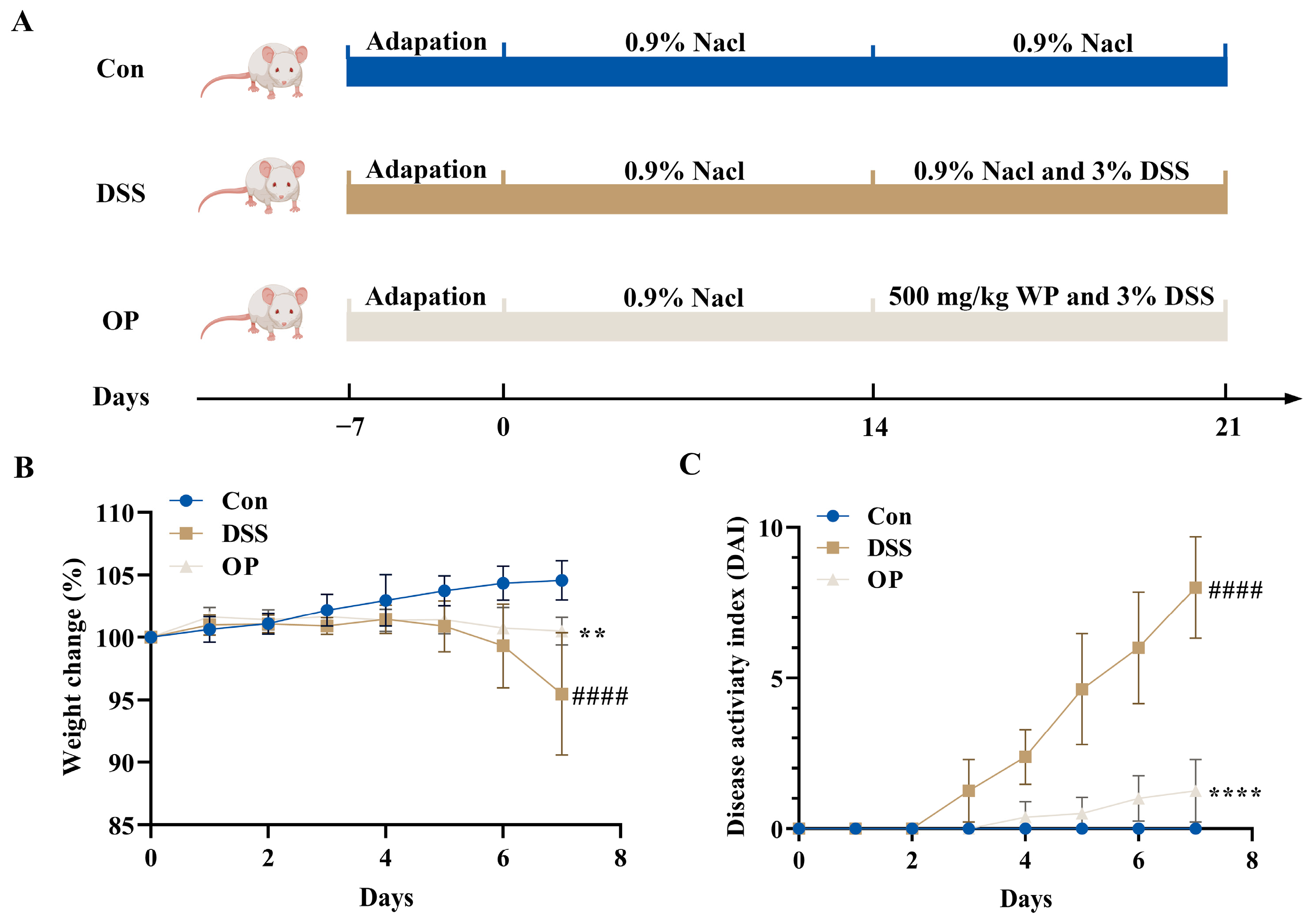
2.4. Animals
2.5. Sample Collection, DAI Scoring, and Histopathology
2.6. Enzyme-Linked Immunosorbent Assays (ELISAs)
2.7. mRNA Expression Level Measurement
2.8. Western Blotting
2.9. The 16S rDNA Gene Sequencing
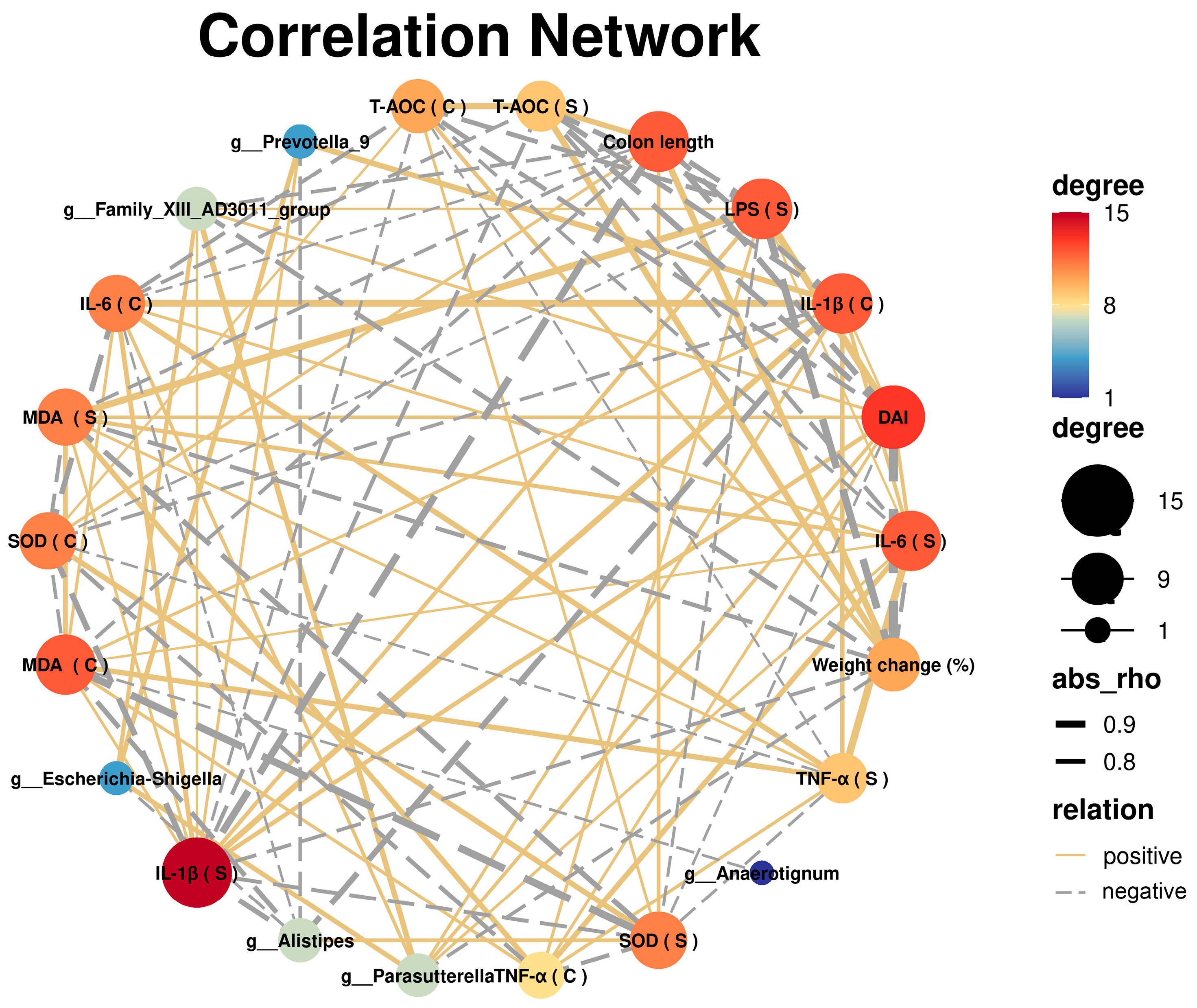
2.10. Network Diagram of Correlation Analysis
2.11. Statistical Analysis
3. Results
3.1. OPs Improve Colitis Symptoms in UC Mice
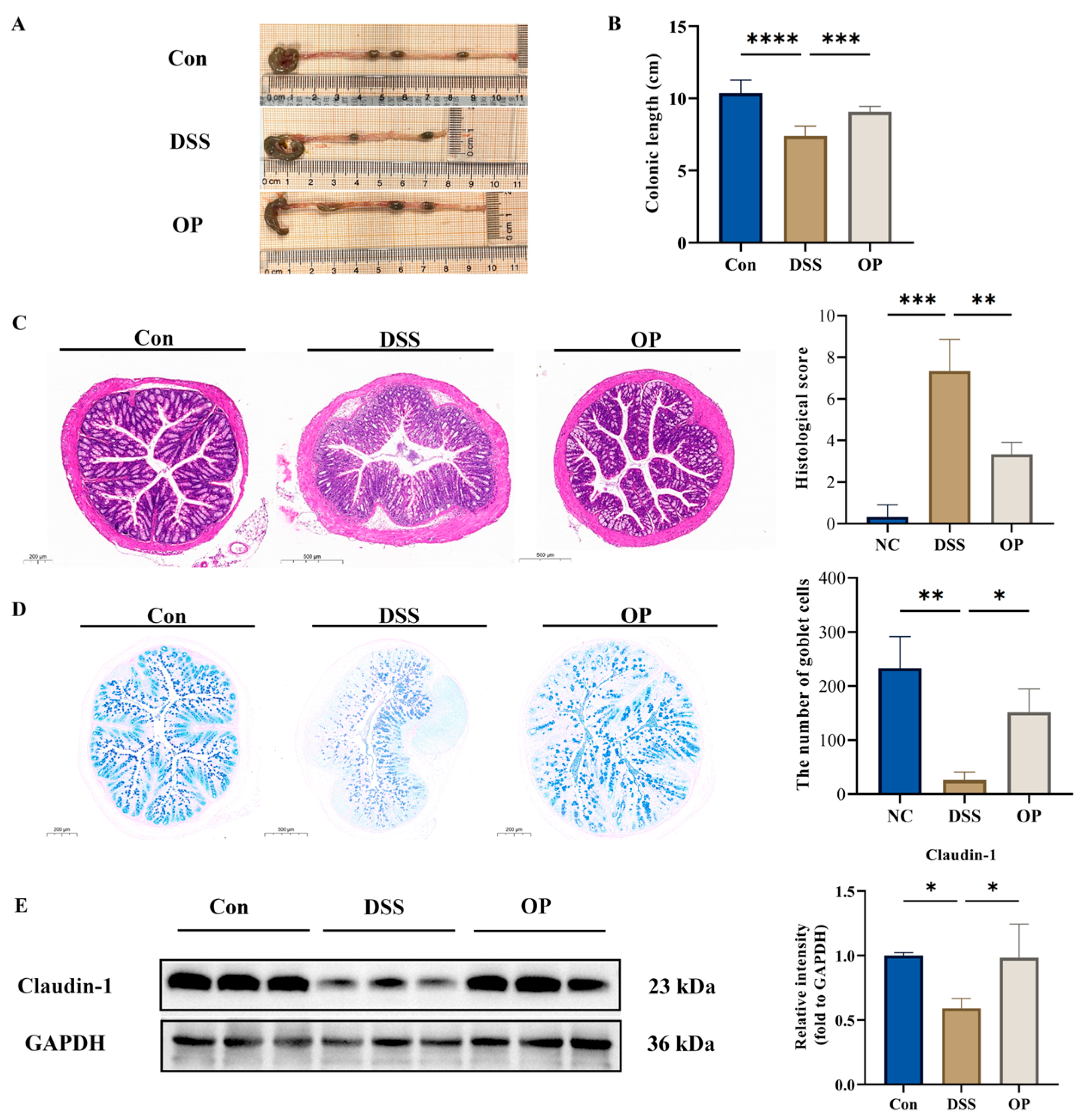
3.2. OPs Ameliorate Colonic Tissue Injury in UC Mice
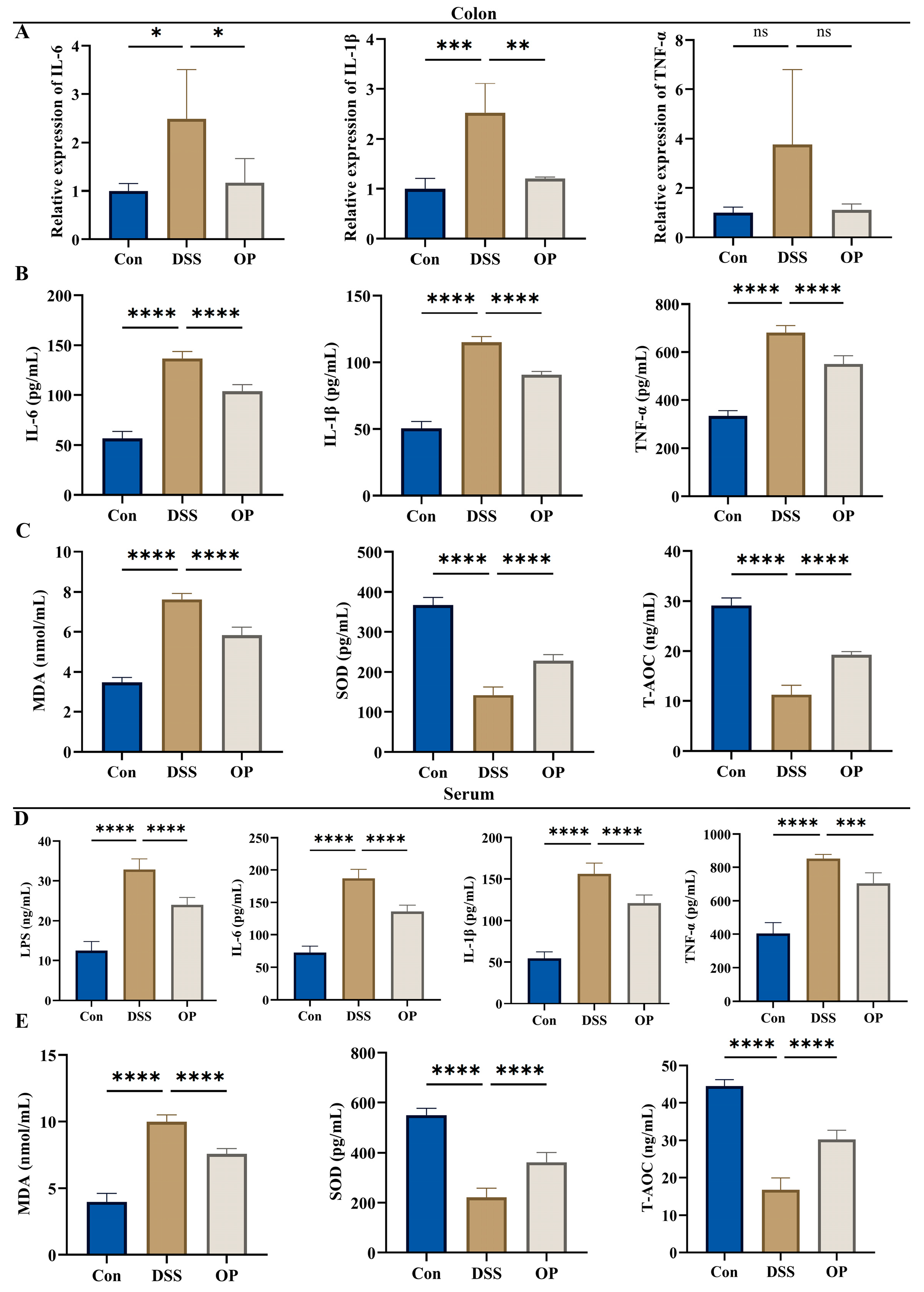
3.3. OPs Inhibit Oxidative Stress Levels and Inflammatory Responses in UC Mice
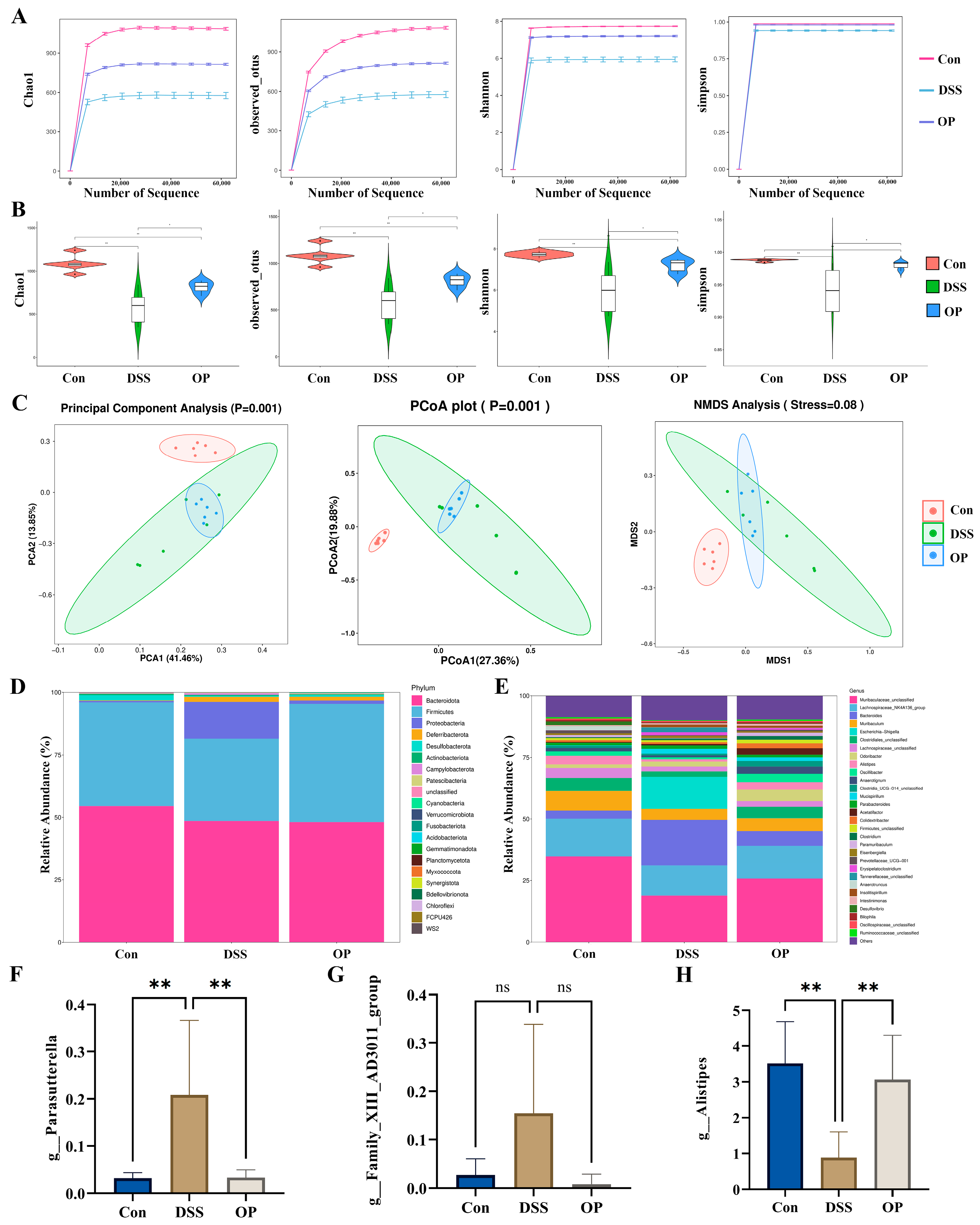
3.4. OPs Ameliorate Gut Microbiota Dysbiosis in UC Mice
3.5. Correlation Analysis between UC and Gut Microbiota
3.6. OPs Alleviated Colonic Inflammation by Inhibiting the TLR4/NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Jay, N.; Natoli, G.; Peyrin-Biroulet, L. Big data in IBD: A look into the future. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ping, H.; Wang, K.; Ding, H.; Zhang, M.; Yang, Z.; Du, Q. Oral delivery of pectin-chitosan hydrogels entrapping macrophage-targeted curcumin-loaded liposomes for the treatment of ulcerative colitis. Int. J. Pharm. 2023, 647, 123510. [Google Scholar] [CrossRef] [PubMed]
- Sebastian Domingo, J.J.; Sanchez Sanchez, C. From the intestinal flora to the microbiome. Rev. Esp. Enferm. Dig. 2018, 110, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Sands, B.E. New Therapeutics for Ulcerative Colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.P.; Cross, R.K. Adverse events in IBD: To stop or continue immune suppressant and biologic treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 223–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, L.; Xie, J.; Wang, Y.; Jiang, H.; Chen, K.; Li, D.; Wang, J.; Liu, Y.; He, J.; Zhou, J.; et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat. Commun. 2022, 13, 4804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vugrin, D.; Whitmore, W.F., Jr.; Nisselbaum, J.; Watson, R.C. Correlation of serum tumor markers and lymphangiography with degrees of nodal involvement in surgical stage II testis cancer. J. Urol. 1982, 127, 683–684. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Rodriguez-Cabezas, M.E.; Galvez, J. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J. Nutr. Biochem. 2018, 61, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes 2012, 3, 332–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, J.; Liu, D.; Huang, Y.; Chen, L.; Li, Y.; Yang, Z.; Fu, S.; Hu, G. Phlorizin Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota and Inhibiting Ferroptosis. J. Agric. Food Chem. 2023, 71, 16043–16056. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, R.; Li, W.; Du, Q.; Deng, Z.; Fan, Y.; Zheng, L. Identification of OLA1 as a Novel Protein Target of Vitexin to Ameliorate Dextran Sulfate Sodium-Induced Colitis with Tissue Thermal Proteome Profiling. J. Agric. Food Chem. 2023, 71, 16057–16066. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Luo, Y.; Chen, W.; He, M.; Deng, J.H.; Zhao, N.; Cao, L.; Wang, L. FAM96A Protects Mice From Dextran Sulfate Sodium (DSS)-Induced Colitis by Preventing Microbial Dysbiosis. Front. Cell. Infect. Microbiol. 2019, 9, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ullah, H.; Deng, T.; Ali, M.; Farooqui, N.A.; Alsholi, D.M.; Siddiqui, N.Z.; Rehman, A.U.; Ali, S.; Ilyas, M.; Wang, L.; et al. Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration. Molecules 2023, 28, 6849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishida, T.; Matsui, H.; Matsuda, Y.; Shimono, T.; Kanda, S.; Nishiyama, T.; Hosomi, R.; Fukunaga, K.; Yoshida, M. Dietary Oyster (Crassostrea gigas) Extract Ameliorates Dextran Sulfate Sodium-Induced Chronic Experimental Colitis by Improving the Composition of Gut Microbiota in Mice. Foods 2022, 11, 2032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishida, T.; Matsui, H.; Matsuda, Y.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Fukunaga, K.; Yoshida, M. Oyster (Crassostrea gigas) Extract Attenuates Dextran Sulfate Sodium-Induced Acute Experimental Colitis by Improving Gut Microbiota and Short-Chain Fatty Acids Compositions in Mice. Foods 2022, 11, 373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Satsu, H.; Fujisawa, M.; Hori, M.; Ishimoto, Y.; Totsuka, M.; Nambu, A.; Kakuta, S.; Ozaki, H.; Shimizu, M. Attenuation by dietary taurine of dextran sulfate sodium-induced colitis in mice and of THP-1-induced damage to intestinal Caco-2 cell monolayers. Amino Acids 2008, 35, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ulagesan, S.; Krishnan, S.; Nam, T.J.; Choi, Y.H. A Review of Bioactive Compounds in Oyster Shell and Tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Y.; Li, M.; Wei, Y.; Jia, F.; Zheng, Y.; Tao, G.; Xiong, F. Oyster-derived dipeptides RI, IR, and VR promote testosterone synthesis by reducing oxidative stress in TM3 cells. Food Sci. Nutr. 2023, 11, 6470–6482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Cho, S.B.; Woo, M.S.; Lee, D.K.; Hong, S.G.; Han, J.; Kang, S.S.; Kim, D.R.; et al. Oyster-Derived Tyr-Ala (YA) Peptide Prevents Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Suppressing Inflammatory, Apoptotic, Ferroptotic, and Pyroptotic Signals. Mar. Drugs 2021, 19, 614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, J.; Zhao, Y.; Wang, L.; Wu, T.; Jin, Y.; Meng, J.; Zhang, M. Sea Cucumber Peptide Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium by Alleviating Gut Microbiota Imbalance and Regulating miR-155/SOCS1 Axis in Mice. Foods 2023, 12, 3434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.; Zhao, C.; Zhang, Y.; Lv, H.; Ji, X.; Wang, J.; Pang, W.; Wang, X.; Wang, S. Protective effects of bioactive peptides in foxtail millet protein hydrolysates against experimental colitis in mice. Food Funct. 2022, 13, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Spatz, M.; Ciocan, D.; Merlen, G.; Rainteau, D.; Humbert, L.; Gomes-Rochette, N.; Hugot, C.; Trainel, N.; Mercier-Nome, F.; Domenichini, S.; et al. Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis. JHEP Rep. 2021, 3, 100230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi-Xiang, M.; Yang, F.; Ze-Hua, H.; Nuo-Ming, Y.; Rui-Long, W.; Bin-Qiang, X.; Jun-Jie, F.; Chun-Lan, H.; Yue, Z. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes 2022, 14, 2112882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudi, K.; Zimonja, M.; Trosvik, P.; Naes, T. Use of multivariate statistics for 16S rRNA gene analysis of microbial communities. Int. J. Food Microbiol. 2007, 120, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Y.; Ji, Z.H.; Ren, W.Z.; Zhao, P.S.; Wei, F.H.; Hu, J.; Yuan, B.; Gao, W. Wheat peptide alleviates DSS-induced colitis by activating the Keap1-Nrf2 signaling pathway and maintaining the integrity of the gut barrier. Food Funct. 2024, 15, 5466–5484. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.X.; Wang, B.B.; Wu, H.Y.; Feng, H.Y.; Zhang, H.Y.; Gao, W.; Yuan, B. Turtle peptide and its derivative peptide ameliorated DSS-induced ulcerative colitis by inhibiting inflammation and modulating the composition of the gut microbiota. Int. Immunopharmacol. 2024, 132, 112024. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.H.; Xie, W.Y.; Zhao, P.S.; Wu, H.Y.; Ren, W.Z.; Hu, J.P.; Gao, W.; Yuan, B. Oat Peptides Alleviate Dextran Sulfate Sodium Salt-Induced Colitis by Maintaining the Intestinal Barrier and Modulating the Keap1-Nrf2 Axis. Nutrients 2023, 15, 5055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Axelsson, L.G.; Landstrom, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.G.; Chang, E.B. The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: New insights into complex disease. Clin. Sci. 2018, 132, 2013–2028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, J. Critical signaling pathways governing colitis-associated colorectal cancer: Signaling, therapeutic implications, and challenges. Dig. Liver Dis. 2023, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodzinski, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carone, M.; Spalinger, M.R.; Gaultney, R.A.; Mezzenga, R.; Hlavackova, K.; Mookhoek, A.; Krebs, P.; Rogler, G.; Luciani, P.; Aleandri, S. Temperature-triggered in situ forming lipid mesophase gel for local treatment of ulcerative colitis. Nat. Commun. 2023, 14, 3489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, X.; Lin, L.; Ding, G.; Yu, F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-kappaB Pathway. Mar. Drugs 2019, 17, 404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, K.; Xia, S.; Xiao, S.; Zhang, M.; Liao, J.; Yu, Q. Kuijie decoction ameliorates ulcerative colitis by affecting intestinal barrier functions, gut microbiota, metabolic pathways and Treg/Th17 balance in mice. J Ethnopharmacol 2023, 19, 117316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, V.; Mahajan, N.; Kaur, J.; Devi, K.; Dharavath, R.N.; Singh, R.P.; Kondepudi, K.K.; Bishnoi, M. Mucin secretory action of capsaicin prevents high fat diet-induced gut barrier dysfunction in C57BL/6 mice colon. Biomed. Pharmacother. 2022, 145, 112452. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Barua, N.; Ip, M. Mucin-degrading gut commensals isolated from healthy faecal donor suppress intestinal epithelial inflammation and regulate tight junction barrier function. Front. Immunol. 2022, 13, 1021094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.W.; Zhou, X.L.; Wang, R.; Shu, C.H.; Zhou, Y.F.; Ying, X.G.; Zheng, B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs 2021, 19, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, L.J.; Cho, J.H.; Gevers, D.; Chu, H. Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 2174–2189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-kappaB-IL6-STAT3 axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, H.; Zhang, Y.; Swallah, M.S.; Wang, S.; Zhang, J.; Fang, J.; Lu, J.; Yu, H. Structural Characteristics of Insoluble Dietary Fiber from Okara with Different Particle Sizes and Their Prebiotic Effects in Rats Fed High-Fat Diet. Foods 2022, 11, 1298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Bai, H.; Deng, F.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Chemically Protected Sodium Butyrate Improves Growth Performance and Early Development and Function of Small Intestine in Broilers as One Effective Substitute for Antibiotics. Antibiotics 2022, 11, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Z.; Hao, Y.; Yang, L.; Yuan, P.; Kang, W.; Liang, T.; Gu, B.; Dong, B. The potential of Klebsiella and Escherichia-Shigella and amino acids metabolism to monitor patients with postmenopausal osteoporosis in northwest China. BMC Microbiol. 2023, 23, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, G.; Chen, T.; Huang, L.; Zhang, Y.; Feng, Y.; Liu, Q.; Yin, X.; Qu, S.; Yang, C.; Wan, J.; et al. Tibetan tea reduces obesity brought on by a high-fat diet and modulates gut flora in mice. Food Sci. Nutr. 2023, 11, 6582–6595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Atractylodes macrocephala Koidz. volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 2023, 14, 1127785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Liu, J.; Wei, J.; Zhang, Y.; Xu, Y.; Yue, T.; Yuan, Y. Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Nutrients 2024, 16, 1178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, S.; Li, P.; Zhao, H.; Li, X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020, 78, 106018. [Google Scholar] [CrossRef] [PubMed]
- Duffuler, P.; Bhullar, K.S.; de Campos Zani, S.C.; Wu, J. Bioactive Peptides: From Basic Research to Clinical Trials and Commercialization. J. Agric. Food Chem. 2022, 70, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Tao, M.; Zhao, X.; Feng, Q.; Fei, X.; Fu, Y. Atrial Natriuretic Peptide Attenuates Colitis via Inhibition of the cGAS-STING Pathway in Colonic Epithelial Cells. Int. J. Biol. Sci. 2022, 18, 1737–1754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Lv, R.; Wang, C.; Ge, Q.; Du, H.; Lin, S. Tricholoma matsutake-derived peptide WFNNAGP protects against DSS-induced colitis by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 2021, 12, 11883–11897. [Google Scholar] [CrossRef] [PubMed]
- Zhi, T.; Hong, D.; Zhang, Z.; Li, S.; Xia, J.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo. Food Res. Int. 2022, 152, 110875. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Wang, B. Marine Bioactive Peptides-Structure, Function and Application. Mar. Drugs 2023, 21, 275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Xie, W.; Ji, Z.; Wang, B.; Ren, W.; Gao, W.; Yuan, B. Oyster Peptides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating the Gut Microbiota and Inhibiting the TLR4/NF-κB Pathway. Nutrients 2024, 16, 1591. https://doi.org/10.3390/nu16111591
Guo H, Xie W, Ji Z, Wang B, Ren W, Gao W, Yuan B. Oyster Peptides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating the Gut Microbiota and Inhibiting the TLR4/NF-κB Pathway. Nutrients. 2024; 16(11):1591. https://doi.org/10.3390/nu16111591
Chicago/Turabian StyleGuo, Haixiang, Wenyin Xie, Zhonghao Ji, Bingbing Wang, Wenzhi Ren, Wei Gao, and Bao Yuan. 2024. "Oyster Peptides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating the Gut Microbiota and Inhibiting the TLR4/NF-κB Pathway" Nutrients 16, no. 11: 1591. https://doi.org/10.3390/nu16111591
APA StyleGuo, H., Xie, W., Ji, Z., Wang, B., Ren, W., Gao, W., & Yuan, B. (2024). Oyster Peptides Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating the Gut Microbiota and Inhibiting the TLR4/NF-κB Pathway. Nutrients, 16(11), 1591. https://doi.org/10.3390/nu16111591





