No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study
Abstract
1. Introduction
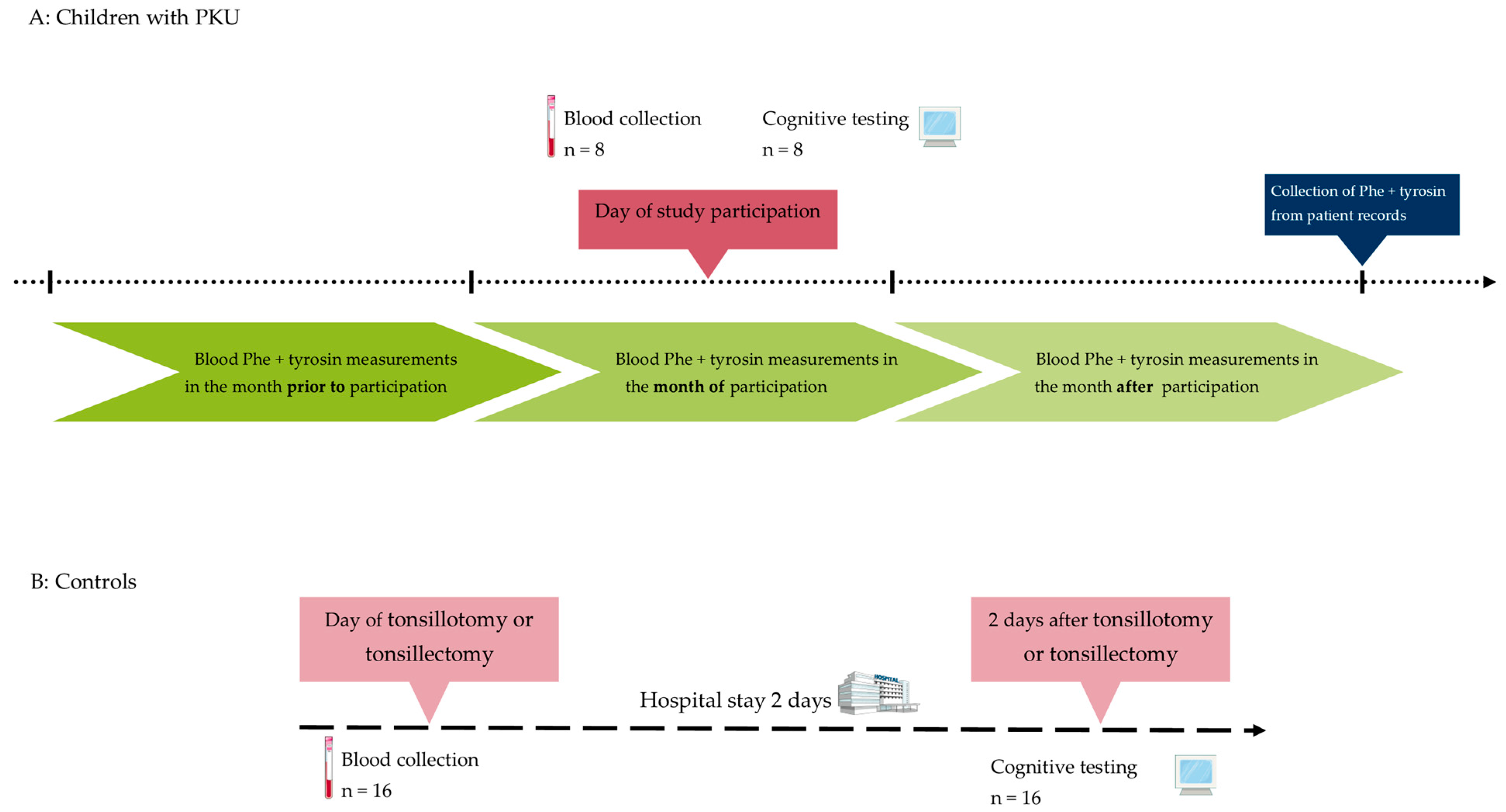
2. Materials and Methods
2.1. Biomaterials
2.2. Cognitive Testing
2.3. Tonic Alertness
2.4. Corsi Block Tapping Task
2.5. Flanker Task
2.6. Switch Task
2.7. Statistical Analyses
3. Results
3.1. Characterization of Children with PKU and Controls
3.2. Characterization of Dietary Control of Patients with PKU
3.3. Executive Functioning
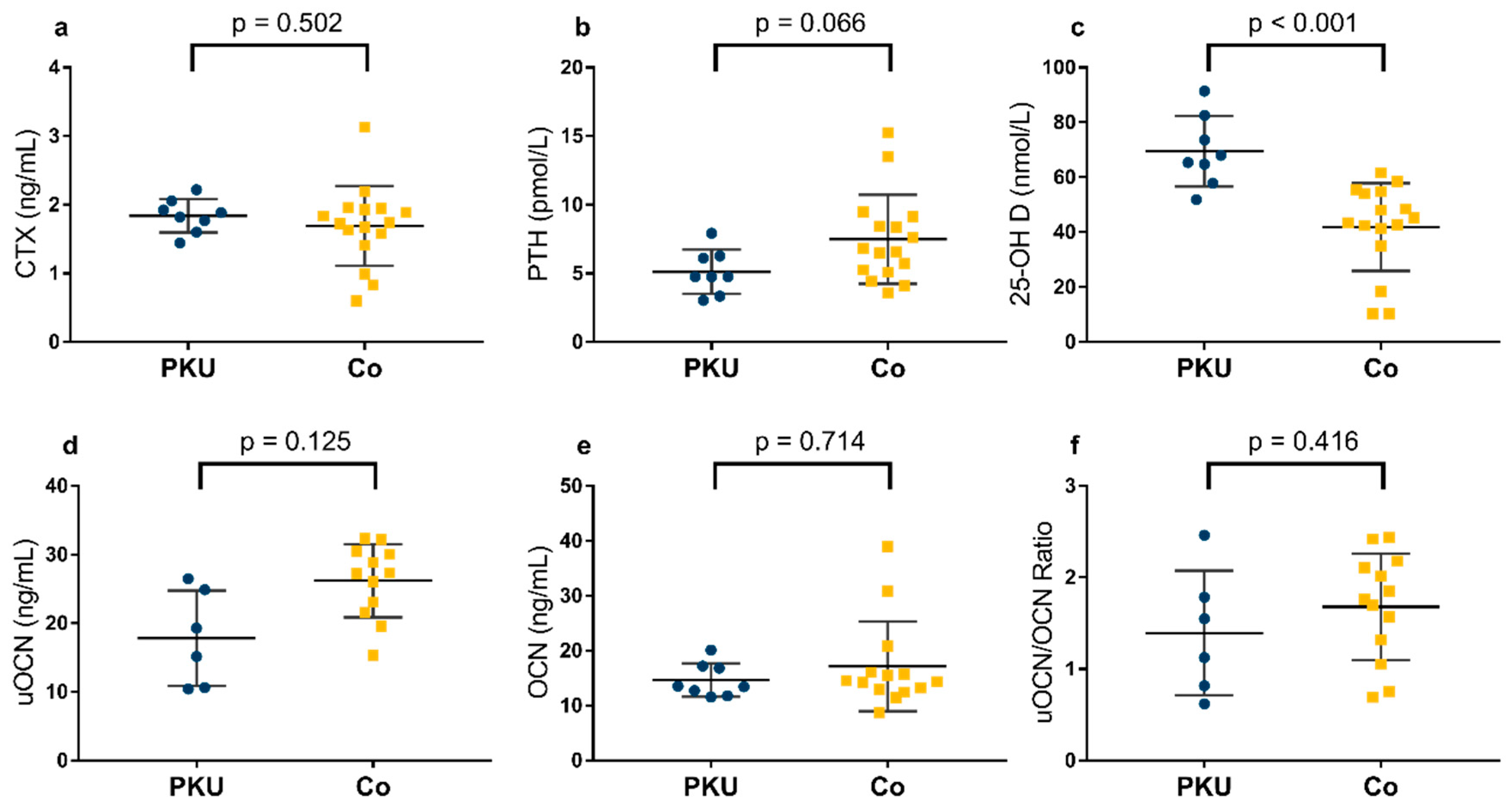
3.4. Bone Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25-OH D | 25-hydroxy vitamin D |
| BH4 | Tetrahydrobiopterin |
| BMD | bone mineral density |
| CTX | C-terminal telopeptide of type 1 collagen |
| DBS | dried blood filter cards |
| IE | inverse efficiency |
| OCN | carboxylated osteocalcin |
| Phe | phenylalanine |
| PKU | phenylketonuria |
| PTH | parathyroid hormone |
| RT | reaction time |
| SD | standard deviation |
| uOCN | undercarboxylated osteocalcin |
| UV | ultraviolet |
| WHO | World Health Organization |
References
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Brockow, I.; Blankenstein, O.; Ceglarek, U.; Ensenauer, R.; Fingerhut, R.; Gramer, G.; Hörster, F.; Janzen, N.; Klein, J.; Lankes, E.; et al. Nationaler Screeningreport Deutschland 2020, 2022. Available online: https://www.screening-dgns.de/Pdf/Screeningreports/DGNS-Screeningreport-d_2020.pdf (accessed on 18 June 2024).
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef]
- Trefz, F.; Maillot, F.; Motzfeldt, K.; Schwarz, M. Adult phenylketonuria outcome and management. Mol. Genet. Metab. 2011, 104, S26–S30. [Google Scholar] [CrossRef]
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591–609. [Google Scholar] [CrossRef]
- Jahja, R.; Huijbregts, S.C.J.; de Sonneville, L.M.J.; van der Meere, J.J.; Legemaat, A.M.; Bosch, A.M.; Hollak, C.E.M.; Rubio-Gozalbo, M.E.; Brouwers, M.C.G.J.; Hofstede, F.C.; et al. Cognitive profile and mental health in adult phenylketonuria: A PKU-COBESO study. Neuropsychology 2017, 31, 437–447. [Google Scholar] [CrossRef]
- White, D.A.; Connor, L.T.; Nardos, B.; Shimony, J.S.; Archer, R.; Snyder, A.Z.; Moinuddin, A.; Grange, D.K.; Steiner, R.D.; McKinstry, R.C. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: A DTI study of the corpus callosum. Mol. Genet. Metab. 2010, 99 (Suppl. S1), S41–S46. [Google Scholar] [CrossRef]
- Hawks, Z.; Hood, A.M.; Lerman-Sinkoff, D.B.; Shimony, J.S.; Rutlin, J.; Lagoni, D.; Grange, D.K.; White, D.A. White and gray matter brain development in children and young adults with phenylketonuria. Neuroimage Clin. 2019, 23, 101916. [Google Scholar] [CrossRef]
- Bartus, A.; Palasti, F.; Juhasz, E.; Kiss, E.; Simonova, E.; Sumanszki, C.; Reismann, P. The influence of blood phenylalanine levels on neurocognitive function in adult PKU patients. Metab. Brain Dis. 2018, 33, 1609–1615. [Google Scholar] [CrossRef]
- Clocksin, H.E.; Abbene, E.E.; Christ, S. A comprehensive assessment of neurocognitive and psychological functioning in adults with early-treated phenylketonuria. J. Int. Neuropsychol. Soc. 2023, 29, 641–650. [Google Scholar] [CrossRef]
- Demirdas, S.; Maurice-Stam, H.; Boelen, C.C.A.; Hofstede, F.C.; Janssen, M.C.H.; Langendonk, J.G.; Mulder, M.F.; Rubio-Gozalbo, M.E.; van Spronsen, F.J.; de Vries, M.; et al. Evaluation of quality of life in PKU before and after introducing tetrahydrobiopterin (BH4); a prospective multi-center cohort study. Mol. Genet. Metab. 2013, 110, S49–S56. [Google Scholar] [CrossRef]
- Leuzzi, V.; Pansini, M.; Sechi, E.; Chiarotti, F.; Carducci, C.; Levi, G.; Antonozzi, I. Executive function impairment in early-treated PKU subjects with normal mental development. J. Inherit. Metab. Dis. 2004, 27, 115–125. [Google Scholar] [CrossRef]
- Huijbregts, S.C.J.; de Sonneville, L.M.J.; Licht, R.; van Spronsen, F.J.; Verkerk, P.H.; Sergeant, J.A. Sustained attention and inhibition of cognitive interference in treated phenylketonuria: Associations with concurrent and lifetime phenylalanine concentrations. Neuropsychologia 2002, 40, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Das, A.M.; Goedecke, K.; Meyer, U.; Kanzelmeyer, N.; Koch, S.; Illsinger, S.; Lücke, T.; Hartmann, H.; Lange, K.; Lanfermann, H.; et al. Dietary habits and metabolic control in adolescents and young adults with phenylketonuria: Self-imposed protein restriction may be harmful. JIMD Rep. 2014, 13, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Weglage, J.; Fromm, J.; van Teeffelen-Heithoff, A.; Möller, H.E.; Koletzko, B.; Marquardt, T.; Rutsch, F.; Feldmann, R. Neurocognitive functioning in adults with phenylketonuria: Results of a long term study. Mol. Genet. Metab. 2013, 110, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Dowsett, C.J.; Claessens, A.; Magnuson, K.; Huston, A.C.; Klebanov, P.; Pagani, L.S.; Feinstein, L.; Engel, M.; Brooks-Gunn, J.; et al. School readiness and later achievement. Dev. Psychol. 2007, 43, 1428–1446. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Bidzan-Bluma, I.; Lipowska, M. Physical Activity and Cognitive Functioning of Children: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 800. [Google Scholar] [CrossRef]
- Ardila, A. Is intelligence equivalent to executive functions? Psicothema 2018, 30, 159–164. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Dios-Fuentes, E.; Gonzalo Marin, M.; Remón-Ruiz, P.; Benitez Avila, R.; Bueno Delgado, M.A.; Blasco Alonso, J.; Doulatram Gamgaram, V.K.; Olveira, G.; Soto-Moreno, A.; Venegas-Moreno, E. Cardiometabolic and Nutritional Morbidities of a Large, Adult, PKU Cohort from Andalusia. Nutrients 2022, 14, 1311. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Agurto, E.; Leal-Witt, M.J.; Arias, C.; Cabello, J.F.; Bunout, D.; Cornejo, V. Muscle and Bone Health in Young Chilean Adults with Phenylketonuria and Different Degrees of Compliance with the Phenylalanine Restricted Diet. Nutrients 2023, 15, 2939. [Google Scholar] [CrossRef] [PubMed]
- Lubout, C.M.A.; Arrieta Blanco, F.; Bartosiewicz, K.; Feillet, F.; Gizewska, M.; Hollak, C.; van der Lee, J.H.; Maillot, F.; Stepien, K.M.; Wagenmakers, M.A.E.M.; et al. Bone mineral density is within normal range in most adult phenylketonuria patients. J. Inherit. Metab. Dis. 2020, 43, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Choukair, D.; Kneppo, C.; Feneberg, R.; Schönau, E.; Lindner, M.; Kölker, S.; Hoffmann, G.F.; Tönshoff, B. Analysis of the functional muscle-bone unit of the forearm in patients with phenylketonuria by peripheral quantitative computed tomography. J. Inherit. Metab. Dis. 2017, 40, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Demirdas, S.; Coakley, K.E.; Bisschop, P.H.; Hollak, C.E.M.; Bosch, A.M.; Singh, R.H. Bone health in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2015, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Porta, F.; Mussa, A.; D’Amico, L.; Fiore, L.; Garelli, D.; Spada, M.; Ferracini, R. Bone impairment in phenylketonuria is characterized by circulating osteoclast precursors and activated T cell increase. PLoS ONE 2010, 5, e14167. [Google Scholar] [CrossRef] [PubMed]
- Stroup, B.M.; Hansen, K.E.; Krueger, D.; Binkley, N.; Ney, D.M. Sex differences in body composition and bone mineral density in phenylketonuria: A cross-sectional study. Mol. Genet. Metab. Rep. 2018, 15, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Geiger, K.E.; Koeller, D.M.; Harding, C.O.; Huntington, K.L.; Gillingham, M.B. Normal vitamin D levels and bone mineral density among children with inborn errors of metabolism consuming medical food-based diets. Nutr. Res. 2016, 36, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Singh, R.H. Natural history of children and adults with phenylketonuria in the NBS-PKU Connect registry. Mol. Genet. Metab. 2021, 134, 243–249. [Google Scholar] [CrossRef]
- Demirdas, S.; van Spronsen, F.J.; Hollak, C.E.M.; van der Lee, J.H.; Bisschop, P.H.; Vaz, F.M.; ter Horst, N.M.; Rubio-Gozalbo, M.E.; Bosch, A.M. Micronutrients, Essential Fatty Acids and Bone Health in Phenylketonuria. Ann. Nutr. Metab. 2017, 70, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F.; Alexy, U.; Schoenau, E.; Wudy, S.A.; Shi, L. Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J. Clin. Endocrinol. Metab. 2011, 96, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Stroup, B.M.; Murali, S.G.; Schwahn, D.J.; Sawin, E.A.; Lankey, E.M.; Bächinger, H.P.; Ney, D.M. Sex effects of dietary protein source and acid load on renal and bone status in the Pahenu2 mouse model of phenylketonuria. Physiol. Rep. 2019, 7, e14251. [Google Scholar] [CrossRef] [PubMed]
- Zerjav Tansek, M.; Bertoncel, A.; Sebez, B.; Zibert, J.; Groselj, U.; Battelino, T.; Avbelj Stefanija, M. Anthropometry and bone mineral density in treated and untreated hyperphenylalaninemia. Endocr. Connect. 2020, 9, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Martins, A.; Monjardino, T.; Caetano-Lopes, J.; Fonseca, J.E. Bone Markers throughout Sexual Development: Epidemiological Significance and Population-Based Findings. In Biomarkers in Bone Disease; Preedy, V.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–34. ISBN 978-94-007-7745-3. [Google Scholar]
- Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T. A study of bone turnover markers in prepubertal children with phenylketonuria. Eur. J. Pediatr. 2004, 163, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Post, T.M.; Cremers, S.C.L.M.; Kerbusch, T.; Danhof, M. Bone physiology, disease and treatment: Towards disease system analysis in osteoporosis. Clin. Pharmacokinet. 2010, 49, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Monjardino, T.; Silva, P.; Amaro, J.; Carvalho, O.; Guimarães, J.T.; Santos, A.C.; Lucas, R. Bone formation and resorption markers at 7 years of age: Relations with growth and bone mineralization. PLoS ONE 2019, 14, e0219423. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Institute of Medicine (Ed.) Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Harper, G.W.; Ottinger, D.R. The performance of hyperactive and control preschoolers on a new computerized measure of visual vigilance: The Preschool Vigilance Task. J. Child. Psychol. Psychiatry 1992, 33, 1365–1372. [Google Scholar] [CrossRef]
- Cremone, A.; McDermott, J.M.; Spencer, R.M.C. Naps Enhance Executive Attention in Preschool-Aged Children. J. Pediatr. Psychol. 2017, 42, 837–845. [Google Scholar] [CrossRef]
- Espy, K.A.; Cwik, M.F. The development of a trial making test in young children: The TRAILS-P. Clin. Neuropsychol. 2004, 18, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Farrell Pagulayan, K.; Busch, R.M.; Medina, K.L.; Bartok, J.A.; Krikorian, R. Developmental normative data for the Corsi Block-tapping task. J. Clin. Exp. Neuropsychol. 2006, 28, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Drozdowska, A.; Sinningen, K.; Falkenstein, M.; Rudolf, H.; Libuda, L.; Buyken, A.E.; Lücke, T.; Kersting, M. Impact of lunch with carbohydrates differing in glycemic index on children’s cognitive functioning in the late postprandial phase: A randomized crossover study. Eur. J. Nutr. 2022, 61, 1637–1647. [Google Scholar] [CrossRef]
- Drozdowska, A.; Falkenstein, M.; Jendrusch, G.; Platen, P.; Luecke, T.; Kersting, M.; Jansen, K. Water Consumption during a School Day and Children’s Short-Term Cognitive Performance: The CogniDROP Randomized Intervention Trial. Nutrients 2020, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Tempes, J.; Drozdowska, A.; Gutmann, M.; Falkenstein, M.; Buyken, A.E.; Libuda, L.; Rudolf, H.; Lücke, T.; Kersting, M. Short-term effects of carbohydrates differing in glycemic index (GI) consumed at lunch on children’s cognitive function in a randomized crossover study. Eur. J. Clin. Nutr. 2020, 74, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Müller, K.; Falkenstein, M.; Stehle, P.; Kersting, M.; Libuda, L. Short-term effects of lunch on children’s executive cognitive functioning: The randomized crossover Cognition Intervention Study Dortmund PLUS (CogniDo PLUS). Physiol. Behav. 2015, 152, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Bruyer, R.; Brysbaert, M. Combining Speed and Accuracy in Cognitive Psychology: Is the Inverse Efficiency Score (IES) a Better Dependent Variable than the Mean Reaction Time (RT) and the Percentage of Errors (PE)? Psychol. Belg. 2013, 51, 5. [Google Scholar] [CrossRef]
- Hogg, J.A.; Riehm, C.D.; Wilkerson, G.B.; Tudini, F.; Peyer, K.L.; Acocello, S.N.; Carlson, L.M.; Le, T.; Sessions, R.; Diekfuss, J.A.; et al. Changes in dual-task cognitive performance elicited by physical exertion vary with motor task. Front. Sports Act. Living 2022, 4, 989799. [Google Scholar] [CrossRef]
- Paermentier, L.; Cano, A.; Chabrol, B.; Roy, A. Executive functions in preschool children with moderate hyperphenylalaninemia and phenylketonuria: A prospective study. Orphanet J. Rare Dis. 2023, 18, 175. [Google Scholar] [CrossRef]
- Huijbregts, S.C.J.; de Sonneville, L.M.J.; van Spronsen, F.J.; Licht, R.; Sergeant, J.A. The neuropsychological profile of early and continuously treated phenylketonuria: Orienting, vigilance, and maintenance versus manipulation-functions of working memory. Neurosci. Biobehav. Rev. 2002, 26, 697–712. [Google Scholar] [CrossRef]
- Huijbregts, S.; de Sonneville, L.; Licht, R.; Sergeant, J.; van Spronsen, F. Inhibition of prepotent responding and attentional flexibility in treated phenylketonuria. Dev. Neuropsychol. 2002, 22, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Jahja, R.; van Spronsen, F.J.; de Sonneville, L.M.J.; van der Meere, J.J.; Bosch, A.M.; Hollak, C.E.M.; Rubio-Gozalbo, M.E.; Brouwers, M.C.G.J.; Hofstede, F.C.; de Vries, M.C.; et al. Long-Term Follow-Up of Cognition and Mental Health in Adult Phenylketonuria: A PKU-COBESO Study. Behav. Genet. 2017, 47, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, B.; Garde, S.; Dehghan Nayyeri, M.; Weglage, J.; Rau, J.; Pfleiderer, B.; Feldmann, R. Approaching altered inhibitory control in phenylketonuria: A functional MRI study with a Go-NoGo task in young female adults. Eur. J. Neurosci. 2020, 52, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Muri, R.; Maissen-Abgottspon, S.; Reed, M.B.; Kreis, R.; Hoefemann, M.; Radojewski, P.; Pospieszny, K.; Hochuli, M.; Wiest, R.; Lanzenberger, R.; et al. Compromised white matter is related to lower cognitive performance in adults with phenylketonuria. Brain Commun. 2023, 5, fcad155. [Google Scholar] [CrossRef] [PubMed]
- Hood, A.; Rutlin, J.; Shimony, J.S.; Grange, D.K.; White, D.A. Brain White Matter Integrity Mediates the Relationship between Phenylalanine Control and Executive Abilities in Children with Phenylketonuria. JIMD Rep. 2017, 33, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.L.; Jurecki, E.R.; McCandless, S.E.; Stahl, S.M.; Bilder, D.A.; Sanchez-Valle, A.; Dimmock, D. Neuropsychiatric Function Improvement in Pediatric Patients with Phenylketonuria. J. Pediatr. 2023, 260, 113526. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Intemann, T.; Russo, P.; Moreno, L.A.; Molnár, D.; Veidebaum, T.; Tornaritis, M.; de Henauw, S.; Eiben, G.; Ahrens, W.; et al. 25-Hydroxyvitamin D reference percentiles and the role of their determinants among European children and adolescents. Eur. J. Clin. Nutr. 2022, 76, 564–573. [Google Scholar] [CrossRef]
- Thierfelder, W.; Dortschy, R.; Hintzpeter, B.; Kahl, H.; Scheidt-Nave, C. Biochemische Messparameter im Kinder- und Jugendgesundheitssurvey (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Schnabel, D.; Wabitsch, M.; Bechtold-Dalla Pozzalla, S.; Bührer, C.; Heidtmann, B.; Jochum, F.; Kauth, T.; Körner, A.; Mihatsch, W.; et al. Vitamin-D-Supplementierung jenseits des zweiten Lebensjahres. Monatsschrift Kinderheilkd. 2018, 166, 814–822. [Google Scholar] [CrossRef]
- Kunz, C.; Hower, J.; Knoll, A.; Ritzenthaler, K.L.; Lamberti, T. No improvement in vitamin D status in German infants and adolescents between 2009 and 2014 despite public recommendations to increase vitamin D intake in 2012. Eur. J. Nutr. 2019, 58, 1711–1722. [Google Scholar] [CrossRef]
- Pfotenhauer, K.M.; Shubrook, J.H. Vitamin D Deficiency, Its Role in Health and Disease, and Current Supplementation Recommendations. J. Am. Osteopath. Assoc. 2017, 117, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Geserick, M.; Vogel, M.; Eckelt, F.; Schlingmann, M.; Hiemisch, A.; Baber, R.; Thiery, J.; Körner, A.; Kiess, W.; Kratzsch, J. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations—Results derived from new pediatric reference ranges. Bone 2020, 132, 115124. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Haftenberger, M.; Lage Barbosa, C.; Brettschneider, A.-K.; Lehmann, F.; Frank, M.; Heide, K.; Moosburger, R.; Patelakis, E.; Perlitz, H. EsKiMo II-Die Ernährungsstudie als KiGGS-Modul; Robert Koch-Institut: Berlin, Germany, 2020. [Google Scholar]
- Sahin, O.N.; Serdar, M.; Serteser, M.; Unsal, I.; Ozpinar, A. Vitamin D levels and parathyroid hormone variations of children living in a subtropical climate: A data mining study. Ital. J. Pediatr. 2018, 44, 40. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Cavalli, L.; Ricci, S.; Mola, M.; Marchi, C.; Seminara, S.; Brandi, M.L.; de Martino, M. Parathyroid Hormone Levels in Healthy Children and Adolescents. Horm. Res. Paediatr. 2015, 84, 124–129. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Alexy, U.; Kersting, M.; Andler, W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur. J. Endocrinol. 2007, 157, 225–232. [Google Scholar] [CrossRef]
- Dortschy, R.; Schaffrath Rosario, A.; Scheidt-Nave, C.; Thierfelder, W.; Thamm, M.; Gutsche, J.; Markert, A. Bevölkerungsbezogene Verteilungswerte Ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS): Beiträge zur Gesundheitsberichterstattung des Bundes; Gesundheitsberichterstattung für Deutschland: Berlin, Germany, 2009. [Google Scholar]
- Vissing Landgrebe, A.; Asp Vonsild Lund, M.; Lausten-Thomsen, U.; Frithioff-Bøjsøe, C.; Esmann Fonvig, C.; Lind Plesner, J.; Aas Holm, L.; Jespersen, T.; Hansen, T.; Christian Holm, J. Population-based pediatric reference values for serum parathyroid hormone, vitamin D, calcium, and phosphate in Danish/North-European white children and adolescents. Clin. Chim. Acta 2021, 523, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Giwercman, A.; Hartwell, D.; Nielsen, C.T.; Price, P.A.; Christiansen, C.; Skakkebaek, N.E. Serum bone Gla-protein as a marker of bone growth in children and adolescents: Correlation with age, height, serum insulin-like growth factor I, and serum testosterone. J. Clin. Endocrinol. Metab. 1988, 67, 273–278. [Google Scholar] [CrossRef]
- van Summeren, M.; Braam, L.; Noirt, F.; Kuis, W.; Vermeer, C. Pronounced elevation of undercarboxylated osteocalcin in healthy children. Pediatr. Res. 2007, 61, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Popko, J.; Karpiński, M.; Chojnowska, S.; Maresz, K.; Milewski, R.; Badmaev, V.; Schurgers, L.J. Decreased Levels of Circulating Carboxylated Osteocalcin in Children with Low Energy Fractures: A Pilot Study. Nutrients 2018, 10, 734. [Google Scholar] [CrossRef]
- Tubic, B.; Magnusson, P.; Mårild, S.; Leu, M.; Schwetz, V.; Sioen, I.; Herrmann, D.; Obermayer-Pietsch, B.; Lissner, L.; Swolin-Eide, D. Different osteocalcin forms, markers of metabolic syndrome and anthropometric measures in children within the IDEFICS cohort. Bone 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Thalange, N.K.; Foster, P.J.; Gill, M.S.; Price, D.A.; Clayton, P.E. Model of normal prepubertal growth. Arch. Dis. Child. 1996, 75, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Reidy, N. Assessing executive function in preschoolers. Neuropsychol. Rev. 2012, 22, 345–360. [Google Scholar] [CrossRef] [PubMed]

| Parameter | PKU | Co | p |
|---|---|---|---|
| n | 9 | 18 | |
| Age (years) | 5.4 ± 1.2 | 5.1 ± 0.8 | 0.464 a |
| Female n (%) | 2 (22.2%) | 4 (22.2%) | 1.000 |
| Winter/spring n (%) | 2 (22.2%) | 5 (27.8%) | 1.000 |
| Daily Phe tolerance (mg/d) | 291.1 ± 43.4 | n.a. | - |
| Mean Phe in blood * (µmol/L) | 203.4 ± 82.9 | n.a. | - |
| Mean Tyr in blood * (µmol/L) | 80.0 ± 34.2 | n.a. | - |
| Supplementation with AAS n (%) | 9 (100%) | n.a. | - |
| Parameter of Executive Functioning | PKU | Co | p | Effect Size |
|---|---|---|---|---|
| Tonic Alertness ° | ||||
| Commission error (n) | 6.00 ± 5.60 | 8.27 ± 7.44 | 0.461 | −0.329 a |
| Missing (n) | 2.5 [0.3–4.8] | 5.0 [2.0–9.0] | 0.213 | −0.264 c |
| Average RT correct (ms) | 701.2 [621.3–814.2] | 738.8 [656.9–905.0] | 0.506 | −0.148 c |
| Corsi Block Tapping Task $ | ||||
| Correct order and path (n) | 7.63 ± 1.10 | 7.19 ± 0.60 | 0.706 | 0.165 a |
| Correct boxes (n) | 9.13 ± 0.79 | 9.31 ± 0.49 | 0.835 | −0.092 a |
| Flanker Task ~ | ||||
| IE congruent (ms) | 886.7 ± 226.2 | 1029.9 ± 161.5 | 0.139 | −0.741 b |
| IE incongruent (ms) | 1036.8 ± 194.9 | 1239.9 ± 157.9 | 0.030 | −1.135 b |
| Difference IE (ms) | 150.2 ± 95.6 | 210.0 ± 130.7 | 0.337 | −0.472 b |
| Switch Task *,# | ||||
| Sum RT lblast * (s) | 17.8 [10.2–39.1] | 17.5 [14.2–27.0] | 1.000 | 0.000 c |
| Switch costs # (s) | 29.6 [12.2–45.8] | 21.2 [16.2–24.8] | 0.606 | −0.160 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanusch, B.; Falkenstein, M.; Volkenstein, S.; Dazert, S.; Lücke, T.; Sinningen, K. No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study. Nutrients 2024, 16, 2072. https://doi.org/10.3390/nu16132072
Hanusch B, Falkenstein M, Volkenstein S, Dazert S, Lücke T, Sinningen K. No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study. Nutrients. 2024; 16(13):2072. https://doi.org/10.3390/nu16132072
Chicago/Turabian StyleHanusch, Beatrice, Michael Falkenstein, Stefan Volkenstein, Stefan Dazert, Thomas Lücke, and Kathrin Sinningen. 2024. "No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study" Nutrients 16, no. 13: 2072. https://doi.org/10.3390/nu16132072
APA StyleHanusch, B., Falkenstein, M., Volkenstein, S., Dazert, S., Lücke, T., & Sinningen, K. (2024). No Impairment in Bone Turnover or Executive Functions in Well-Treated Preschoolers with Phenylketonuria—A Pilot Study. Nutrients, 16(13), 2072. https://doi.org/10.3390/nu16132072







