Introduction of Solid Foods in Preterm Infants and Its Impact on Growth in the First Year of Life—A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Visits
2.3. Primary Outcome
2.4. Secondary Outcomes
2.5. Baseline Characteristics
2.6. Statistical Analysis and Machine Learning Model
3. Results
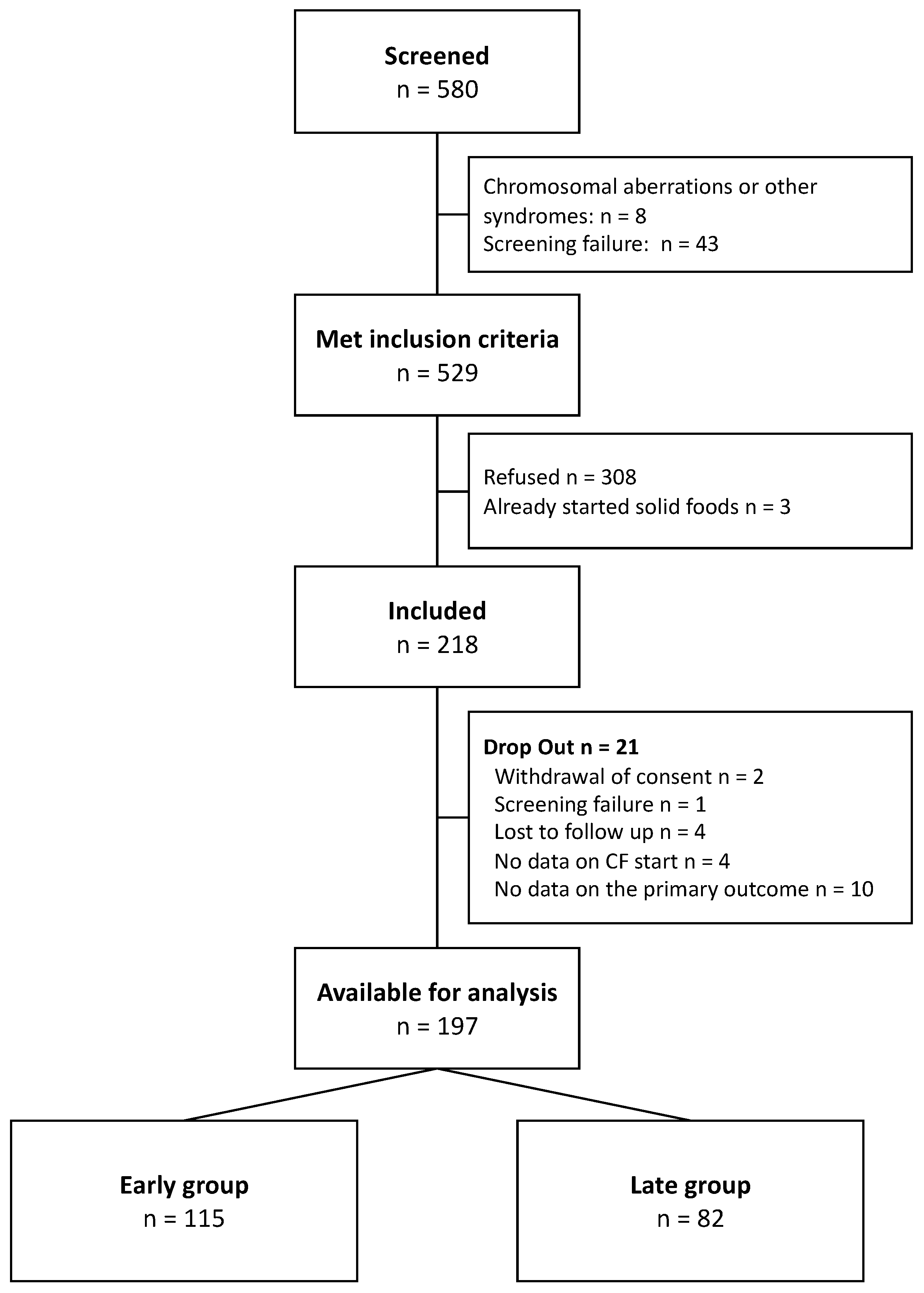
3.1. Screening and Participants
3.2. Baseline Characteristics and Neonatal Morbidity
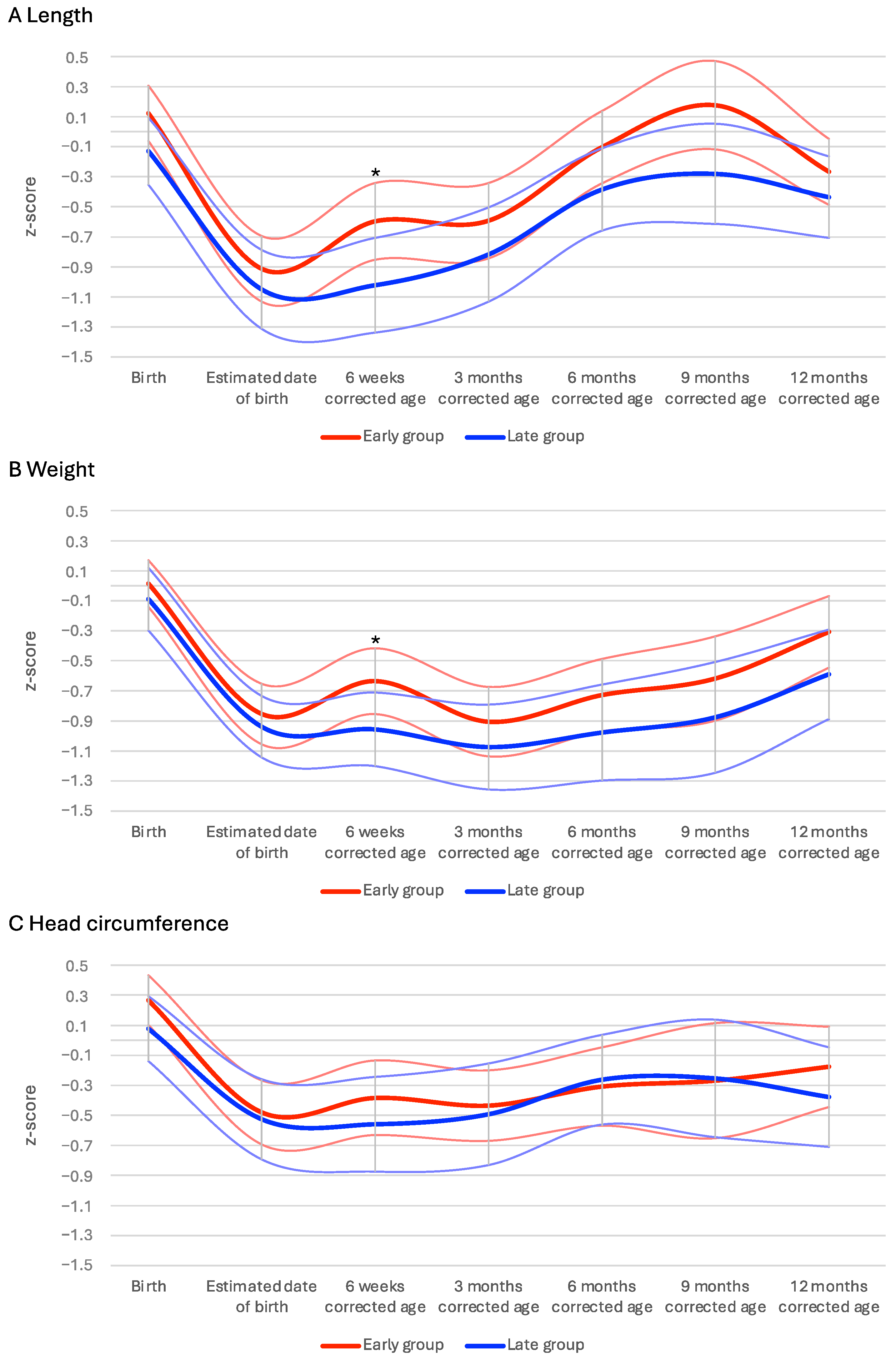
3.3. Primary Outcome
3.4. Secondary Outcomes
3.5. Influence of Comorbidities, Type of Feeding, and Birthweight on Introduction of Solids
3.6. Machine Learning Models
4. Discussion
4.1. Comorbidities
4.2. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Machine Learning Analysis
- (1)
- In the execution of the learning task, Gradient Boosted Decision Tree (GBDT) models were selected due to their demonstrated computational efficiency and high accuracy, as substantiated by Grinsztajn et al. and Ke et al. [16,17]. These models possess an intrinsic capability to capture non-linear associations and variable interactions [17]. Moreover, GBDT models exhibit robustness against multicollinearity and outliers [17].
- (2)
- In order to evaluate the regression performance, particularly focusing on the generalizability and out-of-sample prediction accuracy, a nested cross-validation (CV) procedure was employed. CV is designed to provide a more robust assessment of the model’s predictive capabilities beyond the confines of the training dataset, thereby offering a comprehensive insight into its real-world applicability and reliability [18]. CV implements repeated splits of the data into training and testing sets, whereas a 10 times 5-fold scheme is applied in the main (outer) CV loop. In each repetition of the main CV loop, the respective training set is used for data scaling (standardization) and model complexity tuning. Model complexity tuning is carried out in a nested (inner) CV procedure (10 times 5-fold) using a random search scheme. The complexity parameters that lead to the highest prediction accuracy in the inner CV procedure are subsequently used to train a GBDT model in the main CV loop. The model is subsequently tested on the respective testing set of the main CV loop. Regression performance is measured with the prediction coefficient of determination. Notably, the prediction R2 will be smaller than R2 values of conventional statistical models because the prediction R2 measures prediction performance for unknown data and not post hoc model fit [19].
- (3)
- The importance of single predictors for the model’s performance was assessed with SHAP (Shapely Additive explanations) [20,21]. Originating from the domain of interpretable machine learning, SHAP leverages the concept of Shapley values from cooperative game theory. This approach quantitatively ascertains the impact of each predictor, including interaction effects, on the model’s performance. The SHAP method is instrumental in discerning how individual predictors influence the model’s predictions. By aggregating these contributions across numerous predictions, SHAP facilitates a thorough examination of the pivotal roles played by individual predictors in the context of the predictive task [20,21].
References
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, e05780. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Cheah, F.C.; Domellof, M.; van Goudoever, J.B.; Poindexter, B.B.; Vain, N. Scientific Basis and Practical Application of Nutritional Care for Preterm Infants. World Rev. Nutr. Diet. 2021, 122, XIII–XIV. [Google Scholar] [CrossRef]
- Braid, S.; Harvey, E.M.; Bernstein, J.; Matoba, N. Early introduction of complementary foods in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.; Dalton, S.M.; Harman, A.; Wright, I.M. Current practice in the introduction of solid foods for preterm infants. Public Health Nutr. 2020, 23, 94–101. [Google Scholar] [CrossRef]
- Fanaro, S.; Borsari, G.; Vigi, V. Complementary feeding practices in preterm infants: An observational study in a cohort of Italian infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45 (Suppl. S3), S210–S214. [Google Scholar] [CrossRef]
- Hofstatter, E.; Kottstorfer, V.; Stroicz, P.; Schutz, S.; Auer-Hackenberg, L.; Brandner, J.; Wald, M. Introduction and feeding practices of solid food in preterm infants born in Salzburg! BMC Pediatr. 2021, 21, 56. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Pagano, F.; Di Chiara, M.; Pannucci, C.; Onesta, E.; Prota, R.; Deli, G.; Dito, L.; Regoli, D.; et al. Complementary Feeding and Growth in Infants Born Preterm: A 12 Months Follow-Up Study. Children 2021, 8, 1085. [Google Scholar] [CrossRef]
- Marriott, L.D.; Foote, K.D.; Bishop, J.A.; Kimber, A.C.; Morgan, J.B. Weaning preterm infants: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F302–F307. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, R.; Aggarwal, K.C.; Chellani, H.; Duggal, A.; Arya, S.; Bhatia, S.; Sankar, M.J.; Sreenivas, V.; Jain, V.; et al. Complementary feeding at 4 versus 6 months of age for preterm infants born at less than 34 weeks of gestation: A randomised, open-label, multicentre trial. Lancet Glob. Health 2017, 5, e501–e511. [Google Scholar] [CrossRef]
- Haiden, N.; Thanhaeuser, M.; Eibensteiner, F.; Huber-Dangl, M.; Gsoellpointner, M.; Ristl, R.; Kroyer, B.; Brandstetter, S.; Kornsteiner-Krenn, M.; Binder, C.; et al. Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants. Nutrients 2022, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Cole, C.R.; Hansen, N.I.; Duncan, A.F.; Hintz, S.R.; Adams-Chapman, I.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and Growth Outcomes of Extremely Preterm Infants with Short Bowel Syndrome. J. Pediatr. 2021, 230, 76–83.e75. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Gewolb, I.H.; Sobowale, B.T.; Vice, F.L.; Patwardhan, A.; Solomonia, N.; Reynolds, E.W. The Effect of Severe Intraventricular Hemorrhage on the Biorhythms of Feeding in Premature Infants. Front. Pediatr. 2021, 9, 673152. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, H.; Dincer, A.B.; Lundberg, S.; Kaeberlein, M.; Lee, S.I. Interpretable machine learning prediction of all-cause mortality. Commun. Med. 2022, 2, 125. [Google Scholar] [CrossRef] [PubMed]
- Grinsztajn, L.; Oyallon, E.; Varoquaux, G. Why do tree-based models still outperform deep learning on typical tabular data? In Proceedings of the 36th Conference on Neural Information Processing Systems (NeurIPS 2022), New Orleans, LA, USA, 28 November–9 December 2022; New Orleans Convention Center: New Orleans, LA, USA, 2022. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Cawley, G.C.; Talbot, N.L.C. On Over-fitting in Model Selection and Subsequent Selection Bias in Performance Evaluation. J. Mach. Learn. Res. 2010, 11, 2079–2107. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; p. 745. [Google Scholar]
- Molnar, C. Interpretable Machine Learning: A Guide for Making Black Box Models Explainable. 2019. Available online: https://leanpub.com/interpretable-machine-learning (accessed on 8 June 2024).
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Brion, L.P.; Rosenfeld, C.R.; Heyne, R.; Steven Brown, L.; Lair, C.S.; Heyne, E.; Dohoney, E.L.; Burchfield, P.J.; Caraig, M. Association of age of initiation and type of complementary foods with body mass index and weight-for-length at 12 months of age in preterm infants. J. Perinatol. 2020, 40, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, J.; Eisemann, N.; Ehlers, S.; Orlikowsky, T.; Kannt, O.; Herting, E.; Göpel, W. Length and weight of very low birth weight infants in Germany at 2 years of age: Does it matter at what age they start complementary food? Eur. J. Clin. Nutr. 2015, 69, 662–667. [Google Scholar] [CrossRef]
- Morgan, J.B.; Lucas, A.; Fewtrell, M.S. Does weaning influence growth and health up to 18 months? Arch. Dis. Child. 2004, 89, 728–733. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Di Mauro, A.; Pedico, A.; Rizzo, V.; Capozza, M.; Meneghin, F.; Lista, G.; Laforgia, N.; On behalf of Italian Society of Pediatrics (SIP), Italian Society of Neonatology (SIN), Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition (SIGENP) and Italian Federation of Paediatricians (FIMP). Weaning Time in Preterm Infants: An Audit of Italian Primary Care Paediatricians. Nutrients 2018, 10, 616. [Google Scholar] [CrossRef]
- Ribas, S.A.; de Rodrigues, M.C.C.; Mocellin, M.C.; Marques, E.S.; da Rosa, G.P.C.; Maganha, C.R. Quality of complementary feeding and its effect on nutritional status in preterm infants: A cross-sectional study. J. Hum. Nutr. Diet. 2021, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Early Group (n = 115) | Late Group (n = 82) |
|---|---|---|
| Obstetric and parental parameters | ||
| Multiple pregnancy | 36 (31.3%) | 19 (23.2%) |
| Cesarean delivery | 95 (82.6%) | 70 (85.4%) |
| Prenatal steroids (any) | 105 (91.3%) | 76 (92.7%) |
| Premature rupture of membranes | 43 (37.4%) | 42 (51.2%) * |
| Gestational diabetes | 0 (0%) | 3 (3.7%) * |
| Preeclampsia | 13 (11.3%) | 17 (20.7%) |
| Age of mother at birth | 31.4 (±5.8) | 33.2 (±5.3) |
| Age of father at birth | 35.1 (±7.2) | 35.2 (±6.5) |
| Maternal education | ||
| No graduation/school diploma | 12 (10.4%) | 8 (9.8%) |
| Middle school | 32 (27.8%) | 19 (23.2%) |
| Secondary school | 23 (20%) | 16 (19.5%) |
| Post-secondary school | 43 (37.4%) | 36 (43.9%) |
| Paternal education | ||
| No graduation/school diploma | 10 (8.7%) | 8 (9.8%) |
| Middle school | 45 (39.1%) | 24 (29.3%) |
| Secondary school | 21 (18.3%) | 20 (24.4%) |
| Post-secondary school | 33 (28.7%) | 24 (29.3%) |
| Neonatal parameters | ||
| Male sex | 69 (60%) | 36 (43.9%) * |
| Gestational age (days) | 26 + 6 (±2 + 0) | 26 + 5 (±2 + 2) |
| Birth weight (g) | 926 (±254) | 881 (±262) |
| Small for gestational age | 4 (3.5%) | 4 (4.9%) |
| Neonatal morbidity | ||
| Necrotizing enterocolitis ≥ grade II | 5 (4.3%) | 6 (7.3%) |
| Bronchopulmonary dysplasia | 14 (12.2%) | 23 (28%) * |
| Persisting ductus arteriosus | 51 (44.3%) | 47 (57.3%) |
| Retinopathy of prematurity ≥ grade II | 34 (29.6%) | 27 (32.9%) |
| Sepsis, culture positive | 16 (13.9%) | 19 (23.2%) |
| Intraventricular hemorrhage ≥ grade II | 17 (14.8%) | 12 (14.6%) |
| Periventricular leukomalacia | 0 (0%) | 1 (1.2%) |
| Total | Early Group | Late Group | ||
|---|---|---|---|---|
| Morbidities | NEC ≥ grade II (n = 11) | 17.5 (±2.2) | 15.8 (±1.6) | 19 (±1.6) |
| BPD (n = 37) | 18.1 (±4.5) | 14.1 (±3.2) | 20.6 (±3.3) | |
| ROP ≥ grade II (n = 61) | 16.9 (±4.1) | 14.1 (±2.6) | 20.4 (±2.7) | |
| Sepsis, culture positive (n = 35) | 16.9 (±3.8) | 13.6 (±2.3) | 19.7 (±2.2) | |
| IVH ≥ grade II (n = 29) | 16.9 (±4.2) | 14.2 (±2.5) | 20.8 (±2.9) | |
| Milk | Breast milk (n = 62) | 17.6 (±4.3) | 13.8 (±1.7) | 20.6 (±3.1) |
| Mixed feedings (n = 33) | 16.1 (±4.9) | 12.4 (±3.1) | 20.5 (±2.6) | |
| Formula (n = 97) | 15.5 (±3.9) | 13.5 (±3.1) | 19.6 (±1.8) | |
| Weight | <750 g (n = 62) | 16.8 (±4.6) | 13.7 (±3.2) | 20.5 (±3.0) |
| 750–1000 g (n = 57) | 16.0 (±4.0) | 13.5 (±2.5) | 20.1 (±2.3) | |
| >1000 g (n = 77) | 15.9 (±5.0) | 12.8 (±3.2) | 20.5 (±3.2) |
| Length at 12 Months Corrected Age | Length z-Score at 12 Months Corrected Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Early group vs. Late group | Weeks corrected age at starting solids | Early group vs. Late group | Weeks corrected age at starting solids | |||||
| Model fit | R2 = 0.138 | R2 = 0.134 | R2 = 0.134 | R2 = 0.125 | ||||
| Effect size | p-value | Effect size | p-value | Effect size | p-value | Effect size | p-value | |
| Length z-score at term | 1.03 | <0.001 | 0.99 | <0.001 | 0.39 | <0.001 | 0.39 | <0.001 |
| Female sex | 0.48 | 0.001 | 0.49 | <0.001 | 0.09 | 0.116 | 0.09 | 0.157 |
| Height of mother | 0.3 | 0.039 | 0.26 | 0.039 | 0.11 | 0.015 | 0.1 | 0.066 |
| Age at introduction of solids | 0.19 | 0.181 | 0.14 | 0.560 | 0.04 | 0.542 | 0.03 | 0.843 |
| Nutrition at 6 weeks | 0.14 | 0.633 | 0.11 | 0.912 | 0.05 | 0.719 | 0.05 | 0.680 |
| BPD | 0.08 | 0.549 | 0.11 | 0.278 | 0.02 | 0.675 | 0.03 | 0.541 |
| Height of father | 0.06 | 0.939 | 0.09 | 0.922 | 0.03 | 0.917 | 0.04 | 0.942 |
| Gestational age | 0.05 | 0.885 | 0.07 | 0.858 | 0.02 | 0.921 | 0.03 | 0.916 |
| NEC | 0.03 | 0.915 | 0.01 | 0.894 | 0.01 | 0.905 | 0 | 0.866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanhaeuser, M.; Gsoellpointner, M.; Kornsteiner-Krenn, M.; Steyrl, D.; Brandstetter, S.; Jilma, B.; Berger, A.; Haiden, N. Introduction of Solid Foods in Preterm Infants and Its Impact on Growth in the First Year of Life—A Prospective Observational Study. Nutrients 2024, 16, 2077. https://doi.org/10.3390/nu16132077
Thanhaeuser M, Gsoellpointner M, Kornsteiner-Krenn M, Steyrl D, Brandstetter S, Jilma B, Berger A, Haiden N. Introduction of Solid Foods in Preterm Infants and Its Impact on Growth in the First Year of Life—A Prospective Observational Study. Nutrients. 2024; 16(13):2077. https://doi.org/10.3390/nu16132077
Chicago/Turabian StyleThanhaeuser, Margarita, Melanie Gsoellpointner, Margit Kornsteiner-Krenn, David Steyrl, Sophia Brandstetter, Bernd Jilma, Angelika Berger, and Nadja Haiden. 2024. "Introduction of Solid Foods in Preterm Infants and Its Impact on Growth in the First Year of Life—A Prospective Observational Study" Nutrients 16, no. 13: 2077. https://doi.org/10.3390/nu16132077
APA StyleThanhaeuser, M., Gsoellpointner, M., Kornsteiner-Krenn, M., Steyrl, D., Brandstetter, S., Jilma, B., Berger, A., & Haiden, N. (2024). Introduction of Solid Foods in Preterm Infants and Its Impact on Growth in the First Year of Life—A Prospective Observational Study. Nutrients, 16(13), 2077. https://doi.org/10.3390/nu16132077






