Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Interventions and Randomization
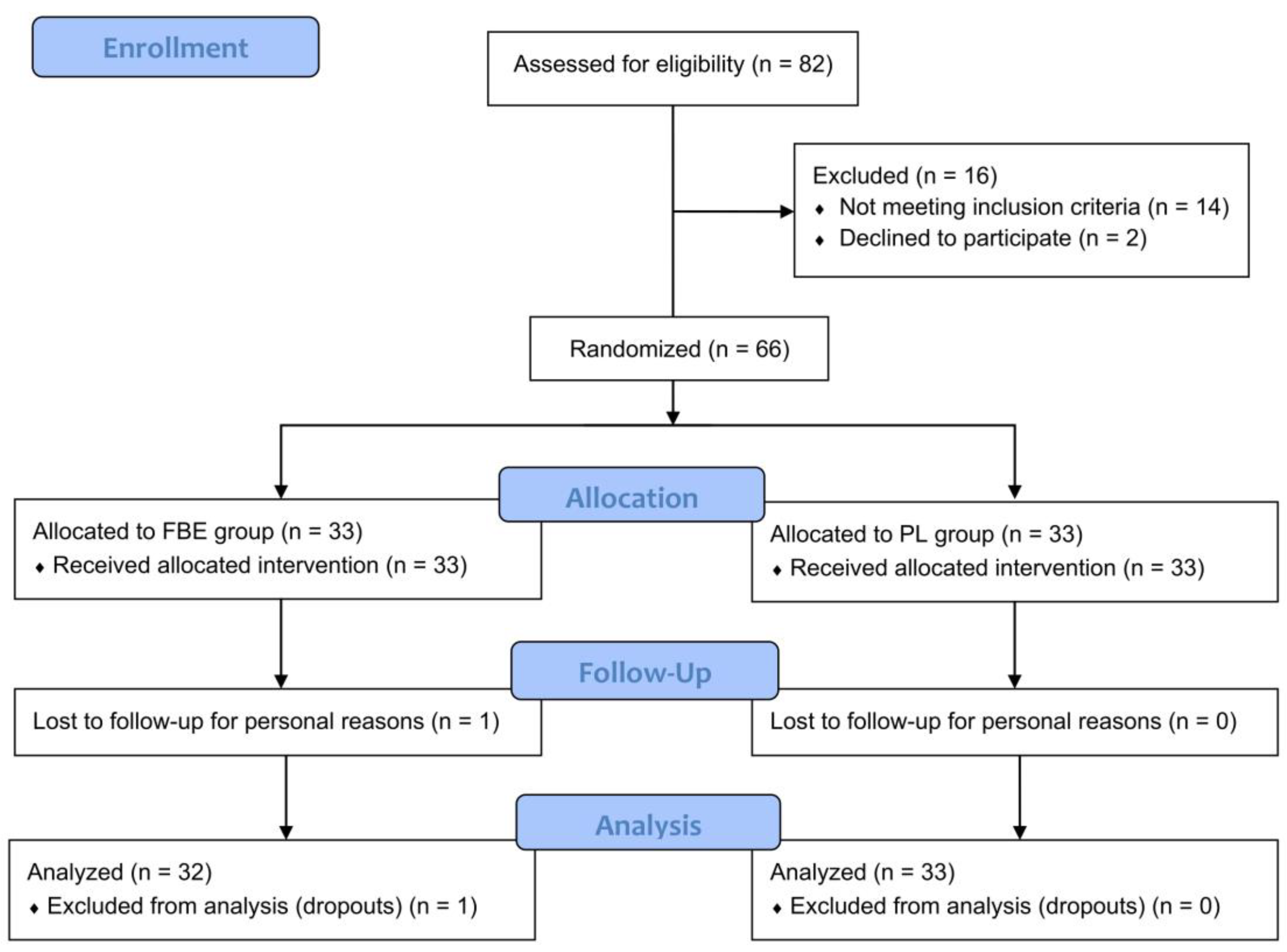
2.3. Participants and Compliance to Treatment
2.4. Primary and Secondary Efficacy Endpoints and Outcome Measures
2.4.1. Skin Texture
2.4.2. Skin Elasticity
2.4.3. Skin Complexion
2.4.4. Skin Antioxidant Capacity
2.4.5. Self-Assessment Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics, Tolerability, and Compliance with Treatment
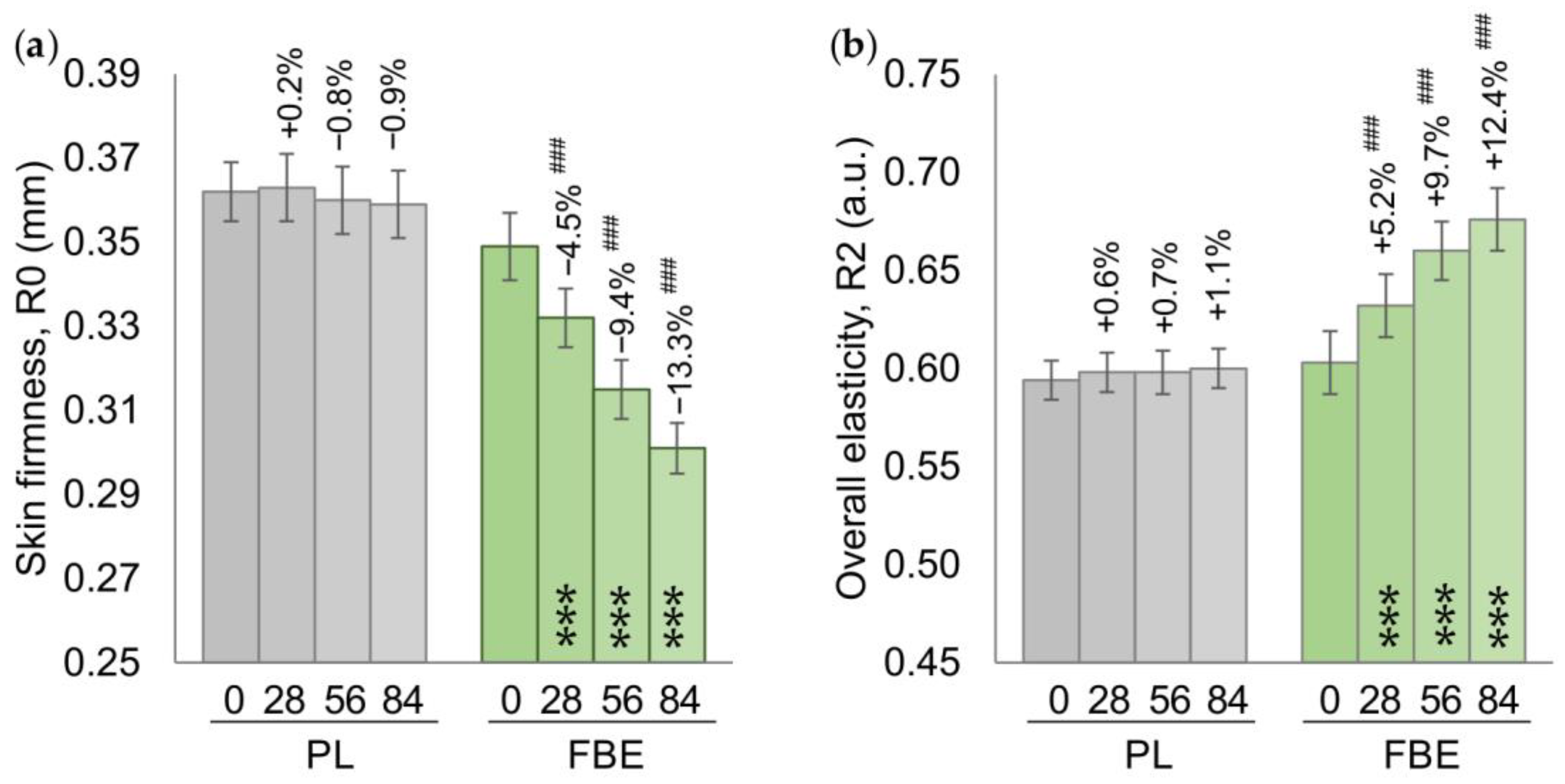
3.2. Primary Endpoints
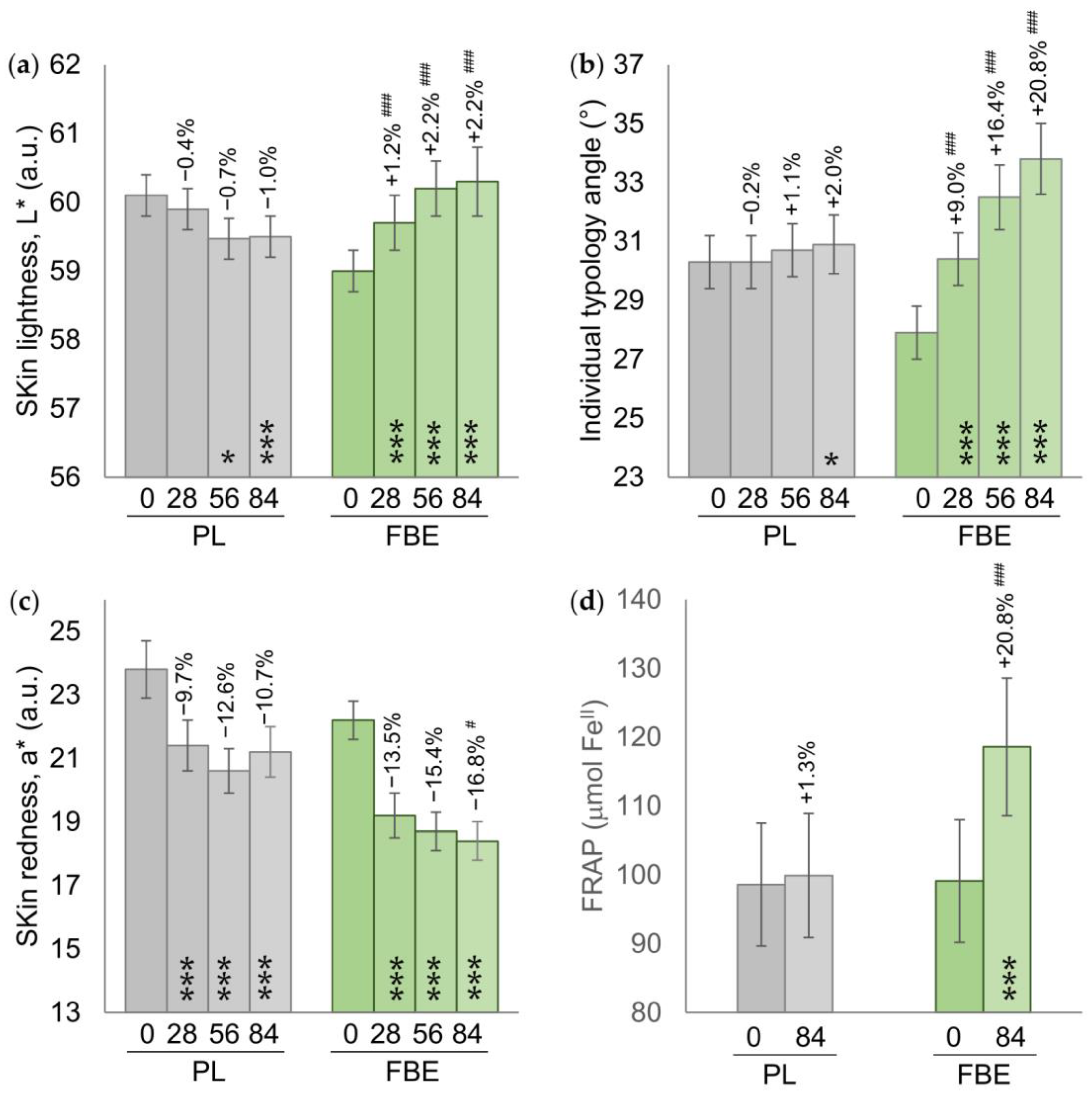
3.3. Secondary Endpoints
3.4. Self-Assessment Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-Dependent Factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Roveda, G.; Cestone, E.; De Gennaro, F.; Poggi, A.; Insolia, V.; Zaccaria, V.; Nobile, V. Artichoke Leaf Extract Effectiveness on the Skin Aging Exposome: Efficacy and Safety Results of a Split-Face Study. Cosmetics 2024, 11, 69. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin Aging from Mechanisms to Interventions: Focusing on Dermal Aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic Aspects of Skin Aging, Prevention, and Local Treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A Antagonizes Decreased Cell Growth and Elevated Collagen-Degrading Matrix Metalloproteinases and Stimulates Collagen Accumulation in Naturally Aged Human Skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.; Liew, S.; Weinkle, S.; Garcia, J.K.; Silberberg, M.B. The Facial Aging Process From the “Inside Out”. Aesthet. Surg. J. 2020, 41, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.J.; Tomsic, J.A.; Fernandez, S.J.; Davison, S.P. Effect of Facial Rejuvenation Surgery on Perceived Attractiveness, Femininity, and Personality. JAMA Facial Plast. Surg. 2015, 17, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Gilchrest, B.A. Psychosocial Aspects of Aging Skin. Dermatol. Clin. 2005, 23, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R. Contemporary Concepts in Brow and Eyelid Aging. Clin. Plast. Surg. 2013, 40, 21–42. [Google Scholar] [CrossRef]
- Pappas, A.; Liakou, A.; Zouboulis, C.C. Nutrition and Skin. Rev. Endocr. Metab. Disord. 2016, 17, 443–448. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and Diet: What Is New in 2021? J. Clin. Aesthetic Dermatol. 2021, 14, 49–54. [Google Scholar]
- Khan, A.; Adalsteinsson, J.; Whitaker-Worth, D.L. Atopic Dermatitis and Nutrition. Clin. Dermatol. 2022, 40, 135–144. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A Brief Overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical Antioxidants in the Prevention of Photocarcinogenesis and Photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Burioli, A.; Yu, S.; Zhifeng, S.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Photoprotective and Antiaging Effects of a Standardized Red Orange (Citrus sinensis (L.) Osbeck) Extract in Asian and Caucasian Subjects: A Randomized, Double-Blind, Controlled Study. Nutrients 2022, 14, 2241. [Google Scholar] [CrossRef]
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Lee, M.-H.; Lee, J.W.; No, K.-O.; Park, S.-K.; Lee, H.-S.; Kang, S.; Cho, W.-G.; Park, H.J.; Oh, K.W.; et al. Anti-Wrinkling Effects of the Mixture of Vitamin C, Vitamin E, Pycnogenol and Evening Primrose Oil, and Molecular Mechanisms on Hairless Mouse Skin Caused by Chronic Ultraviolet B Irradiation. Photodermatol. Photoimmunol. Photomed. 2007, 23, 155–162. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Klemettilä, H.; Jaakola, L. Bilberry (Vaccinium myrtillus L.) ecotypes. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–99. [Google Scholar]
- Sharma, A.; Lee, H.-J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef]
- Thibado, S.P.; Thornthwaite, J.T.; Ballard, T.K.; Goodman, B.T. Anticancer Effects of Bilberry Anthocyanins Compared with NutraNanoSphere Encapsulated Bilberry Anthocyanins. Mol. Clin. Oncol. 2018, 8, 330–335. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishihara, K. Investigation of the Antimicrobial Activity of Bilberry (Vaccinium myrtillus L.) Extract against Periodontopathic Bacteria. J. Oral. Biosci. 2020, 62, 169–174. [Google Scholar] [CrossRef]
- Ashour, O.M.; Elberry, A.A.; Alahdal, A.; Al Mohamadi, A.M.; Nagy, A.A.; Abdel-Naim, A.B.; Abdel-Sattar, E.A.; Mohamadin, A.M. Protective Effect of Bilberry (Vaccinium myrtillus) against Doxorubicin-Induced Oxidative Cardiotoxicity in Rats. Med. Sci. Monit. 2011, 17, BR110–BR115. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective Effect of Bilberry Extract in a Murine Model of Photo-Stressed Retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry Polyphenols Metabolism and Impact on Human Gut Microbiota and Health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, N.; Neelakandan, N.; Rupasinghe, H.P.V. Potential Health Benefits of Fermented Blueberry: A Review of Current Scientific Evidence. Trends Food Sci. Technol. 2023, 132, 103–120. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Pourrat, H. Improvements to Processes for Obtaining Glucosides, in Particular Anthocyanin Glucosides. FR Patent 1300869, 10 August 1961. [Google Scholar]
- Bazin, R.; Doublet, E. Skin Aging Atlas: Caucasian Type; Éd. MED’COM: Paris, France, 2007. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Hooda, R.; Madke, B.; Choudhary, A. Photoaging: Reversal of the Oxidative Stress Through Dietary Changes and Plant-Based Products. Cureus 2023, 15, e37321. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, M.A.; Hadi, H. Aging and Wound Healing of the Skin: A Review of Clinical and Pathophysiological Hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-Derived Bioactive Compounds with Anti-Aging Potential for Nutricosmetic and Cosmeceutical Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef]
- Mauray, A.; Milenkovic, D.; Besson, C.; Caccia, N.; Morand, C.; Michel, F.; Mazur, A.; Scalbert, A.; Felgines, C. Atheroprotective effects of bilberry extracts in apo E-deficient mice. J. Agric. Food Chem. 2009, 57, 11106–11111. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 39, 4581–4609. [Google Scholar] [CrossRef]
- Haslam, E. In vino veritas: Oligomeric procyanidins and the ageing of red wines. Phytochem. 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Salas, E.; Atanasova, V.; Poncet-Legrand, C.; Meudec, E.; Mazauric, J.P.; Cheynier, V. Demonstration of the occurrence of flavanol–anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta 2004, 513, 325–332. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Angeles Varo, M.; Martín-Gómez, J.; Merida, J.; Serratosa, M.P. Exploring the Impact of Temperature and Fermentation Time on the Evolution of Bioactive Compounds, Antioxidant Activity, and Color Evolution in Blueberry Wines. Food Sci. Technol. 2024, 4, 1301–1309. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Cuevas-Rodríguez, E.O.; Reyes-Moreno, C.; Ríos-Iribe, É.Y.; Hernández-Álvarez, A.J.; León-López, L.; Milán-Carrillo, J. Profiling modifications in physicochemical, chemical and antioxidant properties of wild blackberry (Rubus sp.) during fermentation with EC 1118 yeast. J. Food Sci. Technol. 2021, 58, 4654–4665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Ye, J.; Zhang, S.; Chen, Z.; Jiang, S. Effects of Dietary Supplementation with Bilberry Extract on Growth Performance, Immune Function, Antioxidant Capacity, and Meat Quality of Yellow-Feathered Chickens. Animals 2021, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Graf, B.; Curran-Celentano, J.M.; Blumberg, J.B. Bilberry (Vaccinium myrtillus) Anthocyanins Modulate Heme Oxygenase-1 and Glutathione S-Transferase-Pi Expression in ARPE-19 Cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Choo, P.P.; Woi, P.J.; Bastion, M.-L.C.; Omar, R.; Mustapha, M.; Md Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. Biomed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Wei, Y.; Jia, S.; Ding, Y.; Xia, S.; Giunta, S. Balanced Basal-Levels of ROS (Redox-Biology), and Very-Low-Levels of pro-Inflammatory Cytokines (Cold-Inflammaging), as Signaling Molecules Can Prevent or Slow-down Overt-Inflammaging, and the Aging-Associated Decline of Adaptive-Homeostasis. Exp. Gerontol. 2023, 172, 112067. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Roopchand, D.E.; Graf, B.; Cheng, D.M.; Ribnicky, D.; Fridlender, B.; Raskin, I. Role of anthocyanins in skin aging and UV-induced skin damage. In Anthocyanins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2013; pp. 309–321. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral Intake of Proanthocyanidin-Rich Extract from Grape Seeds Improves Chloasma. Phytother. Res. 2004, 18, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Furumura, M.; Sato, N.; Kusaba, N.; Takagaki, K.; Nakayama, J. Oral Administration of French Maritime Pine Bark Extract (Flavangenol(®)) Improves Clinical Symptoms in Photoaged Facial Skin. Clin. Interv. Aging 2012, 7, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Fukui, Y.; Izumi, R.; Numano, K.; Zeida, M. Effects of Oligomeric Proanthocyanidins (OPCs) of Red Wine to Improve Skin Whitening and Moisturizing in Healthy Women—A Placebo-Controlled Randomized Double-Blind Parallel Group Comparative Study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1571–1584. [Google Scholar] [CrossRef]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Ohtake, Y.; Kanda, T. Administration of Apple Polyphenol Supplements for Skin Conditions in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 1071. [Google Scholar] [CrossRef]
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.-X. Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2020, 12, 3252. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed]
| PL (n = 33) | FBE (n = 32) | Units | |
|---|---|---|---|
| Age | 52.5 ± 1.3 | 51.4 ± 1.4 | Years |
| Postmenopausal population | 57.6% (19) | 62.5% (20) | % (no.) |
| Phototype | |||
| I | 0.0% (0) | 3.1% (1) | % (no.) |
| II | 36.4% (12) | 34.4% (11) | % (no.) |
| III | 63.6% (21) | 62.5% (20) | % (no.) |
| Wrinkles scoring | 3.7 ± 0.1 | 3.4 ± 0.1 | score |
| Skin texture | |||
| Wrinkle depth | 318.9 ± 14.9 | 299.9 ± 20.7 | μm |
| Skin smoothness (Ra) | 34.9 ± 1.2 | 34.5 ± 1.2 | μm |
| Skin roughness (Rz) | 247.1 ± 10.2 | 245.4 ± 9.5 | μm |
| Skin elasticity | |||
| R0 (Uf) | 0.362 ± 0.007 | 0.349 ± 0.008 | mm |
| R2 (Ua/Uf) | 0.594 ± 0.010 | 0.603 ± 0.016 | a.u. |
| Skin coloration | |||
| ITA° | 30.3 ± 0.9 | 27.9 ± 0.9 | ° |
| L* | 60.1 ± 0.3 | 59.0 ± 0.3 | a.u. |
| b* | 17.4 ± 0.3 | 16.8 ± 0.3 | a.u. |
| a* | 23.8 ± 0.9 | 22.2 ± 0.6 | a.u. |
| Skin antioxidant capacity (FRAP) | 98.6 ± 8.8 | 99.1 ± 8.9 | μmol FeII |
| D0 | D28 | D56 | D84 | |
|---|---|---|---|---|
| Wrinkle depth (mm) | ||||
| PL (n = 33) | 318.9 ± 14.9 | 319.5 ± 14.7 (+0.4%) | 315.7 ± 15.0 (−1.0%) | 312.6 ± 15.7 * (−2.1%) |
| FBE (n = 32) | 299.9 ± 20.7 | 289.1 ± 20.5 ** (−3.8%) ## | 277.6 ± 20.3 *** (−7.7%) ### | 268.9 ± 20.3 *** (−10.6%) ### |
| Skin smoothness, Ra (mm) | ||||
| PL (n = 33) | 34.9 ± 1.2 | 35.0 ± 1.2 (+0.4) | 34.6 ± 1.2 (−0.8%) | 34.4 ± 1.2 * (−1.3%) |
| FBE (n = 32) | 34.5 ± 1.2 | 33.0 ± 1.3 * (−4.6%) ## | 32.0 ± 1.2 *** (−7.5%) ### | 32.0 ± 1.5 *** (−7.9%) ### |
| Skin roughness, Rz (mm) | ||||
| PL (n = 33) | 247.1 ± 10.2 | 248.2 ± 10.2 (+0.6%) | 245.3 ± 10.4 (−0.8%) | 243.4 ± 10.3 * (−1.5%) |
| FBE (n = 32) | 245.4 ± 9.5 | 239.9 ± 9.7 ** (−2.4%) ## | 233.5 ± 9.6 *** (−5.1%) ### | 228.1 ± 9.5 *** (−7.3%) ### |
| You Feel … | PL (n = 33) | FBE (n = 32) |
|---|---|---|
| that your skin complexion is improved | 72.7% | 96.9% ## |
| that your skin has less redness | 66.7% | 96.9% ## |
| that your skin is brighter/more luminous | 66.7% | 87.5% ## |
| that your skin has a healthier glow | 69.7% | 90.6% |
| that your skin has fewer imperfections | 66.7% | 87.5% |
| that your skin is more plumped | 66.7% | 93.8% # |
| that your skin is softer | 78.8% | 100.0% ## |
| that your skin is smoother | 69.7% | 96.9% ## |
| that your skin is firmer | 66.7% | 90.6% # |
| that product reduced the appearance of fine wrinkles | 63.6% | 93.8% # |
| that the product reduced the appearance of deep wrinkles | 54.5% | 78.1% |
| that the signs of skin aging seem less visible | 63.6% | 78.1% |
| that your skin looks visibly younger | 63.6% | 75.0% |
| that your skin seems healthier | 75.8% | 90.6% |
| that your skin is more moisturized | 69.7% | 100.0% ### |
| better about yourself in your skin | 69.7% | 90.6% |
| that your skin is more beautiful | 72.7% | 93.8% # |
| that your overall skin appearance is improved | 69.7% | 96.9% ## |
| Was the treatment well tolerated? | 100.0% | 100.0% |
| Are you globally satisfied with the efficacy of the product? | 75.8% | 96.9% # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, V.; Dudonné, S.; Kern, C.; Roveda, G.; Garcia, C. Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 2203. https://doi.org/10.3390/nu16142203
Nobile V, Dudonné S, Kern C, Roveda G, Garcia C. Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(14):2203. https://doi.org/10.3390/nu16142203
Chicago/Turabian StyleNobile, Vincenzo, Stéphanie Dudonné, Catherine Kern, Gloria Roveda, and Christine Garcia. 2024. "Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 14: 2203. https://doi.org/10.3390/nu16142203
APA StyleNobile, V., Dudonné, S., Kern, C., Roveda, G., & Garcia, C. (2024). Antiaging, Brightening, and Antioxidant Efficacy of Fermented Bilberry Extract (Vaccinium myrtillus): A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(14), 2203. https://doi.org/10.3390/nu16142203







