Evaluation of the Effect of Thickeners in Enteral Formulas on the Gastric Emptying Rate of Proteins and Carbohydrates Using a Semi-Dynamic Gastric Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Test Enteral Formulas
2.3. Semi-Dynamic Gastric Digestion
2.4. Protein Content Analysis
2.5. Carbohydrate Content Analysis
2.6. Data Analysis
3. Results
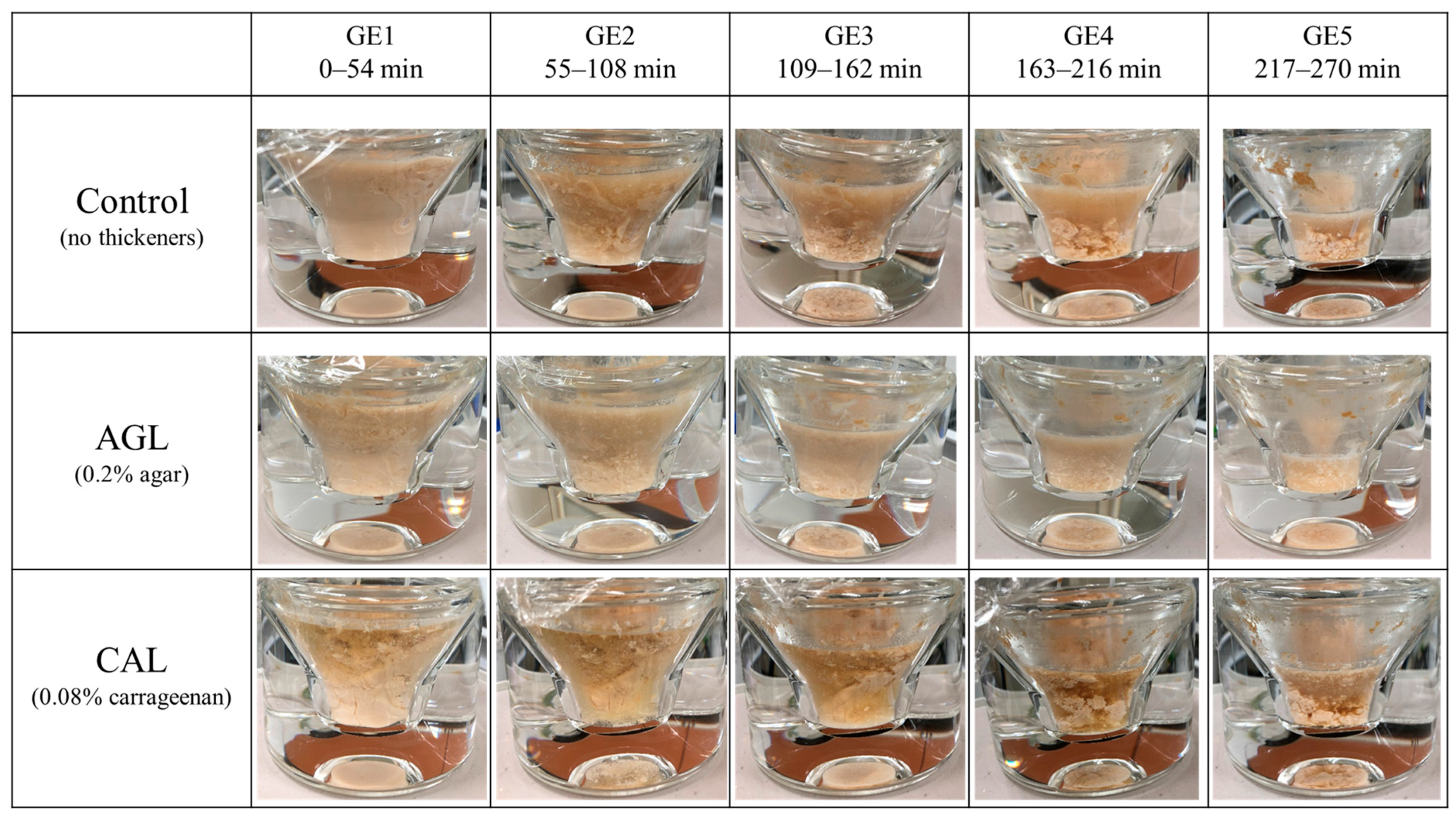
3.1. Formation of Aggregations during Gastric Digestion
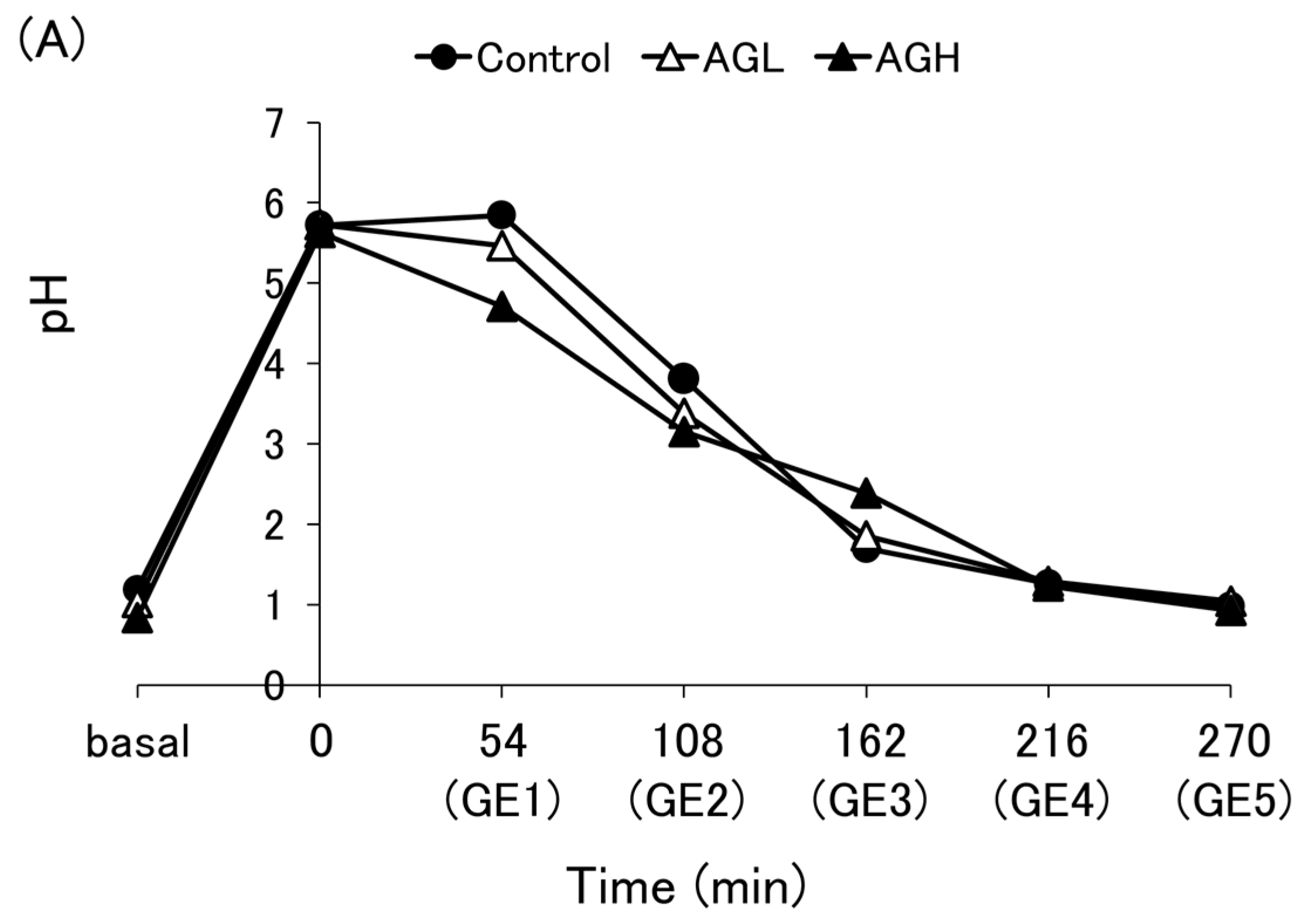
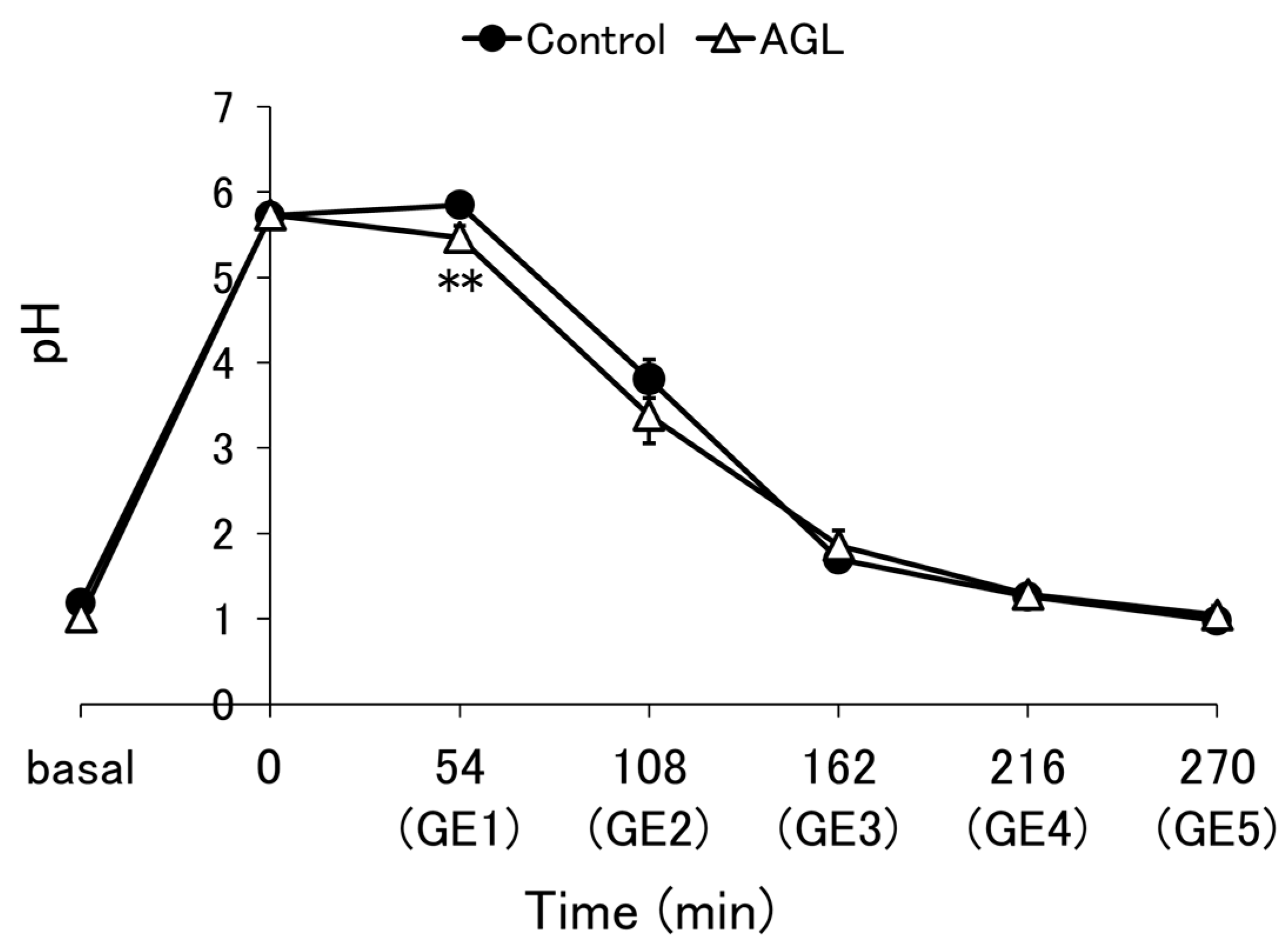
3.2. pH Profile
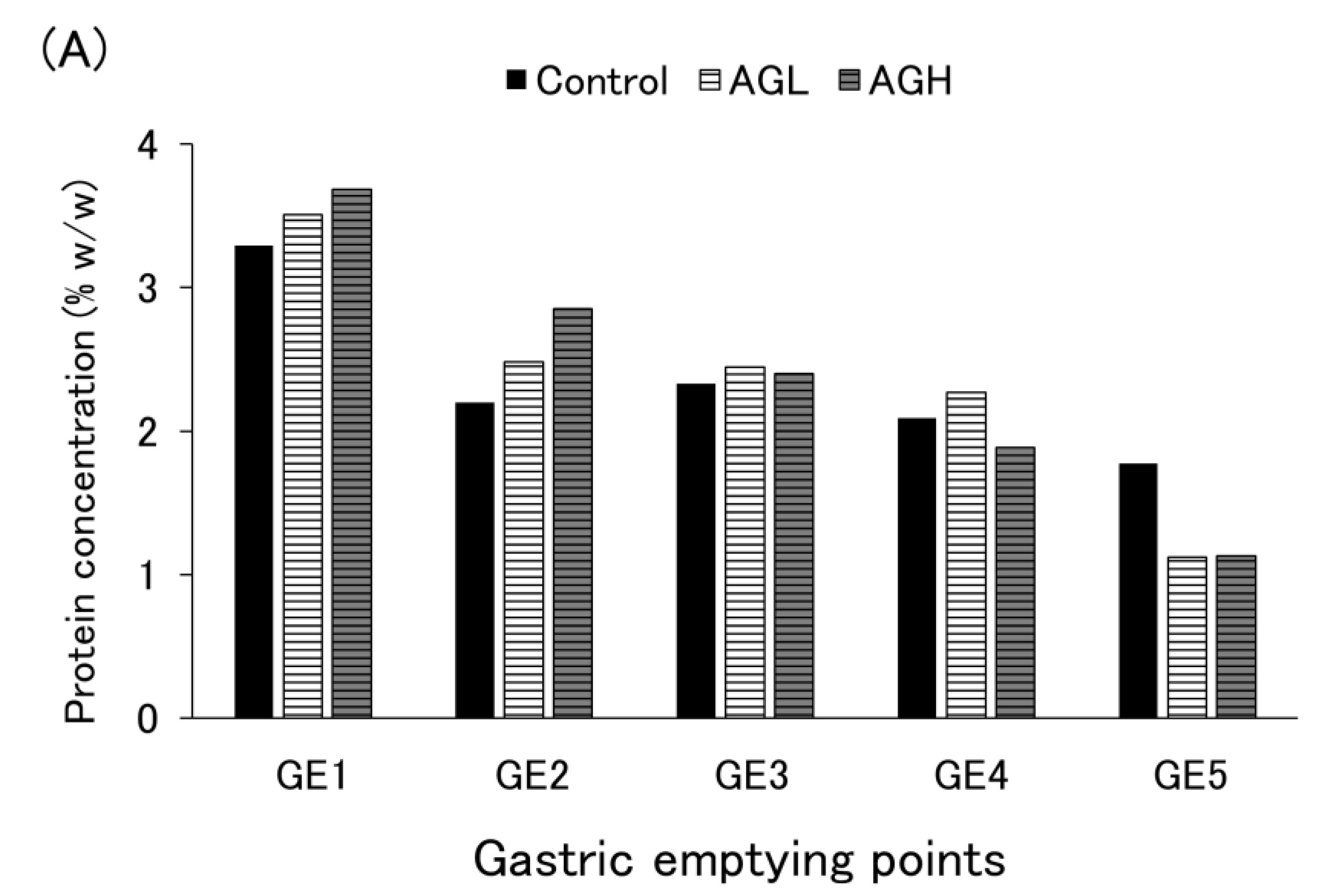
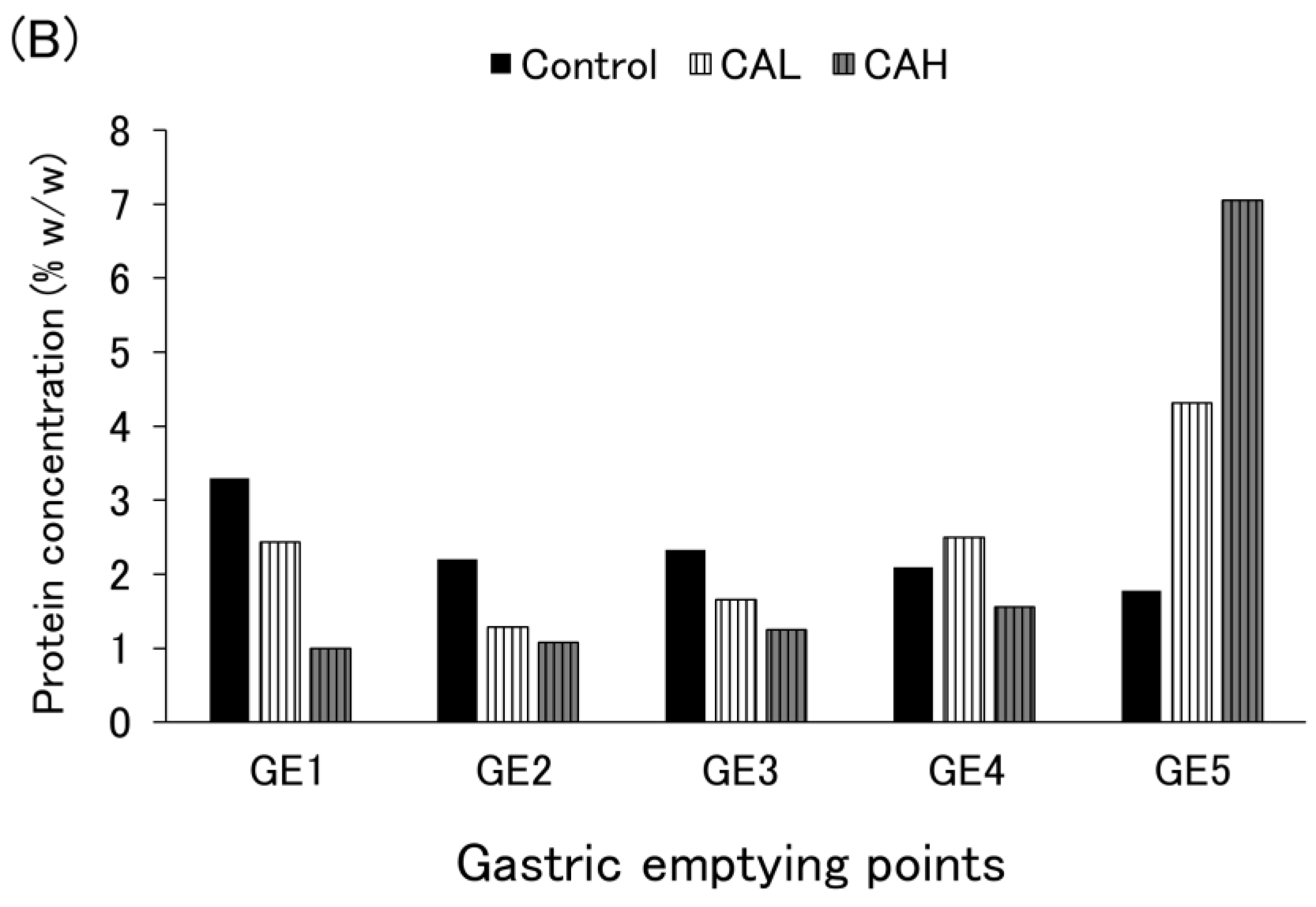
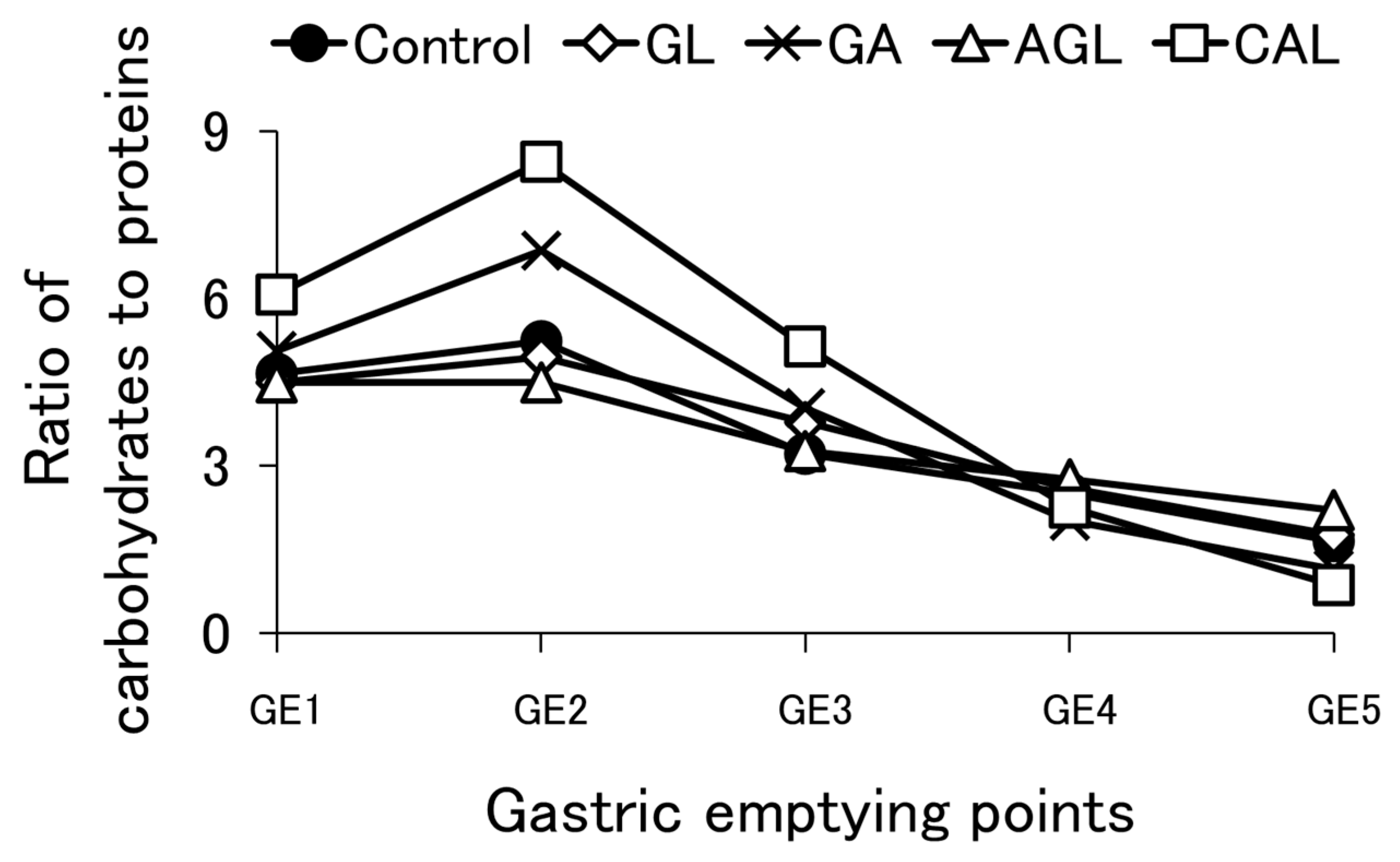
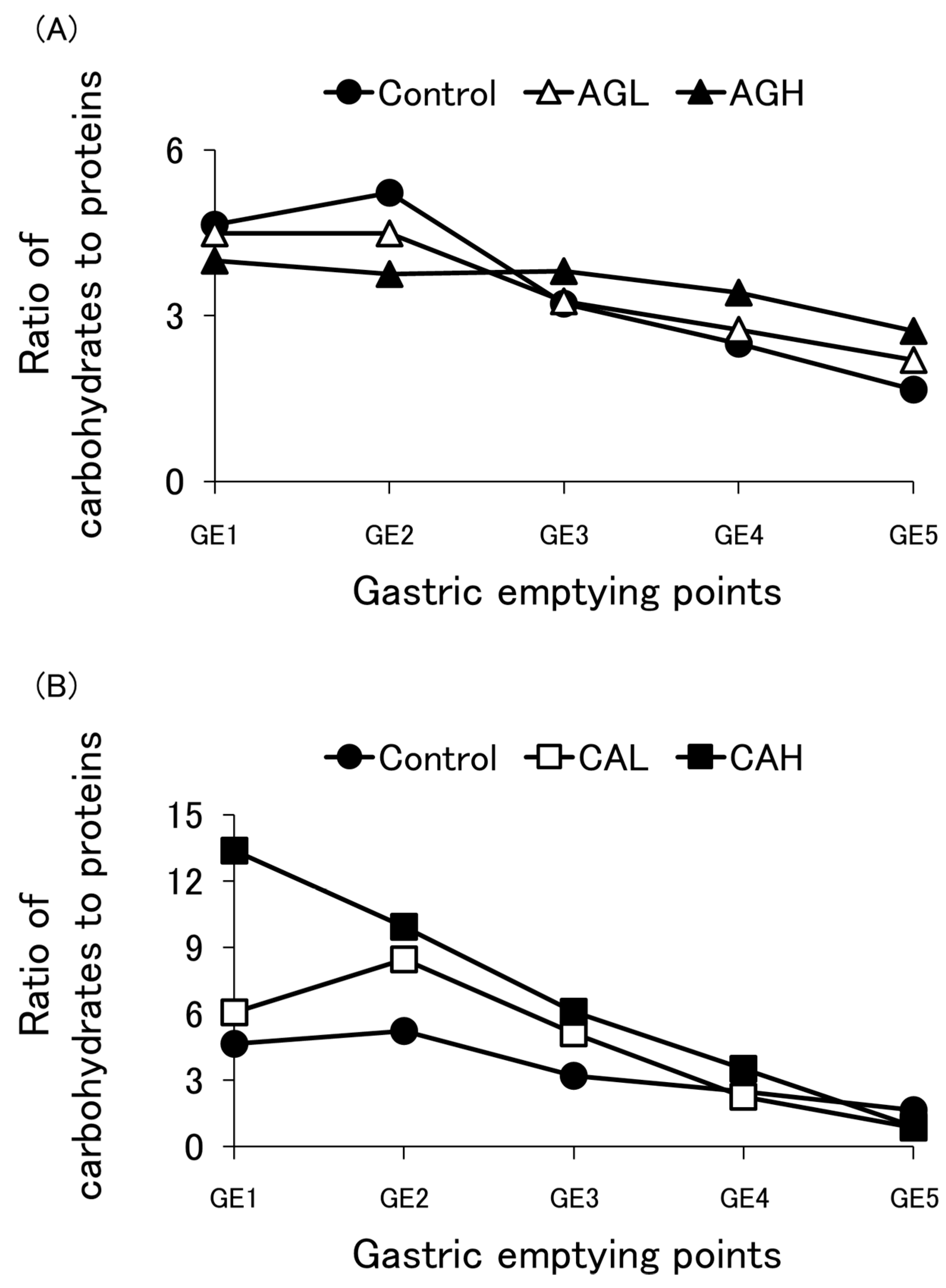
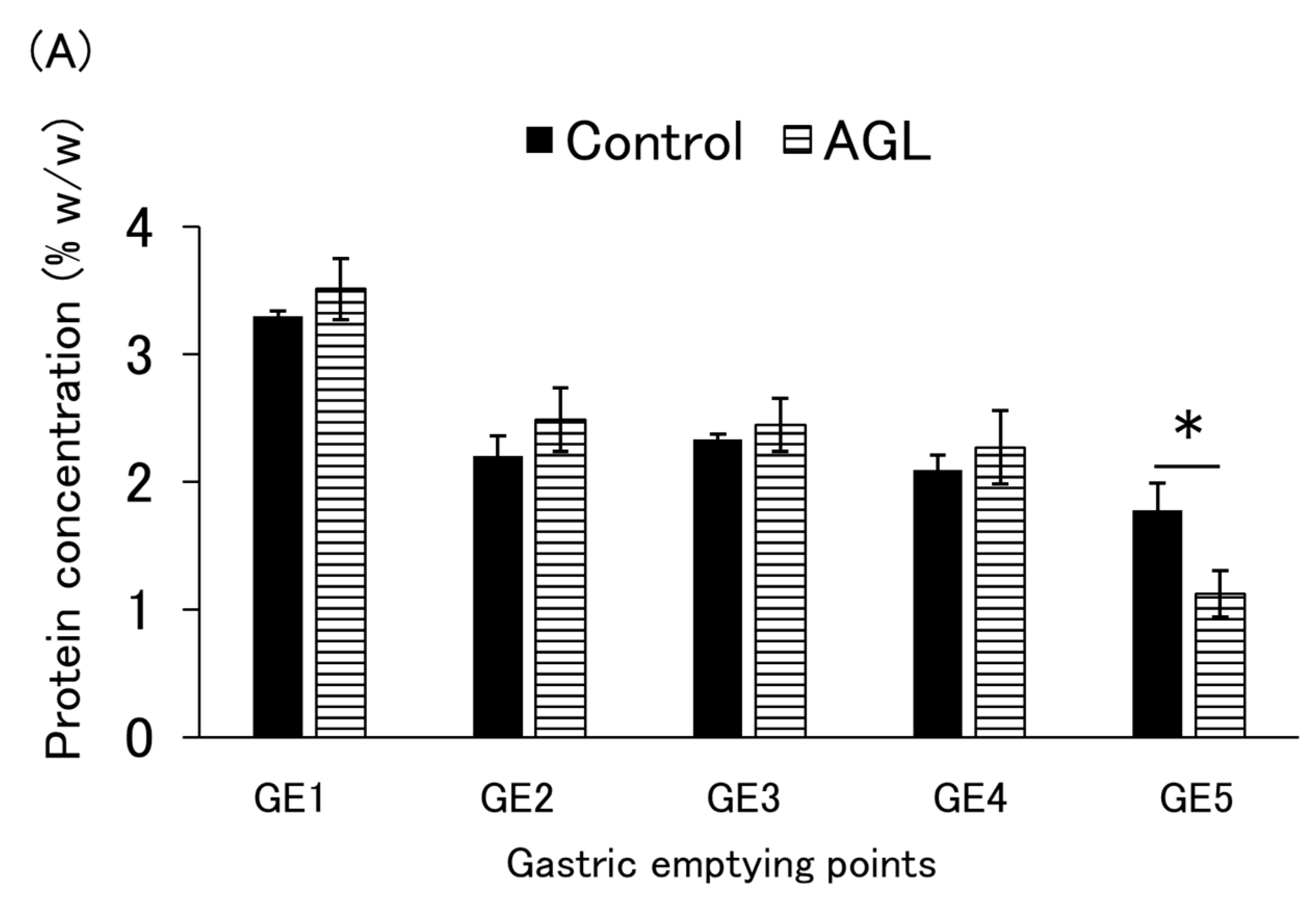
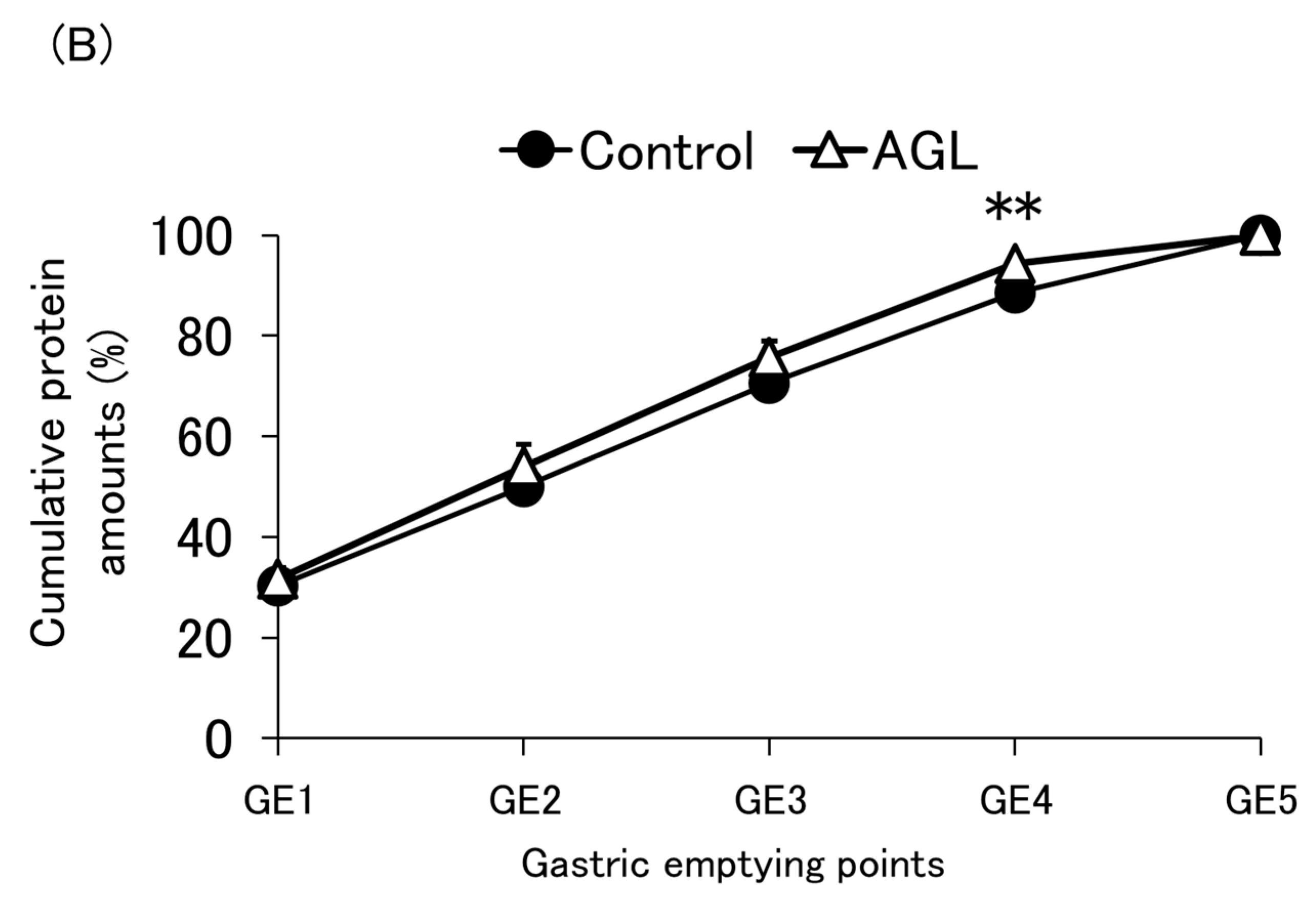
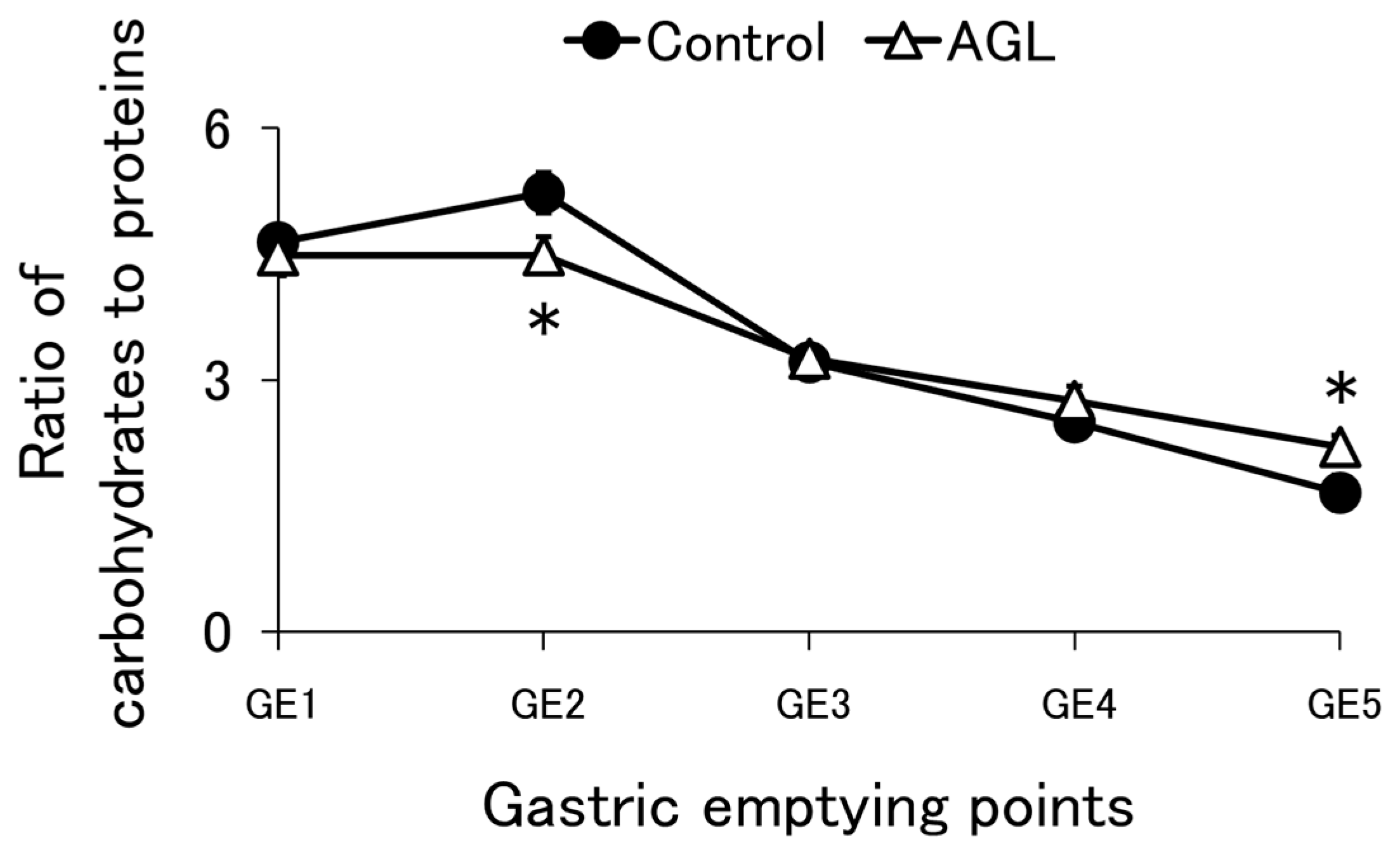
3.3. Protein Emptying
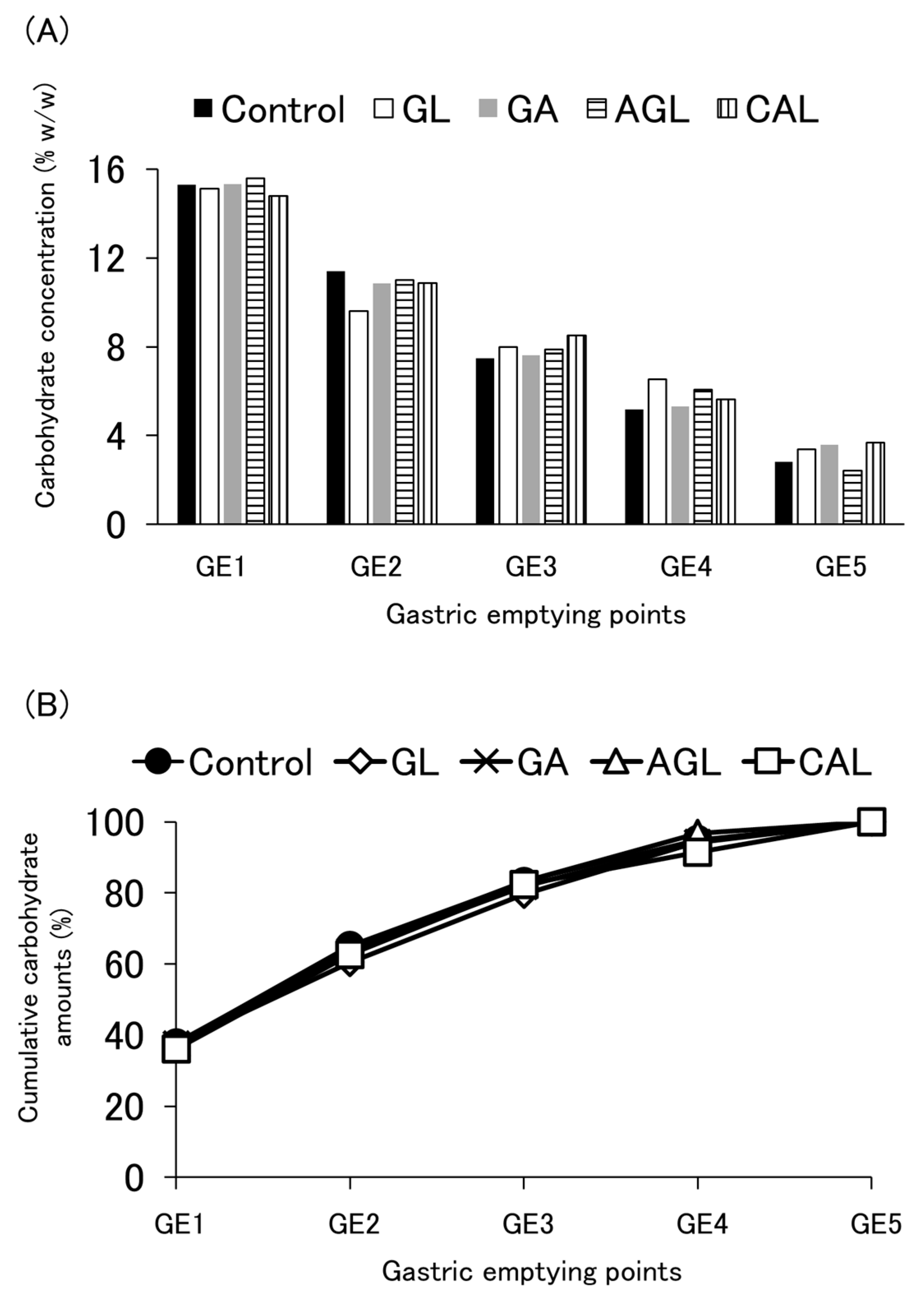
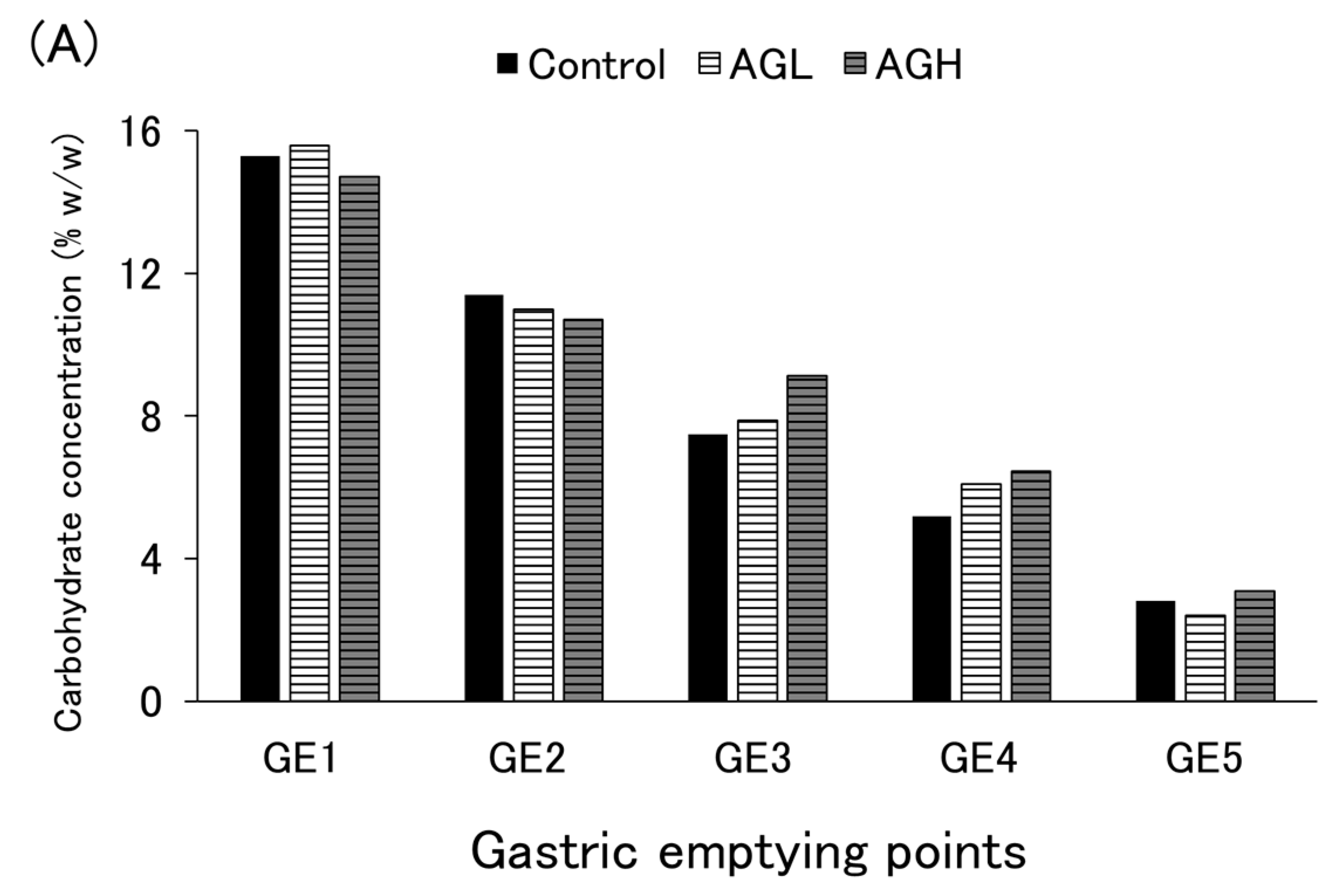
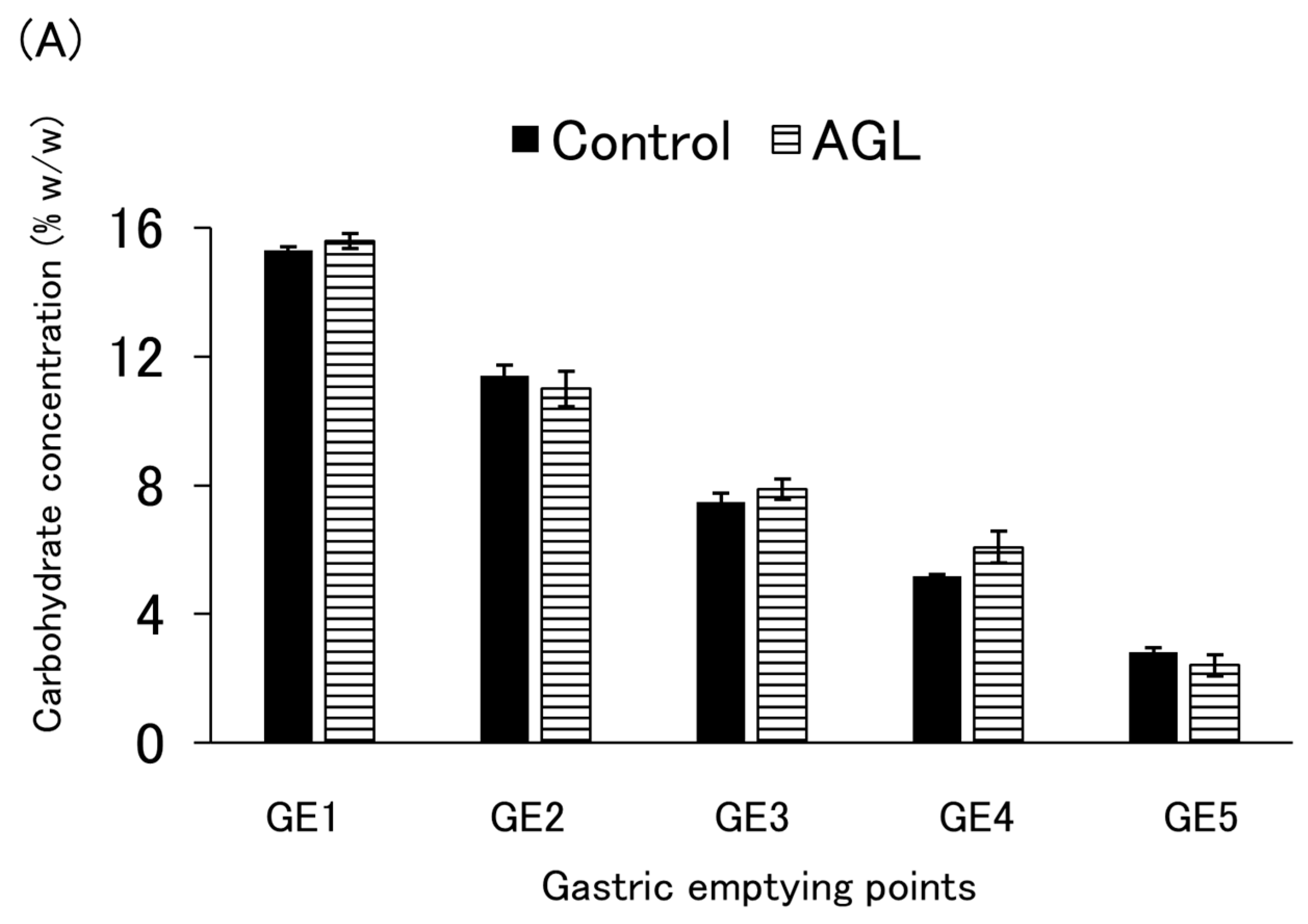
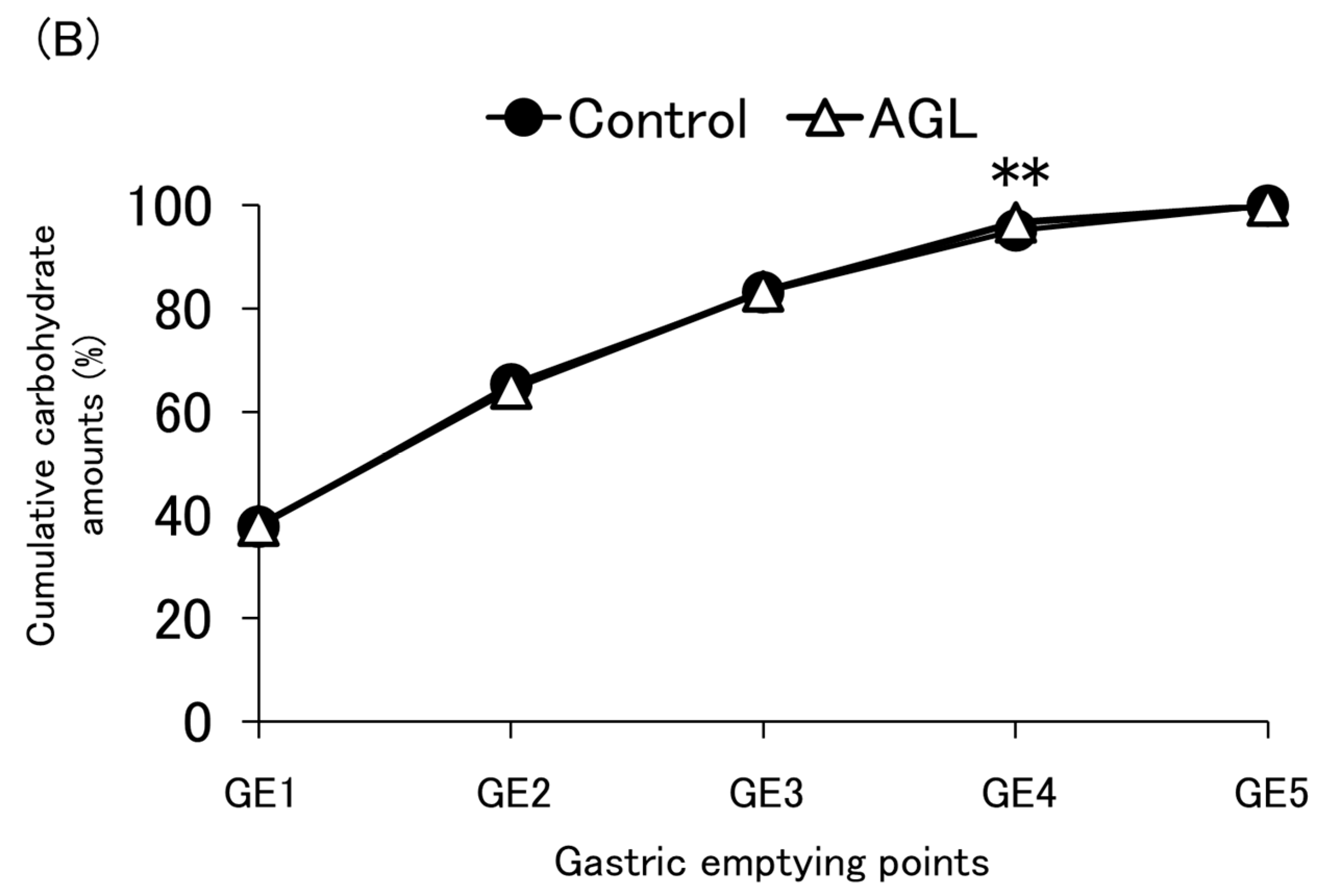
3.4. Carbohydrate Emptying
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A




References
- Doley, J. Enteral Nutrition Overview. Nutrients 2022, 14, 2180. [Google Scholar] [CrossRef] [PubMed]
- Kanie, J.; Kaganu, C.; Yamamoto, T.; Akatsu, H.; Suzuki, Y.; Kuzuya, M.; Iguchi, A. Half-Solid Enteral Nutrient Prevents Chronic Complications of Percutaneous Endoscopic Gastrostomy Tube Feeding. Nippon. Ronen Igakkai Zasshi. Jpn. J. Geriatr. 2002, 39, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Kanie, J.; Suzuki, Y.; Iguchi, A.; Akatsu, H.; Yamamoto, T.; Shimokata, H. Prevention of Gastroesophageal Reflux Using an Application of Half-Solid Nutrients in Patients with Percutaneous Endoscopic Gastrostomy Feeding. J. Am. Geriatr. Soc. 2004, 52, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Suzuki, C.; Wakabayashi, H.; Maeda, K.; Sakai, K.; Momosaki, R. Semi-Solid Nutrients for Prevention of Enteral Tube Feeding-Related Complications in Japanese Population: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1687. [Google Scholar] [CrossRef]
- Kanie, J.; Suzuki, Y.; Akatsu, H.; Kuzuya, M.; Iguchi, A. Prevention of Late Complications by Half-Solid Enteral Nutrients in Percutaneous Endoscopic Gastrostomy Tube Feeding. Gerontology 2004, 50, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Ortega, O.; Martín, A.; Clavé, P. Diagnosis and Management of Oropharyngeal Dysphagia Among Older Persons, State of the Art. J. Am. Med. Dir. Assoc. 2017, 18, 576–582. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef]
- Bisch, E.M.; Logemann, J.A.; Rademaker, A.W.; Kahrilas, P.J.; Lazarus, C.L. Pharyngeal Effects of Bolus Volume, Viscosity, and Temperature in Patients with Dysphagia Resulting from Neurologic Impairment and in Normal Subjects. J. Speech Lang. Hear. Res. 1994, 37, 1041–1049. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Araki, H.; Shirakami, Y.; Kawaguchi, J.; Kawade, N.; Iwashita, M.; Tagami, A.; Hatakeyama, H.; Hayashi, T.; Maeda, T.; et al. Inhibition of Gastroesophageal Reflux by Semi-solid Nutrients in Patients with Percutaneous Endoscopic Gastrostomy. J. Parenter. Enter. Nutr. 2009, 33, 513–519. [Google Scholar] [CrossRef]
- Okabe, K.; Kaneko, R.; Kawai, T.; Ohta, Y.; Ohara, G.; Hibi, H. Efficacy of Semi-Solidification of Enteral Nutrients for Postoperative Nutritional Management with a Nasogastric Tube. Nagoya J. Med. Sci. 2022, 84, 366–373. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, T.; Park, H.J.; Cho, J.H.; Byun, M.K. Long-Term Clinical Outcomes of Patients with Chronic Obstructive Pulmonary Disease with Sarcopenia. Life 2023, 13, 1628. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.H.; Huppertz, T. Milk Proteins: Processing, Gastric Coagulation, Amino Acid Availability and Muscle Protein Synthesis. Crit. Rev. Food Sci. Nutr. 2023, 63, 10267–10282. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.; Pachigolla, S.; Drincic, A. Glycemic Management of Hospitalized Patients Receiving Nutrition Support. Diabetes Spectr. 2022, 35, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahrén, B. Incretin and Islet Hormonal Responses to Fat and Protein Ingestion in Healthy Men. Am. J. Physiol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, H.; Yamamoto, T.; Suzuki, Y.; Kanie, J. Better Control of Blood Sugar with Treatment Using Half-Solid Nutrients: A Case Report. Nippon. Ronen Igakkai Zasshi. Jpn. J. Geriatr. 2005, 42, 564–566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markussen, J.Ø.; Madsen, F.; Young, J.F.; Corredig, M. A Semi Dynamic in Vitro Digestion Study of Milk Protein Concentrate Dispersions Structured with Different Polysaccharides. Curr. Res. Food Sci. 2021, 4, 250–261. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.L.; Carrière, F.; et al. A Standardised Semi-Dynamic in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Mackie, A.R.; Wilde, P.J.; Fenelon, M.A.; Brodkorb, A. Structural Mechanism and Kinetics of in Vitro Gastric Digestion Are Affected by Process-Induced Changes in Bovine Milk. Food Hydrocoll. 2019, 86, 172–183. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Thomas, A. Gut Motility, Sphincters and Reflex Control. Anaesth. Intensive Care Med. 2006, 7, 57–58. [Google Scholar] [CrossRef][Green Version]
- Solnes, L.B.; Sheikhbahaei, S.; Ziessman, H.A. Nuclear Scintigraphy in Practice: Gastrointestinal Motility. Am. J. Roentgenol. 2018, 211, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas Methods for Determining Protein Contents of Soybean Products. J. Am. Oil Chem. Soc. 2003, 80, 1169–1173. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Kozu, H.; Nakata, Y.; Nakajima, M.; Neves, M.A.; Uemura, K.; Sato, S.; Kobayashi, I.; Ichikawa, S. Analysis of Disintegration of Agar Gel Particles with Different Textures Using Gastric Digestion Simulator. Japan J. Food Eng. 2015, 16, 161–166. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Ström, A.; Lopez-Sanchez, P.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Sokolova, A.; Gilbert, E.P.; López-Rubio, A. Advanced Structural Characterisation of Agar-Based Hydrogels: Rheological and Small Angle Scattering Studies. Carbohydr. Polym. 2020, 236, 115655. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, F.; Chang, Y.; Wang, P.; Li, Y.; Zhang, H.; Luo, J. Formation and Structural Properties of Acid-Induced Casein–Agar Double Networks: Role of Gelation Sequence. Food Hydrocoll. 2018, 85, 291–298. [Google Scholar] [CrossRef]
- Fontes-Candia, C.; Jiménez-Barrios, P.; Miralles, B.; Recio, I.; López-Rubio, A.; Martínez-Sanz, M. Development of Polysaccharide-Casein Gel-like Structures Resistant to in Vitro Gastric Digestion. Food Hydrocoll. 2022, 127, 107505. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, P.; Liu, Z.; Lin, L.; Zhang, J. Effect of Physiological PH on the Molecular Characteristics, Rheological Behavior, and Molecular Dynamics of κ-Carrageenan/Casein. Front. Nutr. 2023, 10, 1174888. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Lv, P.; Huang, X.; Pang, J. Physicochemical Dynamic Changes and Differences of κ-Carrageenan in Different Vehicles (Aqueous and Casein Solution) during in Vitro Gastrointestinal Digestion. Food Hydrocoll. 2022, 129, 107553. [Google Scholar] [CrossRef]
- Tang, M.; Lei, Y.; Wang, Y.; Li, D.; Wang, L. Rheological and Structural Properties of Sodium Caseinate as Influenced by Locust Bean Gum and κ-Carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Pereyra, R.; Schmidt, K.A.; Wicker, L. Interaction and Stabilization of Acidified Casein Dispersions with Low and High Methoxyl Pectins. J. Agric. Food Chem. 1997, 45, 3448–3451. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Liu, B.; Zheng, M.; Chen, H.; Pang, J. Rheological Behavior and Molecular Dynamics Simulation of κ-Carrageenan/Casein under Simulated Gastrointestinal Electrolyte Conditions. Food Hydrocoll. 2023, 136, 108240. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Hunt, J.N.; Stubbs, D.F. The Volume and Energy Content of Meals as Determinants of Gastric Emptying. J. Physiol. 1975, 245, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, V.; Zamboni, M.; Dioli, A.; Zoico, E.; Mazzali, G.; Omizzolo, F.; Bissoli, L.; Solerte, S.B.; Benini, L.; Bosello, O. Delayed Postprandial Gastric Emptying and Impaired Gallbladder Contraction Together with Elevated Cholecystokinin and Peptide YY Serum Levels Sustain Satiety and Inhibit Hunger in Healthy Elderly Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.; Huet, B.; Lee, E. Effects of Aging and Gastritis on Gastric Acid and Pepsin Secretion in Humans: A Prospective Study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper Gastrointestinal PH in Seventy-Nine Healthy, Elderly, North American Men and Women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Menard, O.; Lesmes, U.; Shani-Levi, C.S.; Araiza Calahorra, A.; Lavoisier, A.; Morzel, M.; Rieder, A.; Feron, G.; Nebbia, S.; Mashiah, L.; et al. Static in Vitro Digestion Model Adapted to the General Older Adult Population: An INFOGEST International Consensus. Food Funct. 2023, 14, 4569–4582. [Google Scholar] [CrossRef] [PubMed]
- Achour, L.; Méance, S.; Briend, A. Comparison of Gastric Emptying of a Solid and a Liquid Nutritional Rehabilitation Food. Eur. J. Clin. Nutr. 2001, 55, 769–772. [Google Scholar] [CrossRef]
- Pal, A.; Indireshkumar, K.; Schwizer, W.; Abrahamsson, B.; Fried, M.; Brasseur, J.G. Gastric Flow and Mixing Studied Using Computer Simulation. Proc. R. Soc. London Ser. B Biol. Sci. 2004, 271, 2587–2594. [Google Scholar] [CrossRef]
- Kozu, H.; Nakata, Y.; Nakajima, M.; Neves, M.A.; Uemura, K.; Sato, S.; Kobayashi, I.; Ichikawa, S. Development of a Human Gastric Digestion Simulator Equipped with Peristalsis Function for the Direct Observation and Analysis of the Food Digestion Process. Food Sci. Technol. Res. 2014, 20, 225–233. [Google Scholar] [CrossRef]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; Schjerling, P.; van Hall, G.; et al. Whey and Casein Labeled with L-[1-13 C]Leucine and Muscle Protein Synthesis: Effect of Resistance Exercise and Protein Ingestion. Am. J. Physiol. Metab. 2011, 300, E231–E242. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukiashi, M.; Koyama, T.; Iwamoto, H.; Sonoki, H.; Miyaji, K. Evaluation of the Effect of Thickeners in Enteral Formulas on the Gastric Emptying Rate of Proteins and Carbohydrates Using a Semi-Dynamic Gastric Model. Nutrients 2024, 16, 2115. https://doi.org/10.3390/nu16132115
Tsukiashi M, Koyama T, Iwamoto H, Sonoki H, Miyaji K. Evaluation of the Effect of Thickeners in Enteral Formulas on the Gastric Emptying Rate of Proteins and Carbohydrates Using a Semi-Dynamic Gastric Model. Nutrients. 2024; 16(13):2115. https://doi.org/10.3390/nu16132115
Chicago/Turabian StyleTsukiashi, Motoki, Takahiro Koyama, Hiroshi Iwamoto, Hirofumi Sonoki, and Kazuhiro Miyaji. 2024. "Evaluation of the Effect of Thickeners in Enteral Formulas on the Gastric Emptying Rate of Proteins and Carbohydrates Using a Semi-Dynamic Gastric Model" Nutrients 16, no. 13: 2115. https://doi.org/10.3390/nu16132115
APA StyleTsukiashi, M., Koyama, T., Iwamoto, H., Sonoki, H., & Miyaji, K. (2024). Evaluation of the Effect of Thickeners in Enteral Formulas on the Gastric Emptying Rate of Proteins and Carbohydrates Using a Semi-Dynamic Gastric Model. Nutrients, 16(13), 2115. https://doi.org/10.3390/nu16132115





