Does Microbiome Matter in Chronic Intestinal Failure Due to Type 1 Short Bowel Syndrome in Adults?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Aspects
2.3. Procedures
2.4. Definitions
2.5. Volatilome Analysis
2.6. DNA Extraction and Metagenomic Sequencing
2.7. Bioinformatic Analyses
2.8. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
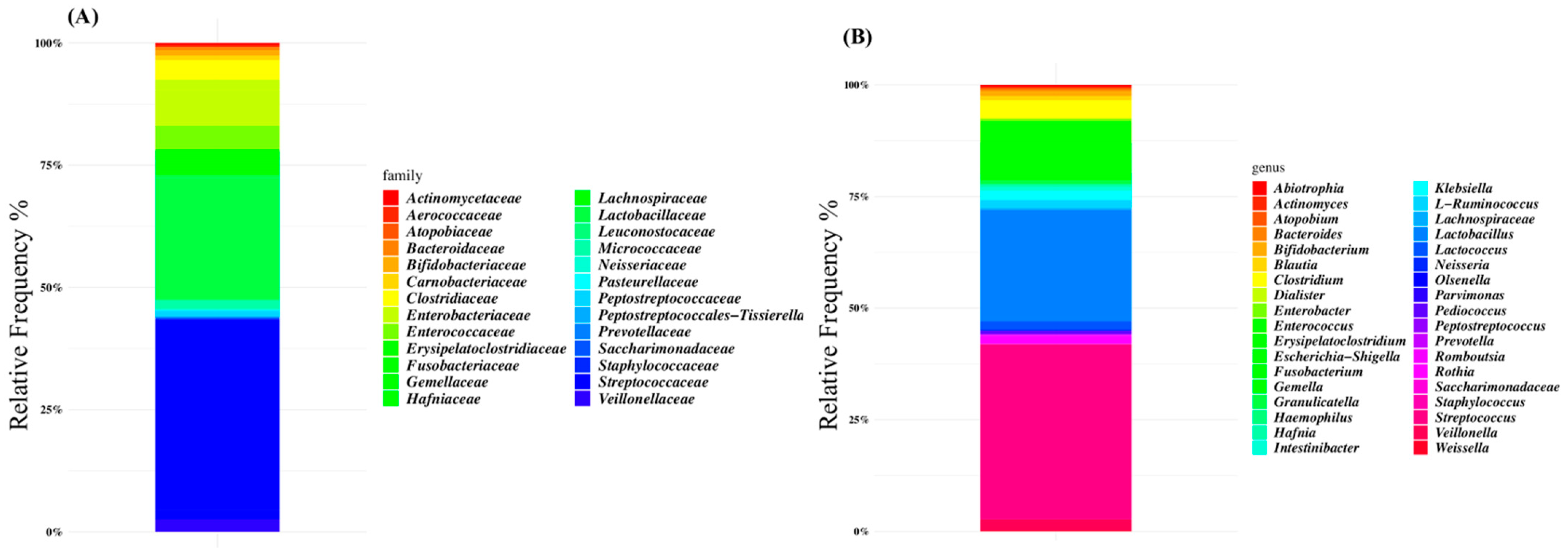
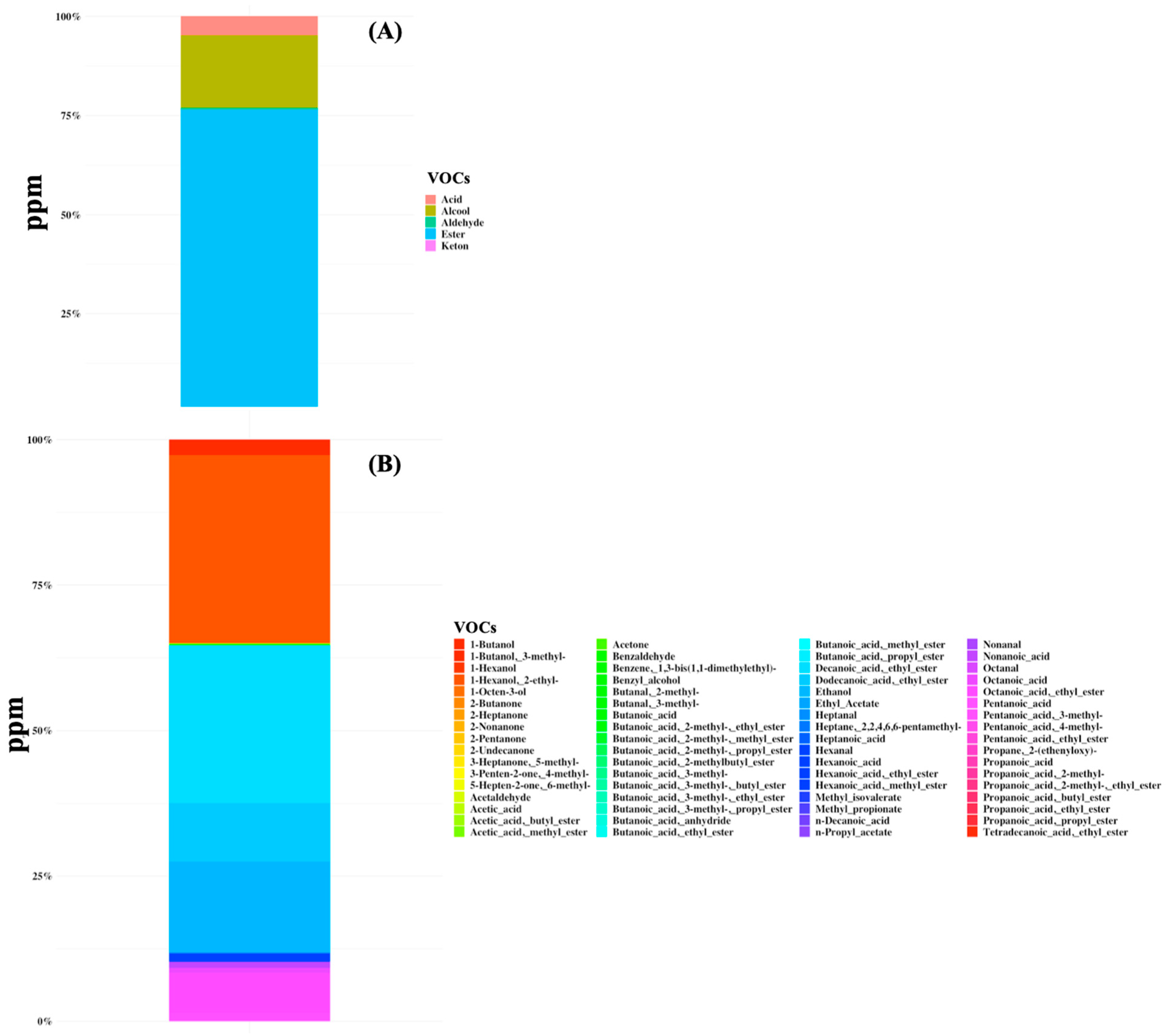
3.2. Microbiome and Volatilome Characteristics of SBS-CIF Patients
3.3. Microbiome and Volatilome Characteristics According to Bowel Length and Underlying Diseases
3.4. Microbiome and Volatilome Characteristics According to Functional Characteristics
3.5. Microbiome and Volatilome Characteristics According to Parenteral Supply
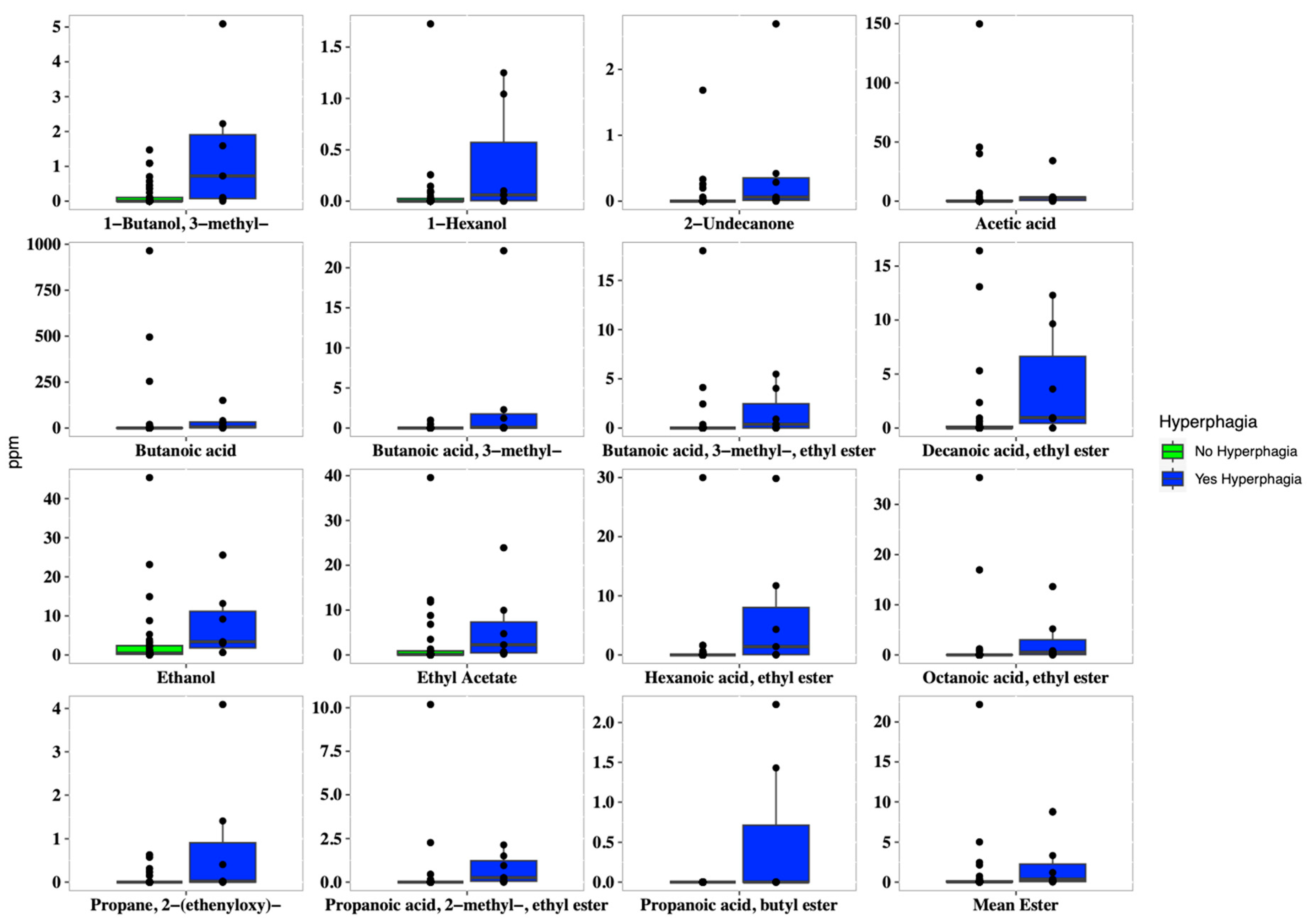
3.6. Microbiome and Volatilome Characteristics According to Hyperphagia and Dietary Intakes
3.7. Microbiome and Volatilome Characteristics According to hs-CRP Values
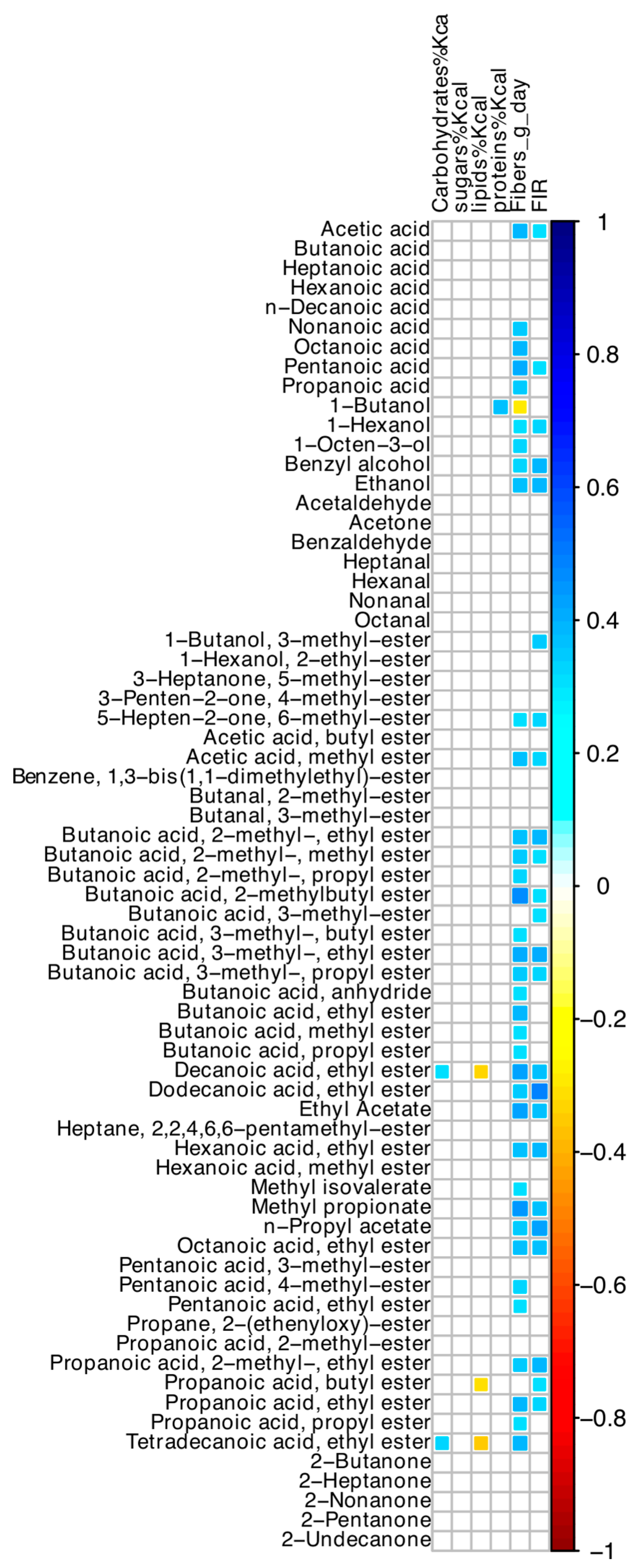
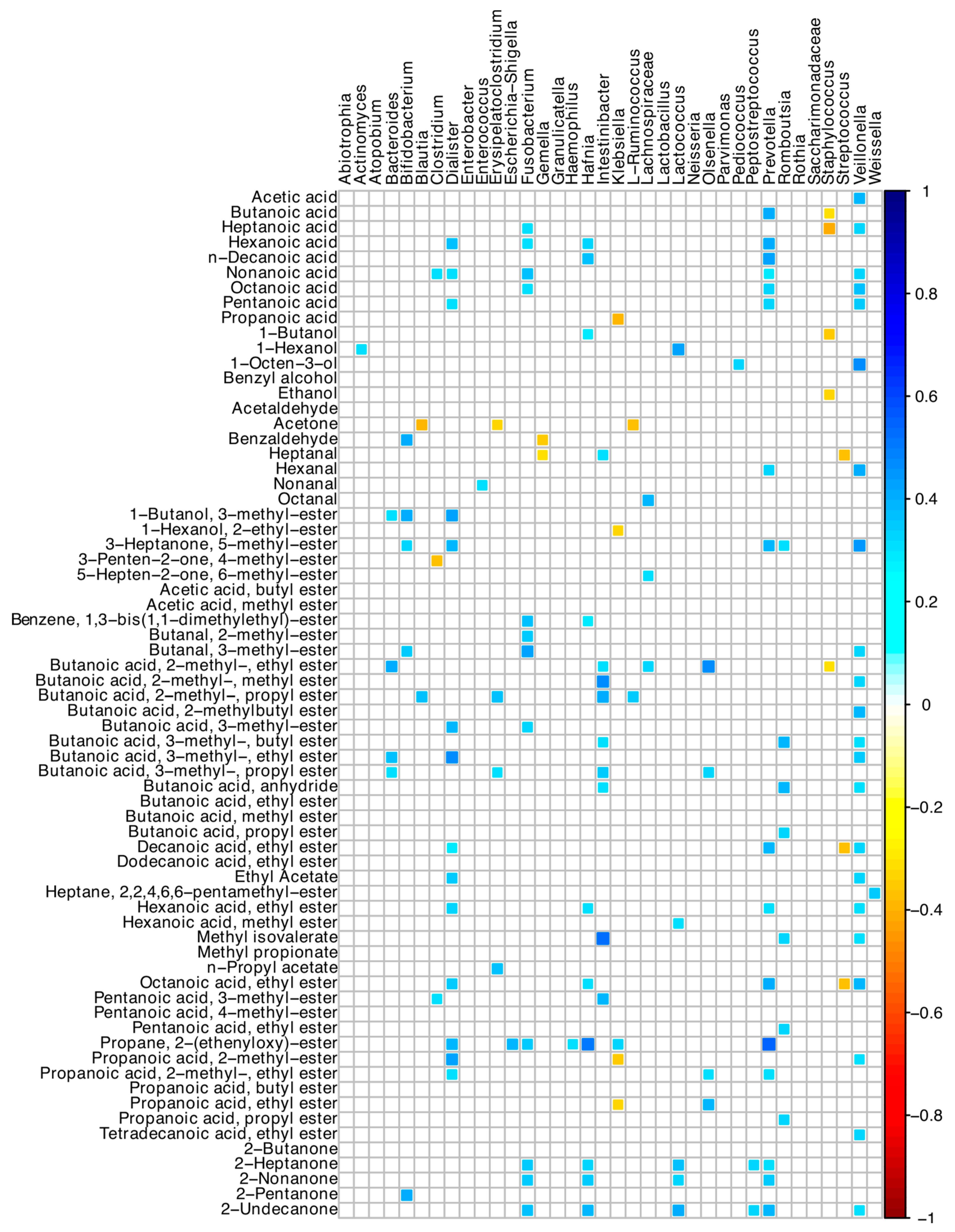
3.8. Correlations among Microbiome and Volatilome Characteristics
4. Discussion
4.1. Microbiota Composition
4.2. Volatile Organic Compounds
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L. Definition, classification, and causes of short bowel syndrome. Nutr. Clin. Pract. 2023, 38, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Verbiest, A.; Jeppesen, P.B.; Joly, F.; Vanuytsel, T. The role of a colon-in-continuity in short bowel syndrome. Nutrients 2023, 15, 628. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Lapthorne, S.; Pereira-Fantini, P.; Foully, F.; Wilson, G.; Thomas, S.L.; Dellios, N.L.; Scurr, M.; O’Sullivan, O.; Ross, R.P.; Stanton, C.; et al. Gut microbial diversity is reduced and is associated with colonic inflammation in a piglet model of short bowel syndrome. Gut Microbes 2013, 4, 212–221. [Google Scholar] [CrossRef]
- Lapthorne, S.; Bines, J.E.; Fouhy, F.; Dellios, N.L.; Wilson, G.; Thomas, S.L.; Scurr, M.; Stanton, C.; Cotter, P.D.; Pereira-Fantini, P. Changes in the colon microbiota and intestinal cytokine gene expression following minimal intestinal surgery. World J. Gastroenterol. 2015, 21, 4150–4158. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.; Byars, S.G.; Pitt, J.; Lapthorne, S.; Foully, F.; Cotter, P.D.; Mines, J.E. Unravelling the metabolic impact of SBS-associated microbial dysbiosis: Insights from the piglet short bowel syndrome model. Sci. Rep. 2017, 7, 43326. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Mayeur, C.; Brunei, A.; Noordine, M.L.; Meylheuc, T.; Langella, P.; Messing, B.; Duèe, P.H.; Cherbuy, C.; Thomas, M. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef]
- Mayeur, C.; Gratadoux, J.J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guy, F.; Li, Y.; Wang, J.; Li, J. Fecal microbiota signatures of adult patients with different types of short bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 1949–1957. [Google Scholar] [CrossRef]
- Gillard, L.; Mayeur, C.; Robert, V.; Pingenot, I.; Le Beyec, J.; Bado, A.; Lepage, P.; Thomas, M.; Joly, F. Microbiota is involved in post-resection adaptation in humans with short bowel syndrome. Front. Physiol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Boccia, S.; Torre, I.; Sanitaria, L.; Iervolino, C.; Del Piano, C.; Puggina, A.; Pastorino, R.; Dragic, M.; Amore, R.; Borriello, T.; et al. Intestinal microbiota in adult patients with short bowel syndrome: Preliminary results from a pilot study. Clin. Nutr. 2017, 36, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Budinska, E.; Gojia, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and metabolome profiles associated with different types of short bowel syndrome: Implications for treatment. J. Parenter. Enter. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Boutte, H.R.; Chen, J.; Wylie, T.N.; Wylie, K.M.; Xie, Y.; Geisman, M.; Prauw, A.; Gazid, V.; Tarr, P.I.; Levin, M.S.; et al. Fecal microbiome and bile acid metabolome in adult short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G154–G168. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.; De Koning, B.; Rings, E.; Wijnen, R.; Nichols, B.; Hults, J.; Gerasimidis, K. The gut microbiome in patients with intestinal failure: Current evidence and implications for clinical practice. J. Parenter. Enter. Nutr. 2019, 43, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, A.; Guy, F.; Wang, J.; Li, Y. Severe intestinal dysbiosis in rat models of short bowel syndrome with ileocecal resection. Dig. Dis. Sci. 2020, 65, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A. Anatomical and physiological considerations in short bowel syndrome: Emphasis on intestinal adaptation and the role of enterohormones. Nutr. Clin. Pract. 2023, 38, S2–S34. [Google Scholar] [CrossRef]
- Piper, H.G.; Fan, D.; Coughlin, L.A.; Ho, X.; McDaniel, M.M.; Channabasappa, N.; Kim, J.; Kim, M.; Shan, X.; Xie, Y.; et al. Severe gut microbiota dysbiosis is associated with poor growth in patients with short bowel syndrome. J. Parenter. Enter. Nutr. 2017, 41, 1202–1212. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, J.; Yao, D.; Guo, F.; Li, Y. Altered fecal microbiome and metabolome profiles in rat models of short bowel syndrome. Front. Microbiol. 2023, 14, 1185463. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Jiang, L.; Wang, Y.; Cai, W. Multi-omics unravels metabolic alterations in the ileal mucosa of neonatal piglets receiving total parenteral nutrition. Metabolites 2023, 13, 555. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Gao, B.; Yan, J.; Cai, W.; Jiang, J. Untargeted metabolomics reveal parenteral nutrition-associated alterations in pediatric patients with short bowel syndrome. Metabolites 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- D’Eusebio, C.; Merlo, F.D.; Ossola, M.; Bioletto, F.; Ippolito, M.; Locatelli, M.; De Francesco, A.; Anrò, M.; Romagnoli, R.; Strignano, P.; et al. Mortality and parenteral nutrition weaning in patients with chronic intestinal failure on home parenteral nutrition: A 30-year retrospective cohort study. Nutrition 2022, 107, 111915. [Google Scholar] [CrossRef] [PubMed]
- Fourati, S.; De Dreuille, B.; Bettolo, J.; Hutinet, C.; Le Gall, M.; Bado, A.; Joly, F.; Le Beyec, J. Hyperphagia is prominent in adult patients with short bowel syndrome: A role for the colon? Clin. Nutr. 2023, 42, 2109–2115. [Google Scholar] [CrossRef]
- Traina, C.; Ferrocino, I.; Bonciolini, A.; Cardenia, V.; Lin, X.; Rantsiou, K.; Cocolin, L. Monitoring the yeasts ecology and volatiles profile throughout the spontaneous fermentation of Taggiasca cv. table olives through culture-dependent and independent methods. Int. J. Food Microbiol. 2024, 417, 110688. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schiere, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Ronsen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bongaerts, G.P.; Severijen, R.S.; Tangerman, A.; Verrips, A.; Tolboom, J.J. Bile acid deconjugation by Lactobacilli and its effects in patients with a short small bowel. J. Gastroenterol. 2000, 35, 801–804. [Google Scholar] [CrossRef]
- Chowdhury, F.; Hill, L.; Shah, N.; Popov, J.; Cheveldayoff, P.; Pai, N. Intestinal microbiome in short bowel syndrome: Diagnostic and therapeutic opportunities. Curr. Opin. Gastroenterol. 2023, 39, 463–471. [Google Scholar] [CrossRef]
- Kastl, A.J.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The structure and function of the human small intestinal microbiota: Current Understanding and Future Directions. Cell Mol. Gastroenterol. Hepat. 2020, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kowlgi, N.G.; Chhabra, L. D-Lactic Acidosis: An underrecognized complication of short bowel syndrome. Gastroent Res. Pract. 2015, 2015, 476215. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Morin, M.C.; Joly, F.; Penven, S.; Thuillier, F.; Messing, B. Net digestive absorption and adaptive hyperphagia in adult short bowel patients. Gut 2004, 53, 1279–1286. [Google Scholar] [CrossRef]
- Bètry, C.; Lauverjat, M.; Mouillot, T.; Bergen, C.; Barnoud, D.; Air, S.; Chamber, C. Hyperphagia in short bowel patients: Fat-free mass is a strong predictor. Nutrition 2019, 62, 146–151. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Mortensen, P.B. Intestinal failure defined by measurements of intestinal energy and wet weight absorption. Gut 2000, 46, 701–706. [Google Scholar] [CrossRef]
- Wolfschluckner, V.; Obermüller, B.; Horvath, A.; Rodriguez-Blanco, G.; Fuchs, P.; Miekisch, W.; Mitte, B.; Flacher, C.; Till, H.; Singer, G. Metabolomic Alterations of volatile organic compounds and bile acids as biomarkers of microbial shifts in a murine model of short bowel syndrome. Nutrients 2023, 15, 4949. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Senanayake, T.; Makanyengo, S.; Hoedt, E.C.; Goggins, B.; Smith, S.R.; Keely, S. Influence of the bile acid/microbioma axis in ideal surgery: A systematic review. Colorect. Dis. 2024, 26, 243–257. [Google Scholar] [CrossRef]

| Variable | Sub-Classification | Number, Mean or Median Values |
|---|---|---|
| Number | 44 | |
| Age (years) | 64.2 ± 14.7 | |
| Females/males | 25/19 | |
| Weight (kg) | 63.2 ± 18.1 | |
| BMI (kg/m2) | 23.2 ± 5.3 | |
| Underlying disease | ||
| Inflammatory bowel diseases | 20 (45.5) | |
| Surgical complications | 16 (36.4) | |
| Mesenteric ischemia | 7 (15.9) | |
| Fibro-adhesive peritonitis | 1 (2.3) | |
| Small bowel length (cm) | 124.2 ± 76.7 | |
| ≤100 cm | 20 (45.5) | |
| >100 cm | 24 (54.5) | |
| Stomal output (mL/day) | 1600; 1200 | |
| ≥1500 mL/day | 25 (56.8) | |
| Year of PN start | ||
| ≤6 months | 10 (22.7) | |
| 6–24 months | 5 (11.4) | |
| ≥24 months | 29 (65.9) | |
| Total volume infused (mL/day) | 1635.5 ± 896.4 | |
| ≥1500 mL/day | 22 (50) | |
| Fluid, oral intake (mL/day) | 1458.0 ± 692.2 | |
| Energy, parenteral supply (kcal/day) | 729.6 ± 435.1 | |
| Energy, parenteral supply/kg body weight (kcal/kg)/day | 13.8 ± 7.1 | |
| ≥15 (kcal/kg) day | 19 (43.2) | |
| Energy, oral intake (kcal/day) | 1439.2 ± 518.0 | |
| Food Intake Ratio (FIR score) | 1.09 ± 0.39 | |
| FIR score > 1.5 | 7 (15.9) | |
| Serum hs-CRP (mg/L) | 5.1; 5.8 | |
| Hs-CRP ≥ 3 mg/L | 29 (65.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossola, M.; Ferrocino, I.; Franciosa, I.; Aimasso, U.; Cravero, L.; Bonciolini, A.; Cardenia, V.; Merlo, F.D.; Anrò, M.; Chiarotto, A.; et al. Does Microbiome Matter in Chronic Intestinal Failure Due to Type 1 Short Bowel Syndrome in Adults? Nutrients 2024, 16, 2282. https://doi.org/10.3390/nu16142282
Ossola M, Ferrocino I, Franciosa I, Aimasso U, Cravero L, Bonciolini A, Cardenia V, Merlo FD, Anrò M, Chiarotto A, et al. Does Microbiome Matter in Chronic Intestinal Failure Due to Type 1 Short Bowel Syndrome in Adults? Nutrients. 2024; 16(14):2282. https://doi.org/10.3390/nu16142282
Chicago/Turabian StyleOssola, Marta, Ilario Ferrocino, Irene Franciosa, Umberto Aimasso, Leila Cravero, Ambra Bonciolini, Vladimiro Cardenia, Fabio Dario Merlo, Marta Anrò, Alessia Chiarotto, and et al. 2024. "Does Microbiome Matter in Chronic Intestinal Failure Due to Type 1 Short Bowel Syndrome in Adults?" Nutrients 16, no. 14: 2282. https://doi.org/10.3390/nu16142282
APA StyleOssola, M., Ferrocino, I., Franciosa, I., Aimasso, U., Cravero, L., Bonciolini, A., Cardenia, V., Merlo, F. D., Anrò, M., Chiarotto, A., Bosa, C., Cocolin, L., & Bo, S. (2024). Does Microbiome Matter in Chronic Intestinal Failure Due to Type 1 Short Bowel Syndrome in Adults? Nutrients, 16(14), 2282. https://doi.org/10.3390/nu16142282









