Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Follow-Up
2.4. Study Outcome
2.5. Definitions
Nutrition Protocol
2.6. Statistical Analysis
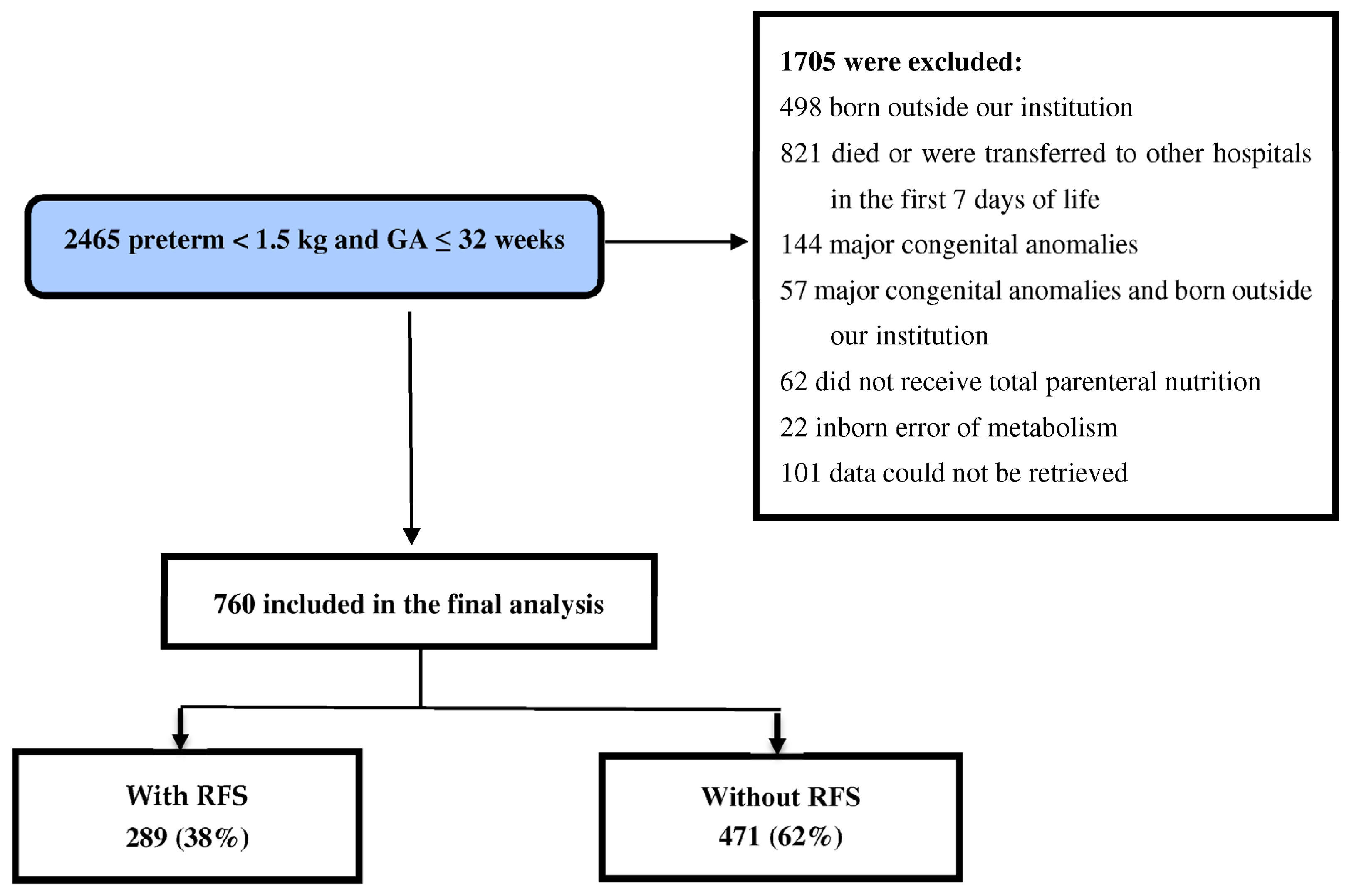
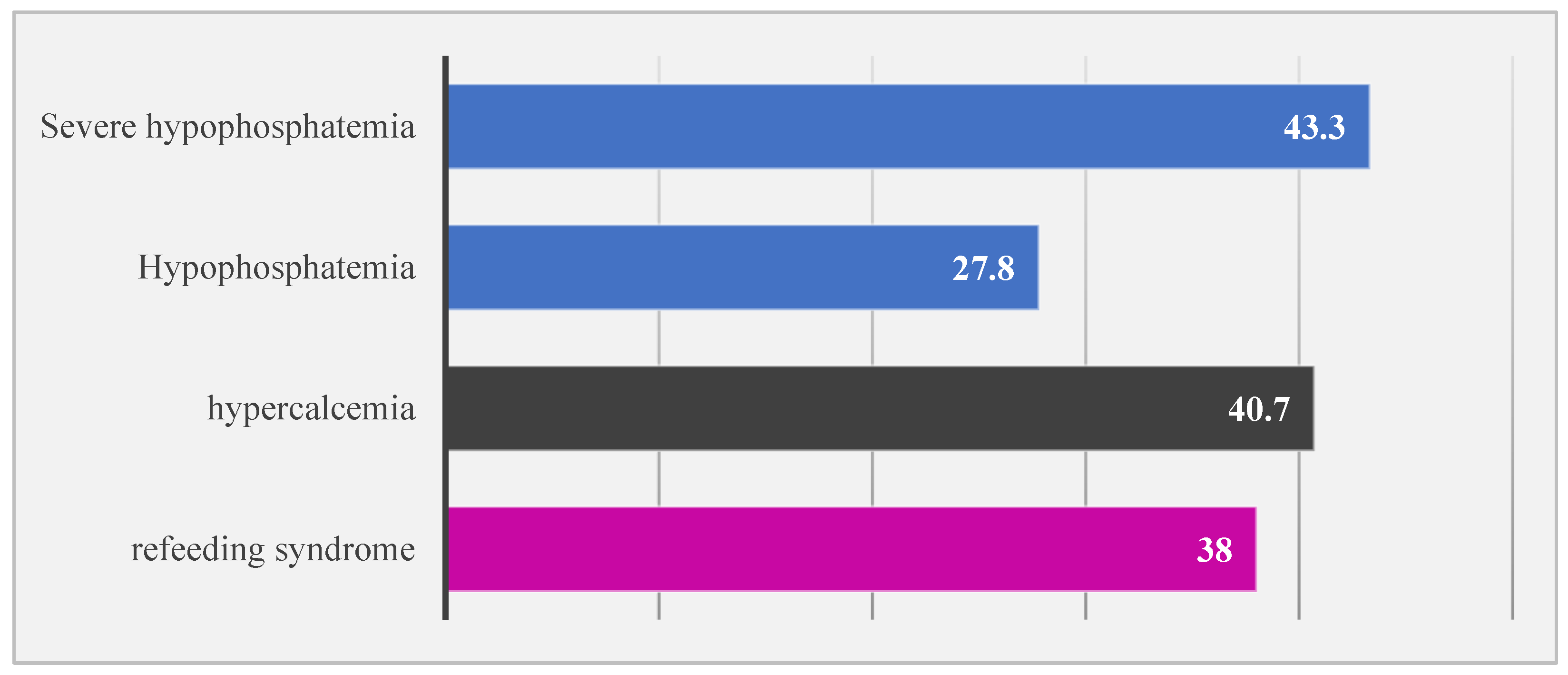
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skipper, A. Refeeding syndrome or refeeding hypophosphatemia: A systematic review of cases. Nutr. Clin. Pract. 2012, 27, 34–40. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN consensus recommendations for refeeding syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- McKnight, C.L.; Newberry, C.; Sarav, M.; Martindale, R.; Hurt, R.; Daley, B. Refeeding syndrome in the critically ill: A literature review and clinician’s guide. Curr. Gastroenterol. Rep. 2019, 21, 58. [Google Scholar] [CrossRef]
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition 2017, 35, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Diekmann, R.; Janssen, G.; Fleiter, O.; Fricke, L.; Kreilkamp, A.; Modreker, M.K.; Marburger, C.; Nels, S.; Pourhassan, M.; et al. Refeeding syndrome: Pathophysiology, risk factors, prevention, and treatment. Internist 2018, 59, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, C.; Siegenthaler, J.; Seres, D.; Owen-Michaane, M.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Adaptation of nutritional risk screening tools may better predict response to nutritional treatment: A secondary analysis of the randomized controlled trial Effect of early nutritional therapy on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial (EFFORT). Am. J. Clin. Nutr. 2024, 119, 800–808. [Google Scholar] [PubMed]
- Bioletto, F.; Pellegrini, M.; Ponzo, V.; Cioffi, I.; De Francesco, A.; Ghigo, E.; Bo, S. Impact of refeeding syndrome on short- and medium-term all-cause mortality: A systematic review and meta-analysis. Am. J. Med. 2021, 134, 1009–1018.e1. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.A.; Hally, V.; Panteli, J.V. The importance of the refeeding syndrome. Nutrition 2001, 17, 632–637. [Google Scholar] [CrossRef]
- Crook, M.; Swaminathan, R. Disorders of plasma phosphate and indications for its measurement. Ann. Clin. Biochem. 1996, 33, 376–396. [Google Scholar] [CrossRef]
- Valla, F.V.; Berthiller, J.; Gaillard-Le-Roux, B.; Ford-Chessel, C.; Ginhoux, T.; Rooze, S.; Cour-Andlauer, F.; Meyer, R.; Javouhey, E. Faltering growth in the critically ill child: Prevalence, risk factors, and impaired outcome. Eur. J. Pediatr. 2018, 177, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Ford-Chessel, C.; Berthiller, J.; Peretti, N.; Javouhey, E.; Valla, F.V. Nutritional status in pediatric intermediate care: Assessment at admission, progression during the stay and after discharge. Arch. Pediatr. 2016, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Bechard, L.J.; Cahill, N.; Wang, M.; Day, A.; Duggan, C.P.; Heyland, D.K. Nutritional practices and their relationship to clinical outcomes in critically ill children—An international multicenter cohort study. Crit. Care Med. 2012, 40, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Mbethe, A.P.; Mda, S. Incidence of refeeding syndrome and its associated factors in South African children hospitalized with severe acute malnutrition. Iran. J. Pediatr. 2017, 27, e8297. [Google Scholar] [CrossRef]
- Ross, J.R.; Finch, C.; Ebeling, M.; Taylor, S.N. Refeeding syndrome in very-low-birth-weight intrauterine growth-restricted neonates. J. Perinatol. 2013, 33, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Bonsante, F.; Iacobelli, S.; Latorre, G.; Rigo, J.; De Felice, C.; Robillard, P.Y.; Gouyon, J.B. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants—It is time to change the composition of the early parenteral nutrition. PLoS ONE 2013, 8, e72880. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia—A randomized, controlled trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Boubred, F.; Herlenius, E.; Bartocci, M.; Jonsson, B.; Vanpée, M. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr. 2015, 104, 1077–1083. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gohil, J.R.; Patel, S.A. Hypophosphatemia in refeeding syndrome in intrauterine growth restricted IUGR neonates who are receiving nutrition: A prospective observational study. Asian J. Pediatr. Res. 2020, 3, 23–29. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H.; ProVIDe Trial Group. Neonatal refeeding syndrome and clinical outcome in extremely low-birth-weight babies: Secondary cohort analysis from the ProVIDe trial. JPEN J. Parenter. Enter. Nutr. 2021, 45, 65–78. [Google Scholar] [CrossRef]
- Sung, S.I.; Chang, Y.S.; Choi, J.H.; Ho, Y.; Kim, J.; Ahn, S.Y.; Park, W.S. Increased risk of refeeding syndrome-like hypophosphatemia with high initial amino acid intake in small-for-gestational-age, extremely-low-birthweight infants. PLoS ONE 2019, 14, e0221042. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, G.; Watabe, Y.; Suzumura, H.; Sairenchi, T.; Muto, T.; Arisaka, O. Hypophosphatemia in small for gestational age extremely low birth weight infants receiving parenteral nutrition in the first week after birth. J. Pediatr. Endocrinol. Metab. 2012, 25, 317–321. [Google Scholar] [CrossRef]
- Al-Wassia, H.; Lyon, A.W.; Rose, S.M.; Sauve, R.S.; Fenton, T.R. Hypophosphatemia is prevalent among preterm infants less than 1500 grams. Am. J. Perinatol. 2019, 36, 1412–1419. [Google Scholar] [PubMed]
- Senterre, T.; Abu Zahirah, I.; Pieltain, C.; de Halleux, V.; Rigo, J. Electrolyte and mineral homeostasis after optimizing early macronutrient intakes in VLBW infants on parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bustos Lozano, G.; Hidalgo Romero, Á.; Melgar Bonis, A.; Ureta Velasco, N.; Orbea Gallardo, C.; Pallás Alonso, C. Early hypophosphataemia in at risk newborns. Frequency and magnitude. Ann. Paediatr. 2018, 88, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Brener Dik, P.H.; Galletti, M.F.; Fernández Jonusas, S.A.; Alonso, G.; Mariani, G.L.; Fustiñana, C.A. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J. Perinatol. 2015, 35, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Bustos Lozano, G.; Soriano-Ramos, M.; Pinilla Martín, M.T.; Chumillas Calzada, S.; García Soria, C.E.; Pallás-Alonso, C.R. Early hypophosphatemia in high-risk preterm infants: Efficacy and safety of sodium glycerophosphate from first Day on parenteral nutrition. JPEN J. Parenter. Enteral Nutr. 2019, 43, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Rigo, J.; Pieltain, C.; Viellevoye, R.; Bagnoli, F. Calcium and phosphorus homeostasis: Pathophysiology. In Neonatology; Buonocore, G., Bracci, R., Weindling, M., Eds.; Springer: Milano, Italy, 2012; pp. 333–353. [Google Scholar]
- Igarashi, A.; Okuno, T.; Ohta, G.; Tokuriki, S.; Ohshima, Y. Risk factors for the development of refeeding syndrome-like hypophosphatemia in very low birth weight infants. Dis. Markers 2017, 2017, 9748031. [Google Scholar] [CrossRef] [PubMed]
- Pająk, A.; Królak-Olejnik, B.; Szafrańska, A. Early hypophosphatemia in very low birth weight preterm infants. Adv. Clin. Exp. Med. 2018, 27, 841–847. [Google Scholar] [CrossRef]
- Mulla, S.; Stirling, S.; Cowey, S.; Close, R.; Pullan, S.; Howe, R.; Radbone, L.; Clarke, P. Severe hypercalcaemia and hypophosphataemia with an optimised preterm parenteral nutrition formulation in two epochs of differing phosphate supplementation. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F451–F455. [Google Scholar] [CrossRef]
- Amissah, E.A.; Brown, J.; Harding, J.E. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst. Rev. 2018, 6, CD000433. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, M.P.; Carmenati, E.; D’Ascenzo, R.; Malatesta, M.; Spagnoli, C.; Biagetti, C.; Burattini, I.; Carnielli, V.P. One extra gram of protein to preterm infants from birth to 1800 g: A single-blinded randomized clinical trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 879–884. [Google Scholar] [CrossRef] [PubMed]
| Variable | ALL | Gestational Age < 28 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| N | without RFS (n = 471) | with RFS (n = 289) | p-Value | N | without RFS (n = 128) | with RFS (n = 136) | p-Value | |
| Gestational age (weeks), median (IQR) | 760 | 29 (27.0–31.0) | 28 (26–30.0) | <0.001 * | 264 | 26 (25.0–27.0) | 26 (25.0–27.0) | 0.02 * |
| Birth weight (grams) (IQR) | 760 | 1180 (950–1370) | 950 (765–1200) | <0.001 * | 264 | 845 (711.25–960) | 775 (661.25–905) | 0.008 * |
| Length (cm), median (IQR) | 760 | 38 (35–40) | 35 (32–38) | <0.001 * | 264 | 33 (31–35) | 33 (31–35) | 0.10 |
| Head circumference (cm) (IQR) | 760 | 27 (25–28) | 25 (23–27) | <0.001 * | 264 | 24 (23–25) | 23 (22–25) | 0.03 * |
| Small for gestational age, n (%) | 760 | 63 (13.4) | 47 (16.3) | 0.29 | 264 | 8 (6.3) | 9 (6.6) | 1 |
| 1 min Apgar score, median (IQR) | 760 | 6 (4–7) | 5 (3–6) | <0.001 * | 264 | 5 (3–6) | 4 (2–6) | 0.03 * |
| 5 min Apgar score, median (IQR) | 760 | 7 (6–8) | 7 (7–8) | <0.001 * | 264 | 5 (6–7) | 4 (6–7) | 0.17 |
| Male, n (%) | 760 | 221 (46.9) | 174 (60.2) | <0.001 * | 264 | 70 (54.7) | 90 (66.2) | 0.06 |
| Antenatal steroid treatment, n (%) | 760 | 253 (53.7) | 147 (50.9) | 0.45 | 264 | 70 (54.7) | 71 (52.2) | 0.71 |
| Gestational diabetes mellitus, n (%) | 760 | 27 (5.7) | 14 (4.8) | 0.37 | 264 | 7 (5.5) | 4 (2.9) | 0.36 |
| Maternal hypertension, n (%) | 760 | 119 (25.3) | 67 (23.2) | 0.54 | 264 | 26 (20.3) | 27 (19.9) | 1.0 |
| Preterm rupture of membrane, n (%) | 760 | 59 (12.5) | 20 (6.9) | 0.01 * | 264 | 17 (13.3) | 12 (8.8) | 0.33 |
| Cesarean section, n (%) | 760 | 223 (47.3) | 146 (50.5) | 0.41 | 264 | 72 (56.3) | 75 (55.1) | 0.90 |
| Expressed breast milk, n (%) | 760 | 247 (52.4) | 145 (50.2) | 0.55 | 264 | 75 (58.6) | 64 (47.1) | 0.06 |
| Respiratory distress syndrome required surfactant, n (%) | 760 | 281 (59.7) | 224 (77.5) | <0.001 * | 264 | 120 (93.8) | 128 (94.1) | 1 |
| Mechanical ventilation, n (%) | 760 | 305 (64.8) | 242 (83.7) | <0.001 * | 264 | 122 (95.3) | 132 (97.1) | 0.53 |
| Patent ductus arteriosus requiring treatment, n (%) | 760 | 35 (7.4) | 34 (11.8) | 0.05 | 264 | 24 (18.8) | 28 (20.6) | 0.76 |
| Intraventricular hemorrhage, n (%) | 760 | 124 (26.3) | 157 (54.5) | <0.001 * | 264 | 64 (50) | 96 (70.6) | 0.001 * |
| Intraventricular hemorrhage grades 3 and 4, n (%) | 760 | 63 (13.3) | 74 (25.6) | <0.001 * | 264 | 41 (32) | 56 (41.2) | 0.13 |
| Variables | ALL | Gestational Age < 28 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| N | without RFS (n = 471) | with RFS (n = 289) | p-Value | N | without RFS (n = 128) | with RFS (n = 136) | p-Value | |
| Average parenteral lipid intake in the 1st 7 days (g/kg/day), median (IQR) | 760 | 2 (1.54–2.35) | 2.1 (1.67–2.5) | 0.02 * | 264 | 1.8 (1.4–2.3) | 1.8 (1.3–2.4) | 0.95 |
| Average parenteral amino acid intake in the 1st 7 days (g/kg/day), median (IQR) | 760 | 3.90 (3.67–4.0) | 3.97 (3.75–4.0) | 0.01 * | 264 | 4 (3.7–4.0) | 4 (3.8–4.0) | 0.13 |
| Average parenteral dextrose intake in the 1st 7 days (mg/kg/min), median (IQR) | 760 | 8.47 (7.74–9.12) | 8.46 (7.65–9.04) | 0.55 | 264 | 8 (7–8.8) | 7.7 (6.4–8.5) | 0.03 * |
| Average parenteral phosphate intake in the 1st 7 days (mg/kg/min), median (IQR) | 760 | 0.30 (0–0.6) | 0.24 (0–0.4) | 0.01 * | 264 | 0.30 (0.02–0.62) | 0.2 (0–0.44) | 0.03 * |
| PN duration, median (IQR) | 760 | 14 (7–29) | 18 (9–35) | 0.002 * | 264 | 28 (12–48) | 23 (11–41) | 0.45 |
| Average magnesium sulfate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.11 (0.07–0.13) | 0.11 (0.07–0.13) | 0.60 | 264 | 0.10 (0.07–0.13) | 0.11 (0.07–0.14) | 0.24 |
| Average calcium gluconate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.71 (0.57–0.92) | 0.79 (0.61–1) | 0.001 * | 264 | 0.77 (0.64–0.96) | 0.86 (0.64–1) | 0.15 |
| Average sodium chloride intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.14 (0–0.60) | 0 (0–0.40) | <0.001 * | 264 | 0.0 (0.0–0.43) | 0.0 (0.0–0.29) | 0.08 |
| Average sodium acetate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.57 (0–1.14) | 0.43 (0–0.96) | 0.08 | 264 | 0.86 (0.104–1.14) | 0.57 (0.0–1.0) | 0.02 * |
| Average sodium Phosphate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.37 (0.14–0.60) | 0.29 (0–0.50) | <0.001 * | 264 | 0.3 (0.1–0.59) | 0.2 (0.0–0.43) | 0.001 * |
| Average potassium chloride intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.4 (0.0–1.0) | 0.2 (0.0–0.8) | 0.001 * | 264 | 0.4 (0.0–1.0) | 0.0 (0.0–0.6) | 0.001 * |
| Average potassium acetate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0 (0–0.43) | 0.14 (0–0.71) | <0.001 * | 264 | 0.14 (0–0.64) | 0.29 (0–0.86) | 0.11 |
| Average potassium phosphate intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0 (0–0.38) | 0.1 (0.0–0.38) | 0.14 | 264 | 0.0 (0.0–0.42) | 0.1 (0.0–0.40) | 0.14 |
| Average calcium-to-phosphate ratio intake in the 1st 7 days (mmol/kg/day), median (IQR) | 760 | 0.61 (0.38–0.90) | 0.87 (0.54–1.44) | <0.001 * | 264 | 0.65 (0.42–0.99) | 0.97 (0.69–1.68) | <0.001 * |
| Risk Factor | All | Gestational Age < 28 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted RR 95% CI | p-Value | Adjusted RR 95% CI | p-Value | Unadjusted RR 95% CI | p-Value | Adjusted RR 95% CI | p-Value | |
| Gestational age | 0.89 (0.86–0.92) | <0.001 * | 1.02 (0.95–1.09) | 0.59 | 0.90 (0.83–0.99) | 0.03 * | 1.0 (0.89–1.13) | 0.98 |
| Birth weight | 0.99 (0.98–1.0) | <0.001 * | 0.99 (0.98–1.0) | 0.07 | 0.99 (0.98–1) | 0.01 * | 1.0 (0.99–1.001) | 0.63 |
| Small for gestational age | 1.15 (0.90–1.46) | 0.25 | – | – | 0.97 (0.61–1.55) | 0.90 | – | – |
| Male | 1.40 (1.16–1.69) | <0.001 * | 1.31 (1.08–1.59) | 0.006 * | 0.78 (0.61–1.01) | 0.06 | – | – |
| Cesarean section | 1.08 (0.90–1.30) | 0.40 | – | – | 1.02 (0.81–1.29) | 0.86 | – | – |
| 1 min Apgar score | 0.89 (0.85–0.93) | <0.001 * | 0.98 (0.91–1.04) | 0.48 | 0.93 (0.88–0.99) | 0.03 * | 0.99 (0.93–1.06) | 0.72 |
| 5 min Apgar score | 0.88 (0.82–0.94) | <0.001 * | 1.04 (0.94–1.14) | 0.47 | 0.95 (0.86–1.04) | 0.28 | – | – |
| Surfactant | 1.74 (1.38–2.19) | <0.001 * | 0.81 (0.58–1.12) | 0.20 | 0.97 (058–1.61) | 0.90 | – | – |
| Patent ductus requiring treatment | 1.34 (1.03–1.73) | 0.03 * | 0.84 (0.64–1.10) | 0.21 | 1.06 (0.79–1.40) | 0.70 | – | – |
| Intraventricular hemorrhage | 2.03 (1.70–2.44) | <0.001 * | 1.71 (1.27–2.13) | <0.001 * | 1.56 (1.19–2.05) | 0.001 * | 0.75 (0.57–1.0) | 0.05 |
| Nutritional and anthropometrics | ||||||||
| Average amino acid intake | 1.38 (1.02–1.88) | 0.03 * | 1.29 (0.93–1.81) | 0.13 | 1.60 (0.98–2.59) | 0.06 | – | – |
| Average dextrose intake | 0.95 (0.89–1.02) | 0.16 | – | – | 0.91 (0.85–0.99) | 0.02 | 0.96 (0.89–1.04) | 0.32 |
| Average lipid intake | 0.88 (0.76–1.03) | 0.11 | – | – | 0.99 (0.82–1.20) | 0.9 | – | – |
| Average magnesium sulfate intake | 1.19 (0.16–8.81) | 0.86 | – | – | 2.36 (0.16–33.78) | 0.53 | – | – |
| Average calcium gluconate intake | 1.81 (1.28–2.54) | 0.001 * | 1.29 (0.91–1.82) | 0.15 | 1.30 (0.83–2.02) | 0.25 | – | – |
| Average sodium chloride intake | 0.64 (0.50–0.82) | <0.001 * | 0.86 (0.68–1.09) | 0.21 | 0.81 (0.57–1.14) | 0.23 | – | – |
| Average sodium acetate intake | 0.86 (0.73–1.01) | 0.07 | – | – | 0.82 (0.66–1.02) | 0.08 | – | – |
| Average sodium phosphate intake | 0.55 (0.37–0.72) | <0.001 * | 0.67 (0.47–0.98) | 0.03 * | 0.50 (0.32–0.78) | 0.002 * | 0.60 (0.38–0.96) | 0.03 * |
| Average potassium chloride intake | 0.82 (0.69–0.97) | 0.02 * | 0.98 (0.83–1.17) | 0.84 | 0.75 (0.55–1.01) | 0.06 | – | – |
| Average potassium acetate intake | 1.40 (1.17–1.67) | <0.001 * | 1.05 (0.86–1.29) | 0.61 | 1.17 (0.94–1.46) | 0.17 | – | – |
| Average potassium phosphate intake | 1.22 (0.91–1.64) | 0.18 | – | – | 1.23 (0.91–1.67) | 0.18 | – | – |
| Average calcium-to-phosphate ratio intake | 1.13 (1.07–1.19) | <0.001 | 1.03 (0.99–1.07) | 0.07 | 1.06 (1.02–1.10) | 0.001 | 1.02 (0.98–1.06) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asfour, S.S.; Alshaikh, B.; Mathew, M.; Fouda, D.I.; Al-Mouqdad, M.M. Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants. Nutrients 2024, 16, 2557. https://doi.org/10.3390/nu16152557
Asfour SS, Alshaikh B, Mathew M, Fouda DI, Al-Mouqdad MM. Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants. Nutrients. 2024; 16(15):2557. https://doi.org/10.3390/nu16152557
Chicago/Turabian StyleAsfour, Suzan S., Belal Alshaikh, Maya Mathew, Dina I. Fouda, and Mountasser M. Al-Mouqdad. 2024. "Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants" Nutrients 16, no. 15: 2557. https://doi.org/10.3390/nu16152557
APA StyleAsfour, S. S., Alshaikh, B., Mathew, M., Fouda, D. I., & Al-Mouqdad, M. M. (2024). Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants. Nutrients, 16(15), 2557. https://doi.org/10.3390/nu16152557





