Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Treatments and Dietary Control
2.4. Blood Pressure Monitoring and Blood Sampling
2.5. HPLC Analysis
2.6. Untargeted Plasma Metabolomic Analysis
2.6.1. Sample Extraction
2.6.2. Data Acquisition
2.7. Data Analysis
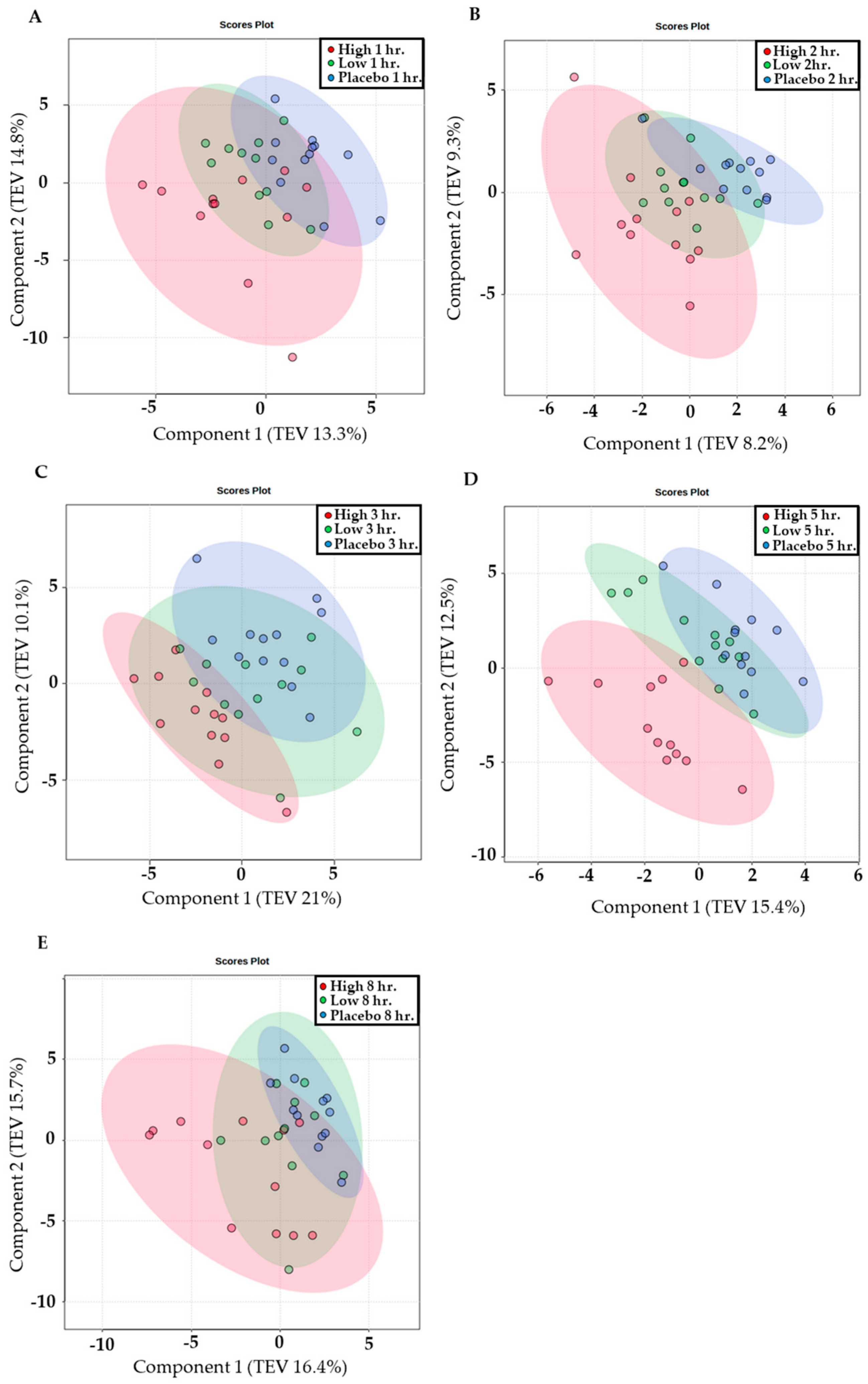
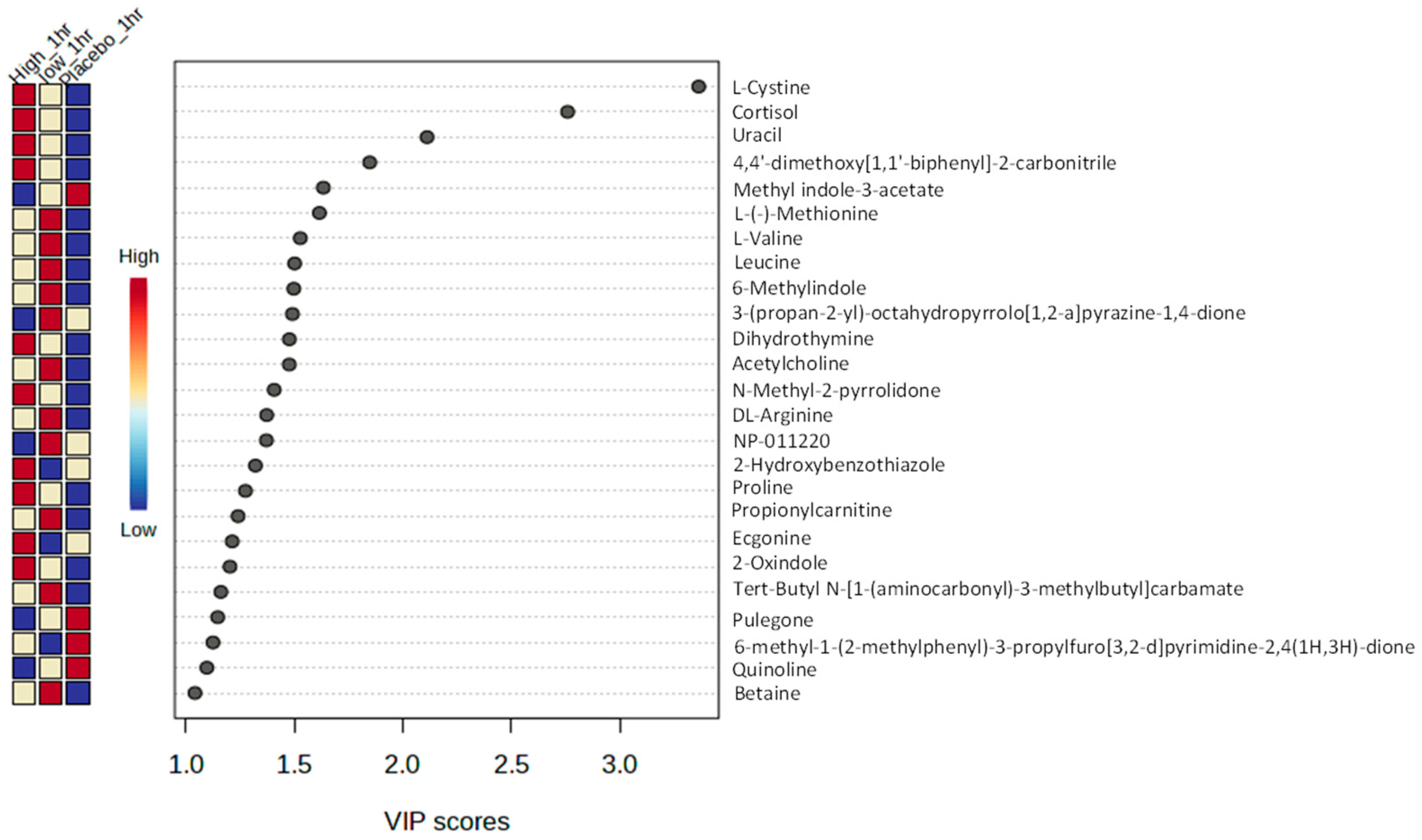
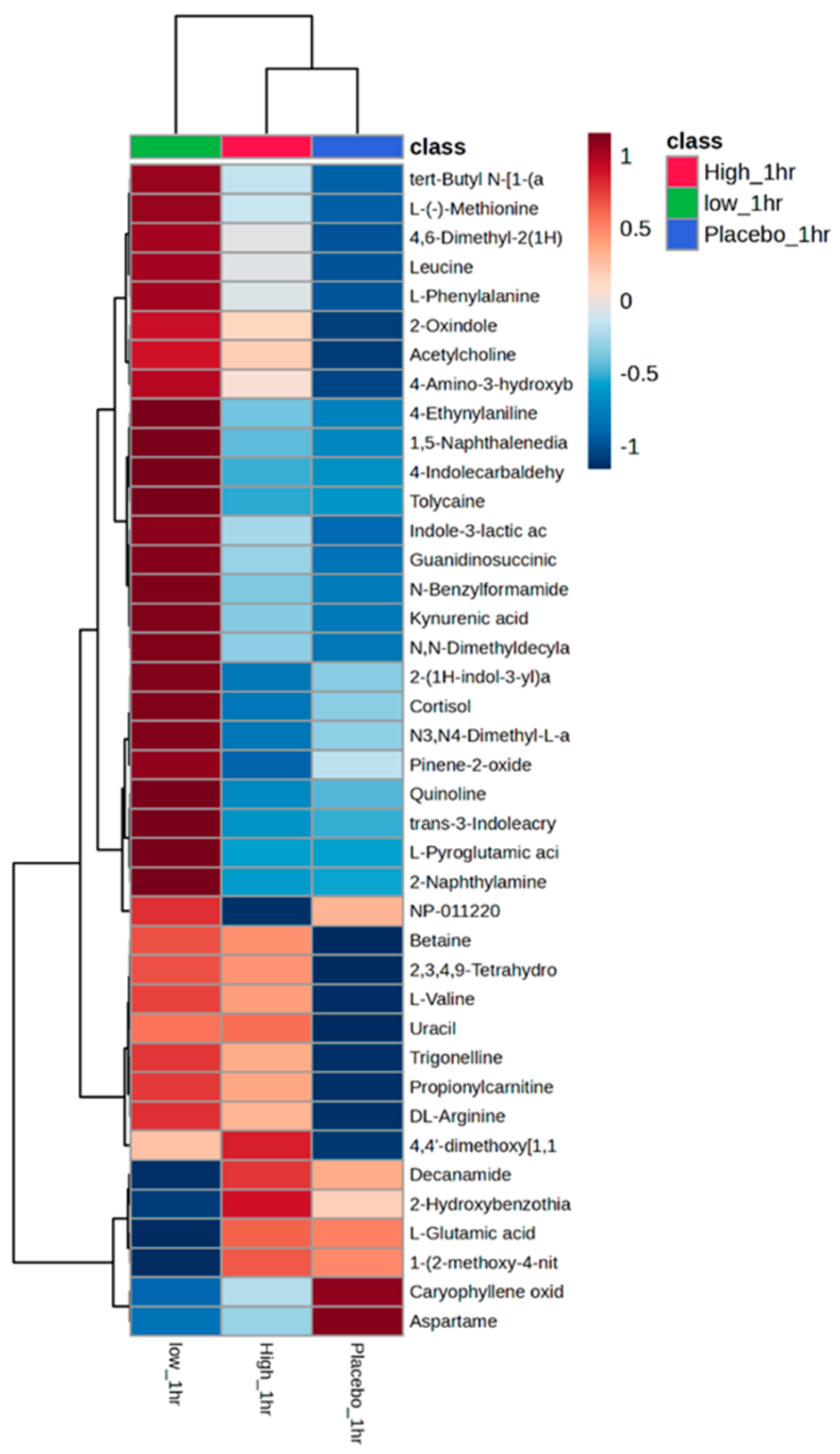
3. Results
3.1. Analysis of the TC Extract
3.2. Bioavailability Results
3.3. Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Ginsburg, I.; Kohen, R.; Koren, E. Microbial and host cells acquire enhanced oxidant-scavenging abilities by binding polyphenols. Arch. Biochem. Biophys. 2011, 506, 12–23. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent. Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.-P.J.; Rezzi, S. Metabolomics View on Gut Microbiome Modulation by Polyphenol-rich Foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced Stress and Improved Sleep Quality Caused by Green Tea Are Associated with a Reduced Caffeine Content. Nutrients 2017, 9, 777. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Alba, C.M.; Daya, M.; Franck, C. Tart Cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2019, 59, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Ataie-Jafari, A.; Hosseini, S.; Karimi, F.; Pajouhi, M. Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: A pilot study. Nutr. Food Sci. 2008, 38, 355–360. [Google Scholar] [CrossRef]
- Garrido, M.; Espino, J.; Toribio-Delgado, A.F.; Cubero, J.; Maynar-Mariño, J.I.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. A jerte valley cherry-based product as a supply of tryptophan. Int. J. Tryptophan Res. 2012, 5, IJTR.S9394. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O’Connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef]
- Connolly; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Wangdi, J.T.; O’Leary, M.F.; Kelly, V.G.; Jackman, S.R.; Tang, J.C.; Dutton, J.; Bowtell, J.L. Tart cherry supplement enhances skeletal muscle glutathione peroxidase expression and functional recovery after muscle damage. Med. Sci. Sports Exerc. 2022, 54, 609–621. [Google Scholar] [CrossRef]
- Quinlan, R.; Hill, J.A. The Efficacy of Tart Cherry Juice in Aiding Recovery after Intermittent Exercise. Int. J. Sports Physiol. Perform. 2020, 15, 368–374. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.-C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Seymour, E.M.; Warber, S.M.; Kirakosyan, A.; Noon, K.R.; Gillespie, B.; Uhley, V.E.; Wunder, J.; Urcuyo, D.E.; Kaufman, P.B.; Bolling, S.F. Anthocyanin pharmacokinetics and dose-dependent plasma antioxidant pharmacodynamics following whole tart cherry intake in healthy humans. J. Funct. Foods 2014, 11, 509–516. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Product. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Kirakosyan; Seymour, E.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Kirakosyan; Seymour, E.M.; Wolforth, J.; McNish, R.; Kaufman, P.B.; Bolling, S.F. Tissue bioavailability of anthocyanins from whole tart cherry in healthy rats. Food Chem. 2015, 171, 26–31. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Cheung, W.; Haskell-Ramsay, C.F.; Howatson, G. Polyphenol-rich tart cherries (Prunus cerasus, cv Montmorency) improve sustained attention, feelings of alertness and mental fatigue and influence the plasma metabolome in middle-aged adults: A randomised, placebo-controlled trial. Br. J. Nutr. 2022, 128, 2409–2420. [Google Scholar] [CrossRef]
- Kimble, R.; Murray, L.; Keane, K.M.; Haggerty, K.; Howatson, G.; Lodge, J.K. The influence of tart cherries (Prunus cerasus) on vascular function and the urinary metabolome: A randomised placebo-controlled pilot study. J. Nutr. Sci. 2021, 10, e73. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Bell; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Bowtell; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Fazzari, M.; Fukumoto, L.; Mazza, G.; Livrea, M.A.; Tesoriere, L.; Marco, L.D. In vitro bioavailability of phenolic compounds from five cultivars of frozen sweet cherries (Prunus avium L.). J. Agric. Food Chem. 2008, 56, 3561–3568. [Google Scholar] [CrossRef]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Quero-García, J.; Iezzoni, A.; Pulawska, J.; Lang, G.A. Cherries: Botany, Production and Uses; CABI: Wallingford, UK, 2017. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- Tang, J.E.; Phillips, S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef]
- Morgan, P.T.; Breen, L. The role of protein hydrolysates for exercise-induced skeletal muscle recovery and adaptation: A current perspective. Nutr. Metab. 2021, 18, 44. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Wyszczelska-Rokiel, M.; Glowacki, R.; Jakubowski, H.; Matthews, T.; Wood, R.; Craig, S.A.; Paolone, V. Effects of betaine on body composition, performance, and homocysteine thiolactone. J. Int. Soc. Sports Nutr. 2013, 10, 39. [Google Scholar] [CrossRef]
- Du, Y.; Li, Y.-Y.; Choi, B.Y.; Fernadez, R.; Su, K.-J.; Sharma, K.; Qi, L.; Yin, Z.; Zhao, Q.; Shen, H. Metabolomic profiles associated with physical activity in White and African American adult men. PLoS ONE 2023, 18, e0289077. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting pyrimidine metabolism in the era of precision cancer medicine. Front. Oncol. 2021, 11, 684961. [Google Scholar] [CrossRef]
- Duley, J.A.; Ni, M.; Shannon, C.; Norris, R.L.; Sheffield, L.; Harris, M.; van Kuilenburg, A.B.; Mead, S.; Cameron, A.; Helsby, N. Towards a test to predict 5-fluorouracil toxicity: Pharmacokinetic data for thymine and two sequential metabolites following oral thymine administration to healthy adult males. Eur. J. Pharm. Sci. 2016, 81, 36–41. [Google Scholar] [CrossRef]
- Dawidzik, J.; Budzinski, E.; Patrzyc, H.; Cheng, H.C.; Iijima, H.; Alderfer, J.; Tabaczynski, W.; Wallace, J.; Box, H. Dihydrothymine lesion in X-irradiated DNA: Characterization at the molecular level and detection in cells. Int. J. Radiat. Biol. 2004, 80, 355–361. [Google Scholar] [CrossRef]
- Gambichler, T.; Schlaffke, A.; Tomi, N.S.; Othlinghaus, N.; Altmeyer, P.; Kreuter, A. Tacrolimus ointment neither blocks ultraviolet B nor affects expression of thymine dimers and p53 in human skin. J. Dermatol. Sci. 2008, 50, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A review of the health benefits of cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Bassi, C.; Saunders, M.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.A.; Vargas, A.A.; Puebla, C.; Fernández, P.; Escamilla, R.; Lagos, C.F.; Matus, M.F.; Vilos, C.; Cea, L.A.; Barnafi, E. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat. Commun. 2020, 11, 1073. [Google Scholar] [CrossRef]
- da Costa, K.-A.; Badea, M.; Fischer, L.M.; Zeisel, S.H. Elevated serum creatine phosphokinase in choline-deficient humans: Mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004, 80, 163–170. [Google Scholar] [CrossRef]


| Chemical Formula | Molecular Weight | Monoisotopic Mass | M-H | Retention Time (min) | Relative Abundance | |
|---|---|---|---|---|---|---|
| Protocatechuic Acid | C7H6O4 | 154.12 | 154.02661 | 153.0187 | 6.89 | 3.68 × 105 |
| Vanillic Acid | C8H8O4 | 168.14 | 168.04226 | 167.0347 | 8.12 | 3.75 × 103 |
| Chlorogenic Acid | C16H18O9 | 354.31 | 354.09058 | 353.0865 | 7.52 | 5.00 × 106 |
| Cyanidine-3-O-Rutinoside | C27H31O15 | 595.52 | 595.16630 | 593.1505 | 7.22 | 5.02 × 106 |
| Peonidin-3-O-Rutinoside | C28H33O15 | 609.6 | 609.18195 | 607.1655 | 7.47 | 5.68 × 105 |
| Cyanidine-3-O-Glucoside | C21H21O11 | 449.38 | 449.10839 | 447.0924 | 7.22 | 3.09 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squires, E.; Walshe, I.H.; Cheung, W.; Bowerbank, S.L.; Dean, J.R.; Wood, J.; McHugh, M.P.; Plattner, S.; Howatson, G. Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption. Nutrients 2024, 16, 1023. https://doi.org/10.3390/nu16071023
Squires E, Walshe IH, Cheung W, Bowerbank SL, Dean JR, Wood J, McHugh MP, Plattner S, Howatson G. Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption. Nutrients. 2024; 16(7):1023. https://doi.org/10.3390/nu16071023
Chicago/Turabian StyleSquires, Emma, Ian H. Walshe, William Cheung, Samantha L. Bowerbank, John R. Dean, Jacob Wood, Malachy P. McHugh, Stephan Plattner, and Glyn Howatson. 2024. "Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption" Nutrients 16, no. 7: 1023. https://doi.org/10.3390/nu16071023
APA StyleSquires, E., Walshe, I. H., Cheung, W., Bowerbank, S. L., Dean, J. R., Wood, J., McHugh, M. P., Plattner, S., & Howatson, G. (2024). Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption. Nutrients, 16(7), 1023. https://doi.org/10.3390/nu16071023







