The Role of Food Matrices Supplemented with Milk Fat Globule Membrane in the Bioaccessibility of Lipid Components and Adaptation of Cellular Lipid Metabolism of Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BM-MFGM
2.2. Experimental Design
2.3. In Vitro Digestion
2.4. Caco-2 Cell Culture, Protein Quantification, and Cell Viability
2.5. Isolation Protocol of Lipids from Food Matrices, Micellar Fractions, and Cell Culture
2.6. Lipids Analyses
2.7. Statistical Analysis
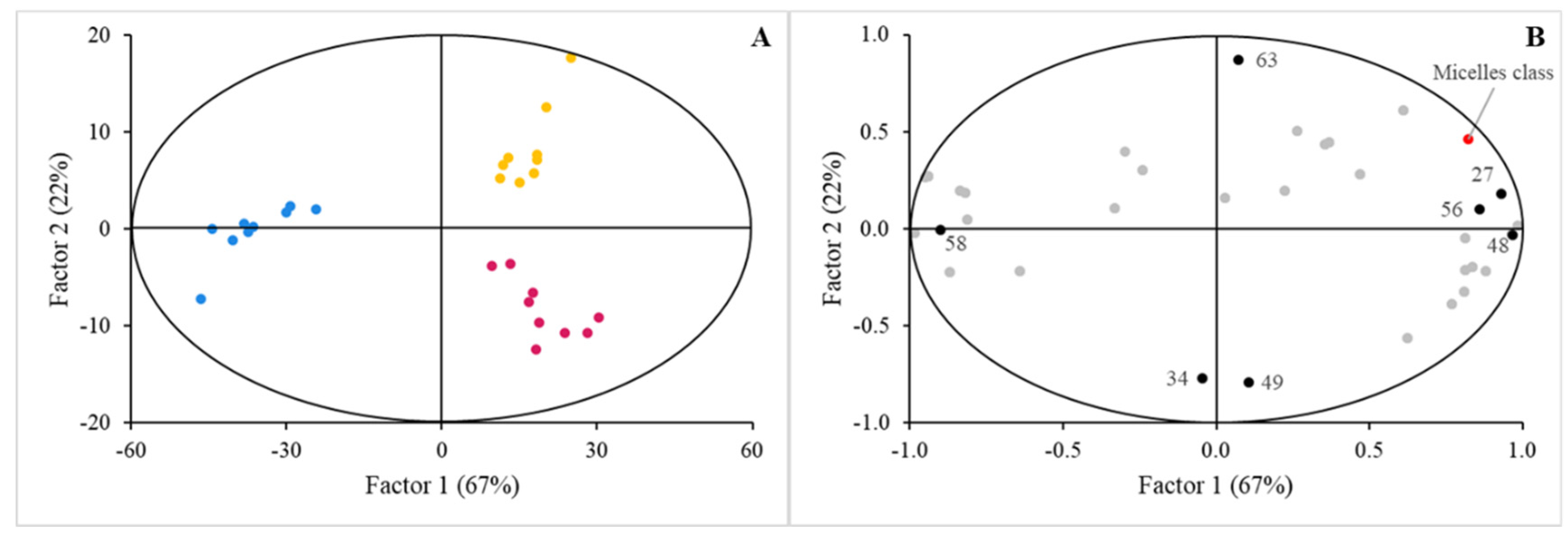
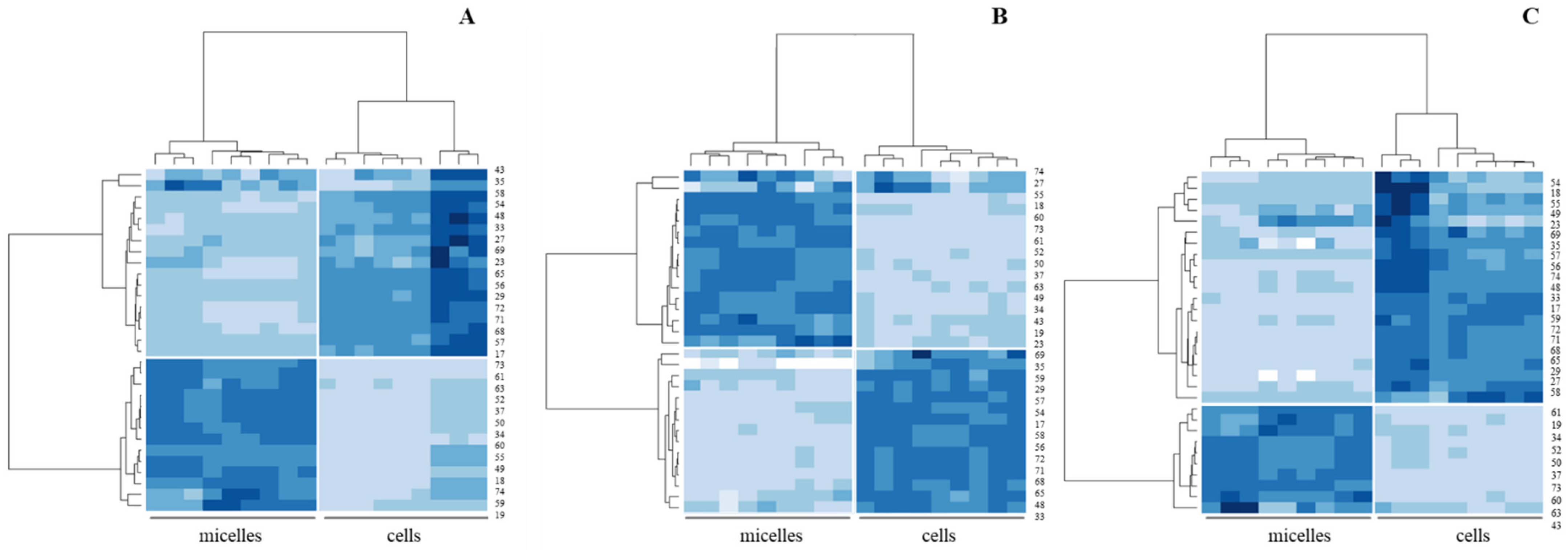
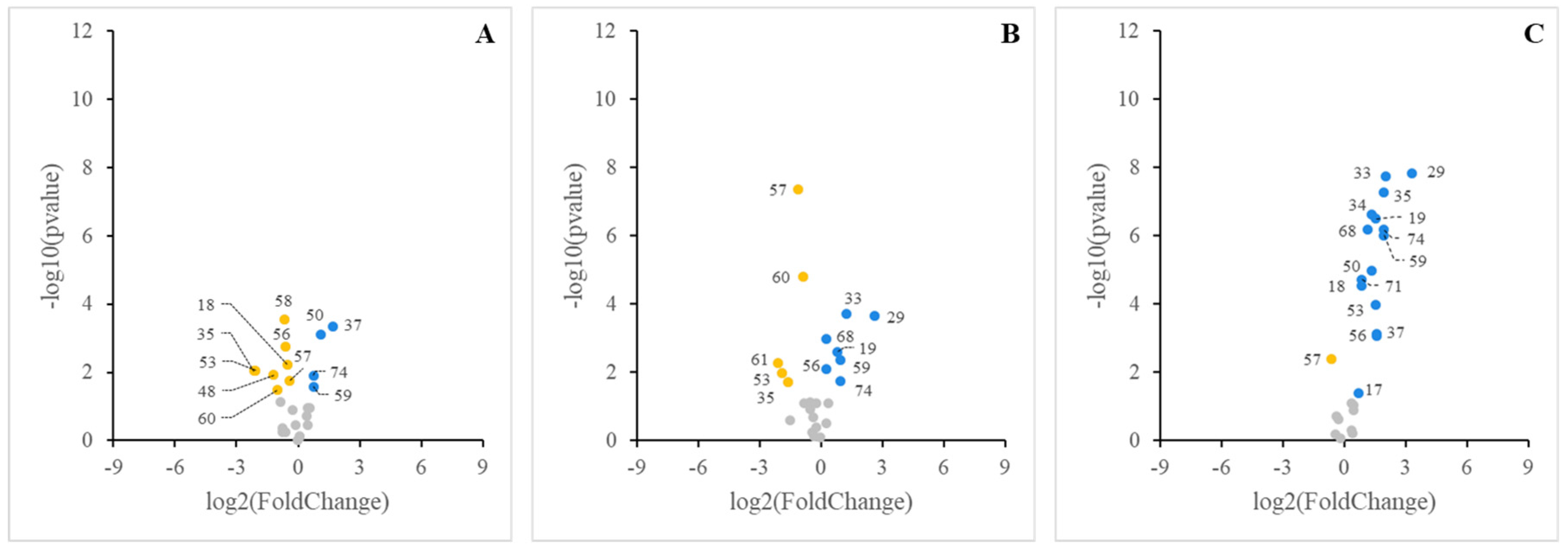
3. Results
3.1. Digestibility of BM-MFGM Lipids
3.2. Sensing of Cell Cultures to Lipid Micellar Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabadán, A.; Nieto, R.; Bernabéu, R. Food innovation as a means of developing healthier and more sustainable foods. Foods 2021, 10, 2069. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Rincón, F.; Pérez-Gálvez, A. Developing an emulsifier system to improve the bioaccessibility of carotenoids. J. Agric. Food Chem. 2008, 56, 10384–10390. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Viera, I.; Herrera, M.; Roca, M. In vitro bioaccessibility protocol for chlorophylls. J. Agric. Food Chem. 2021, 69, 8777–8786. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Ménard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled Trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Lagerqvist, C.; Stenlund, H.; Lönnerdal, B.; Hernell, O.; West, C.E. Serum cytokine patterns are modulated in infants fed formula with probiotics or milk fat globule membranes: A randomized controlled trial. PLoS ONE 2021, 16, e0251293. [Google Scholar] [CrossRef]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Huërou-Luron, I.L.; Lemaire, M.; Blat, S. Health benefits of dairy lipids and MFGM in infant formula. Oilseeds Fats Crops Lipids 2018, 25, D306. [Google Scholar] [CrossRef]
- Norris, G.H.; Porter, C.M.; Jiang, C.; Millar, C.L.; Blesso, C.N. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 40, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr. Metab. 2010, 7, 90. [Google Scholar] [CrossRef]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, P.; García-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Fontecha-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2020, 86, 103386. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Calvo, M.V.; Kohen, V.L.; Díaz-Mardomingo, C.; García-Herranz, S.; Baliyan, S.; Tomé-Carneiro, J.; Colmenarejo, G.; Visioli, F.; Venero, C.; Fontecha, J. Milk fat globule membrane-enriched milk improves episodic memory: A randomized, parallel, double-blind, placebo-controlled trial in older adults. J. Funct. Foods 2023, 111, 105849. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Viera, I.; Herrera, M.; Roca, M. Influence of food composition on chlorophyll bioaccessibility. Food Chem. 2022, 386, 132805. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Martínez-Sánchez, V.; Calvo, M.V.; Viera, I.; Girón-Calle, J.; Fontecha, J.; Pérez-Gálvez, A. Mechanisms for the interaction of the milk fat globule membrane with the plasma membrane of gut epithelial cells. Food Res. Int. 2023, 173, 113330. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Fontecha, J.; Rodríguez-Alcalá, L.M. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta 2014, 128, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Fontecha, J.; Goudjil, H.; Ríos, J.J.; Fraga, M.J.; Juárez, M. Identity of the major triacylglycerols in ovine milk fat. Int. Dairy J. 2005, 15, 1217–1224. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In Vitro Digestion of Lipids in Real Foods: Influence of Lipid Organization Within the Food Matrix and Interactions with Nonlipid Components. J. Food Sci. 2018, 83, 2629–2637. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, F.; Xiao, H. The nutraceutical bioavailability classification scheme: Classifying nutraceuticals according to factors limiting their oral bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef]
- Berry, S.E.; Tydeman, E.A.; Lewis, H.B.; Phalora, R.; Rosborough, J.; Picout, D.R.; Ellis, P.R. Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects1. Am. J. Clin. Nutr. 2008, 88, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.M.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk Fat Globule Membrane Supplementation in Formula-fed Rat Pups Improves Reflex Development and May Alter Brain Lipid Composition. Sci. Rep. 2018, 8, 15277–15279. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Calvo, M.V.; Piquera, D.; Montero, O.; Visioli, F.; Venero, C.; Fontecha, J. Milk fat globule membrane concentrate as a nutritional supplement prevents age-related cognitive decline in old rats: A lipidomic study of synaptosomes. Food Res. Int. 2023, 163, 112163. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.-H.; Huang, S.-L.; Yue, Z.-B.; Yin, X.-S.; Feng, Z.-Q.; Zhang, X.-G.; Song, G.-L. Whey protein powder with milk fat globule membrane attenuates Alzheimer’s disease pathology in 3×Tg-AD mice by modulating neuroinflammation through the peroxisome proliferator-activated receptor γ signaling pathway. J. Dairy Sci. 2023, 106, 5253–5265. [Google Scholar] [CrossRef]
- Luque-Uría, A.; Calvo, M.V.; Visioli, F.; Fontecha, J. Milk fat globule membrane and their polar lipids: Reviewing preclinical and clinical trials on cognition. Food Funct. 2024, 15, 6783–6797. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Excipient foods: Designing food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.S.; Jones, P.J. Dietary fatty acid composition modulates obesity and interacts with obesity-related genes. Lipids 2017, 52, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. The gut: A regulatory hall governing fat-soluble micronutrient absorption. Am. J. Clin. Nutr. 2019, 110, 1045–1046. [Google Scholar] [CrossRef]
- Tremblay, A.J.; Lamarche, B.; Guay, V.; Charest, A.; Lemelin, V.; Couture, P. Short-term, high-fat diet increases the expression of key intestinal genes involved in lipoprotein metabolism in healthy men. Am. J. Clin. Nutr. 2013, 98, 32–41. [Google Scholar] [CrossRef]
- Tovar, A.R.; Díaz-Villaseñor, A.; Cruz-Salazar, N.; Ordáz, G.; Granados, O.; Palacios-González, B.; Tovar-Palacio, C.; López, P.; Torres, N. Dietary type and amount of fat modulate lipid metabolism gene expression in liver and in adipose tissue in high-fat diet-fed rats. Arch. Med. Res. 2011, 42, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Albright, E.J.; Mathur, S.N. Effect of dietary n-3 fatty acids on HMG-CoA reductase and ACAT activities in liver and intestine of the rabbit. J. Lipid Res. 1987, 28, 50–58. [Google Scholar] [CrossRef]
- Le, T.T.; Van de Wiele, T.; Do, T.N.H.; Debyser, G.; Struijs, K.; Devreese, B.; Dewettinck, K.; Van Camp, J. Stability of milk fat globule membrane proteins toward human enzymatic gastrointestinal digestion. J. Dairy Sci. 2012, 95, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Du, X.; Brink, L.; Lönnerdal, B. The role of orally ingested milk fat globule membrane on intestinal barrier functions evaluated with a suckling rat pup supplementation model and a human enterocyte model. J. Nutr. Biochem. 2022, 108, 109084. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.; García-Cano, I.; Chaves, F.; Walsh, K.R.; Jimenez-Flores, R.; Failla, M.L. Efficient in vitro digestion of lipids and proteins in bovine milk fat globule membrane ingredient (MFGMi) and whey-casein infant formula with added MFGMi. J. Dairy Sci. 2023, 106, 3086–3097. [Google Scholar] [CrossRef]
- Lefèvre-Arbogast, S.; Hejblum, B.P.; Helmer, C.; Klose, C.; Manach, C.; Low, D.Y.; Urpi-Sarda, M.; Andres-Lacueva, C.; González-Domínguez, R.; Aigner, L.; et al. Early signature in the blood lipidome associated with subsequent cognitive decline in the elderly: A case-control analysis nested within the Three-City cohort study. eBioMedicine 2021, 64, 103216. [Google Scholar] [CrossRef]
- Ambrożej, D.; Dumycz, K.; Dziechciarz, P.; Ruszczyński, M. Milk fat globule membrane supplementation in children: Systematic review with meta-analysis. Nutrients 2021, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y. Dietary fat, lipogenesis and energy balance. Physiol. Behav. 2004, 83, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Kuksis, A. Biosynthesis of triacylglycerols. Prog. Lipid Res. 1996, 35, 169–201. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | JM | CM | LCM |
|---|---|---|---|
| Jelly | 3 g | - | - |
| All-purpose flour | - | 150 g | 150 g |
| Sunflower oil | - | - | 80 g |
| Sugar | 35 g | 100 g | 100 g |
| Water | 62 g | - | - |
| Egg white | - | 140 g | 140 g |
| BM-MFGM | 0, 2, 5, 10% | 0, 2, 5, 10% | 0, 2, 5, 10% |
| Food Matrices Supplemented with BM-MFGM | Micelles Class | Cell Class | |
|---|---|---|---|
| Lipid classes | |||
| Triacylglycerides | 2.610 | - | 1.706 |
| Diacylglycerides | - 2 | - | 1.355 |
| Free fatty acids plus cholesterol | - | 1.187 | 1.544 |
| Monoacylglycerides | - | 1.623 | - |
| Phosphatidylcholine | - | - | 1.036 |
| Total polar lipids 1 | - | - | 1.379 |
| ƩPolar lipids | - | - | 1.380 |
| ƩNeutral lipids | 2.678 | 2.105 | 2.633 |
| Fatty acids | |||
| C16:0 | - | - | 1.014 |
| C18:1 c9 | 1.482 | 1.271 | 1.246 |
| C18:2 | 1.990 | 1.826 | 1.916 |
| ƩSaturated fatty acids | - | - | 1.420 |
| ƩMonounsaturated fatty acids | 1.510 | 1.283 | 1.596 |
| ƩPolyunsaturated fatty acids | 2.003 | 1.779 | 1.968 |
| ω6 fatty acids | 1.983 | 1.779 | 1.916 |
| ƩLong chain fatty acids | 2.682 | 2.112 | 2.624 |
| Triacylglycerides species | |||
| CN50 | - | - | 1.418 |
| CN52 | 1.287 | - | 2.124 |
| CN54 | 2.304 | - | 2.086 |
| ƩNeutral species | 2.675 | - | 1.824 |
| ƩPolar species | - | 2.097 | 2.349 |
| Cholesterol | - | 1.597 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, V.; Calvo, M.V.; Fontecha, J.; Pérez-Gálvez, A. The Role of Food Matrices Supplemented with Milk Fat Globule Membrane in the Bioaccessibility of Lipid Components and Adaptation of Cellular Lipid Metabolism of Caco-2 Cells. Nutrients 2024, 16, 2798. https://doi.org/10.3390/nu16162798
Martínez-Sánchez V, Calvo MV, Fontecha J, Pérez-Gálvez A. The Role of Food Matrices Supplemented with Milk Fat Globule Membrane in the Bioaccessibility of Lipid Components and Adaptation of Cellular Lipid Metabolism of Caco-2 Cells. Nutrients. 2024; 16(16):2798. https://doi.org/10.3390/nu16162798
Chicago/Turabian StyleMartínez-Sánchez, Victoria, María Visitación Calvo, Javier Fontecha, and Antonio Pérez-Gálvez. 2024. "The Role of Food Matrices Supplemented with Milk Fat Globule Membrane in the Bioaccessibility of Lipid Components and Adaptation of Cellular Lipid Metabolism of Caco-2 Cells" Nutrients 16, no. 16: 2798. https://doi.org/10.3390/nu16162798
APA StyleMartínez-Sánchez, V., Calvo, M. V., Fontecha, J., & Pérez-Gálvez, A. (2024). The Role of Food Matrices Supplemented with Milk Fat Globule Membrane in the Bioaccessibility of Lipid Components and Adaptation of Cellular Lipid Metabolism of Caco-2 Cells. Nutrients, 16(16), 2798. https://doi.org/10.3390/nu16162798








