Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View
Abstract
1. Introduction
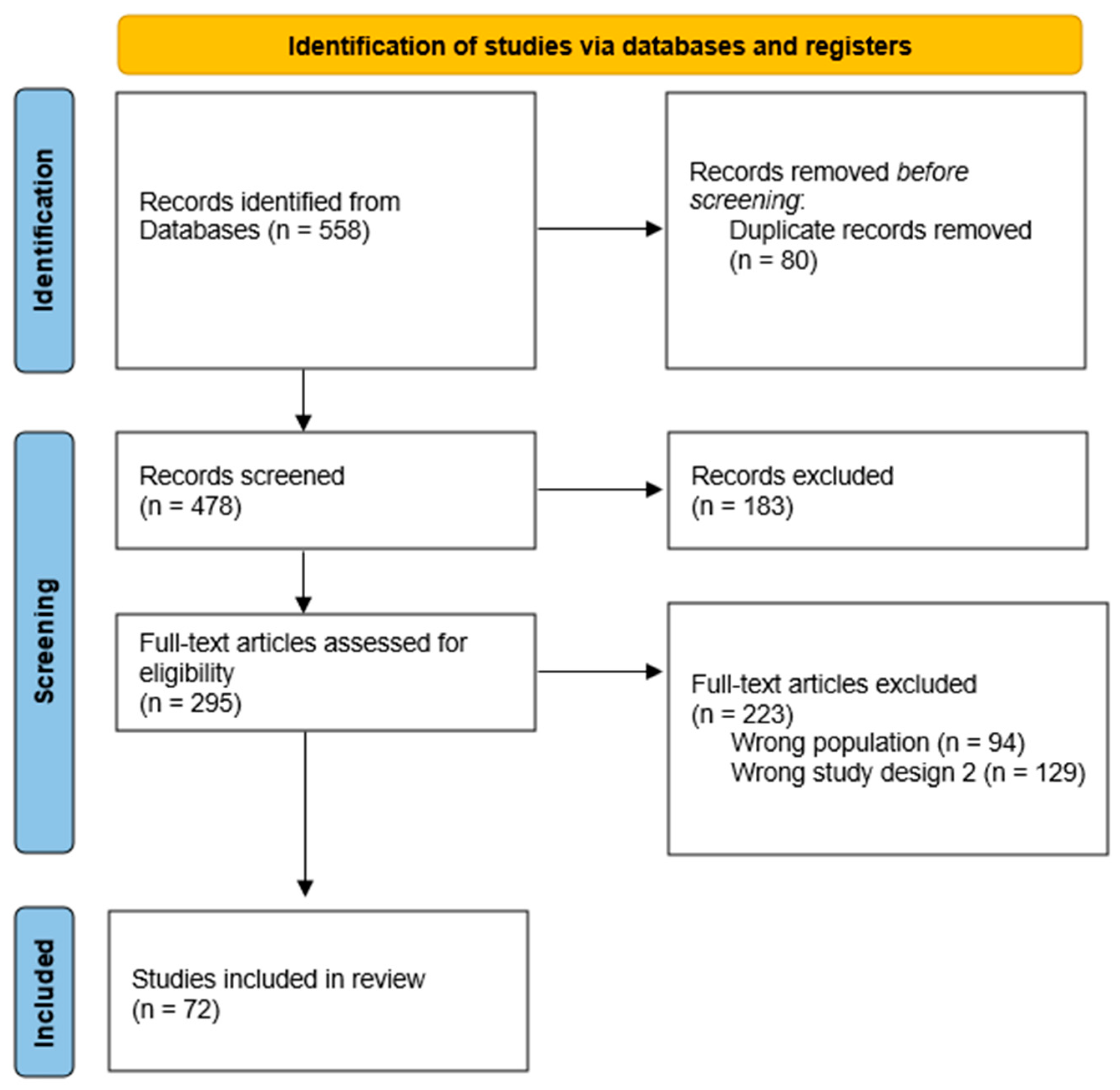
2. Materials and Methods
2.1. Data Sources and Study Selection
2.2. Data Extraction
2.3. Results
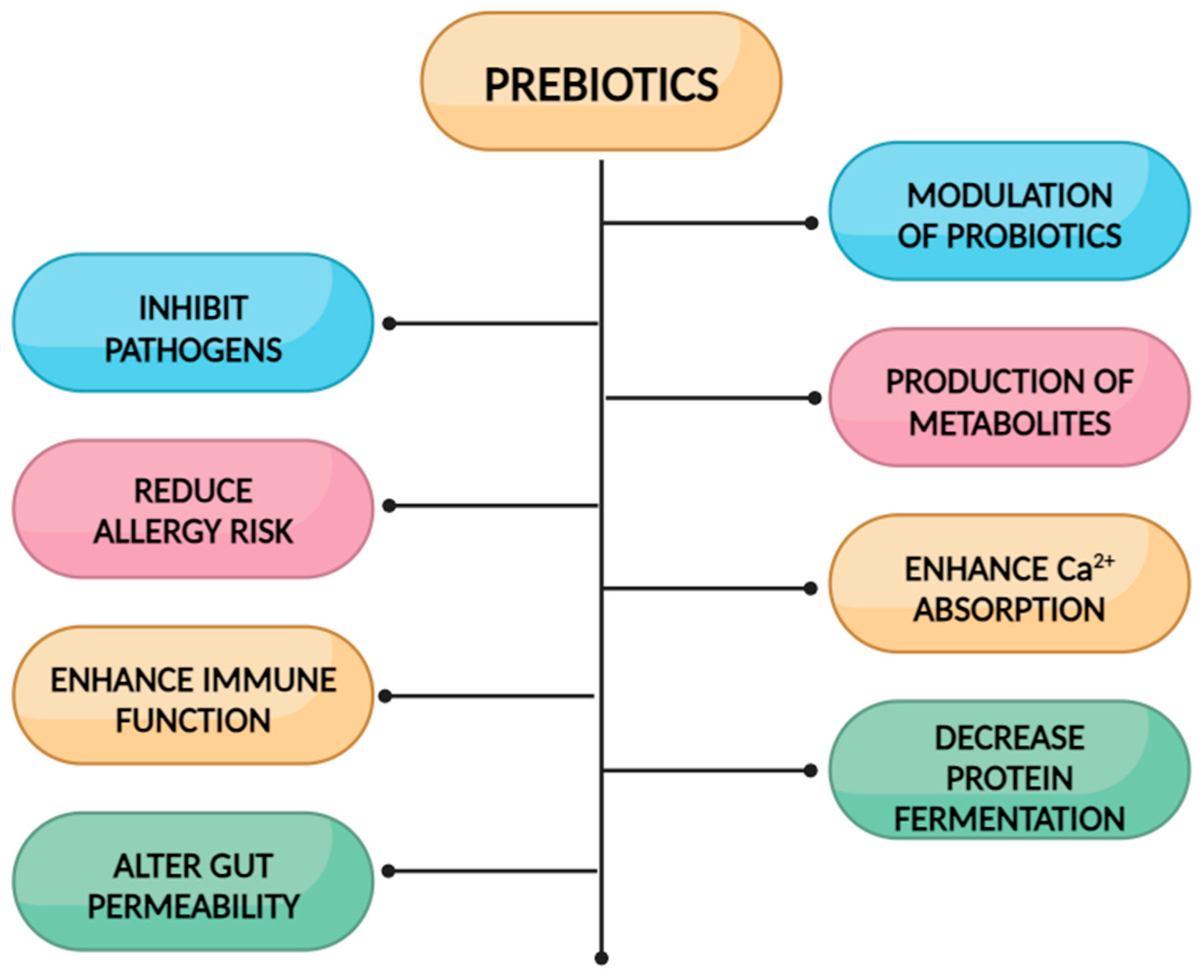
3. Dietary Fibers as Bioactive Compounds in Oral Nutritional Supplements
3.1. Prebiotic Fibers in Oral Nutritional Supplementations for Children
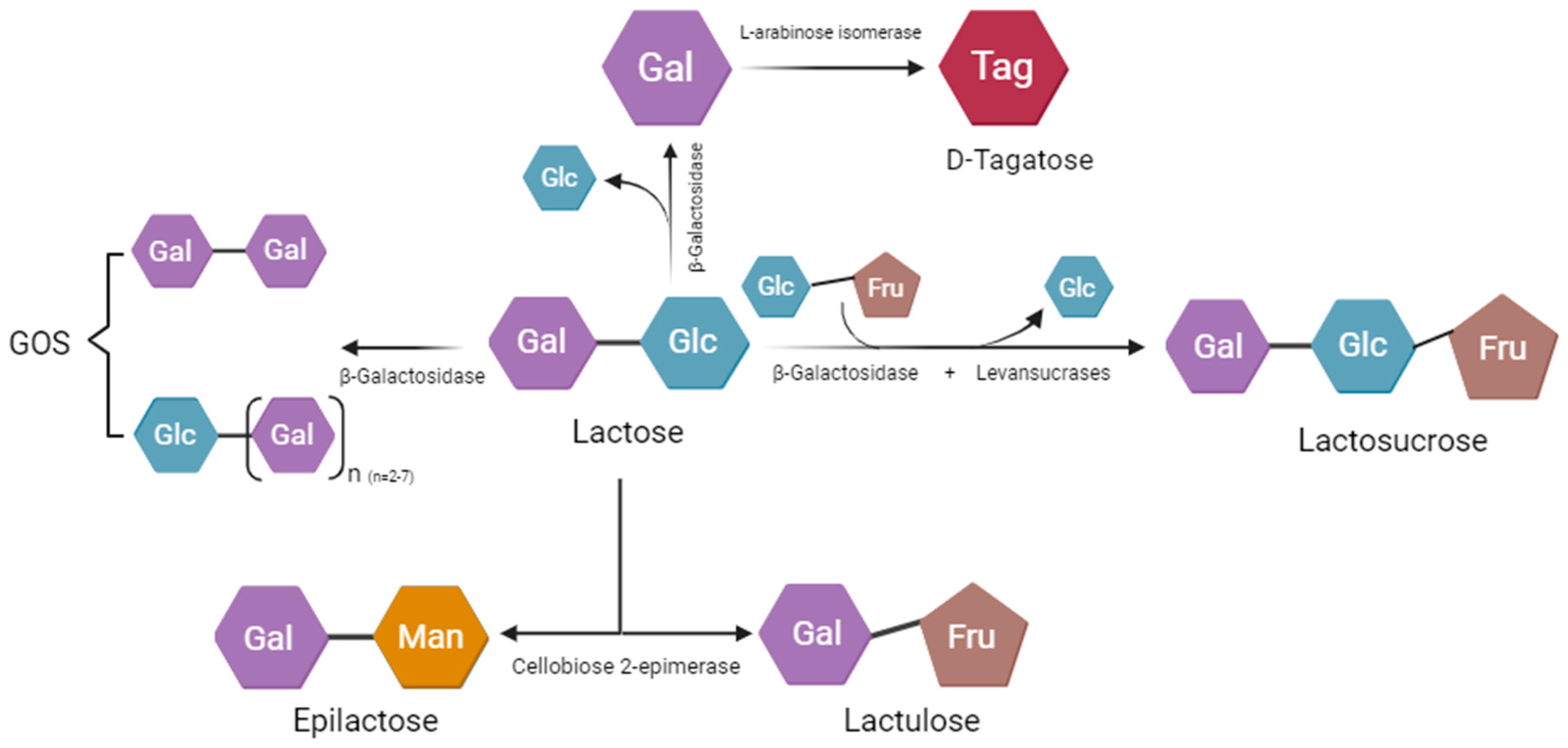
3.1.1. Fructo-Oligosaccharides (FOSs)
3.1.2. Biological Effects of Galacto-Oligosaccharides (GOSs)
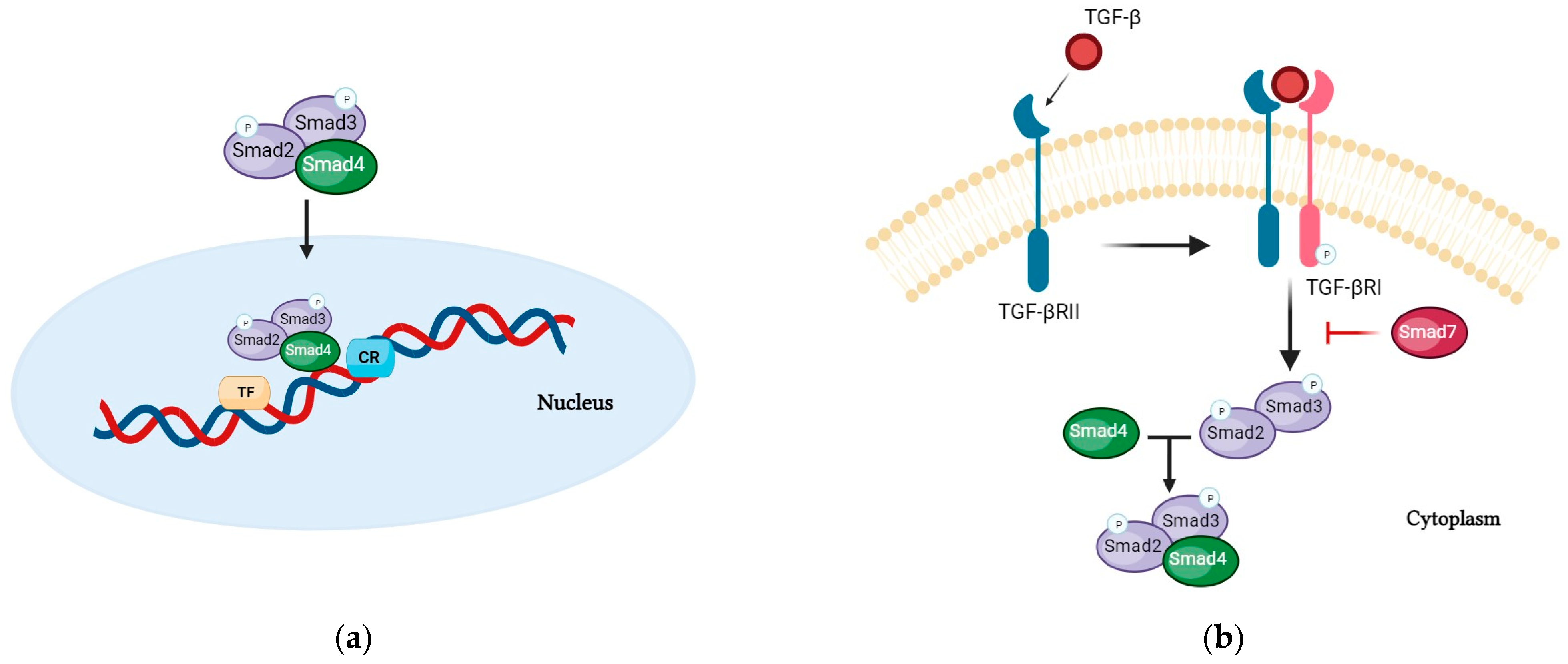
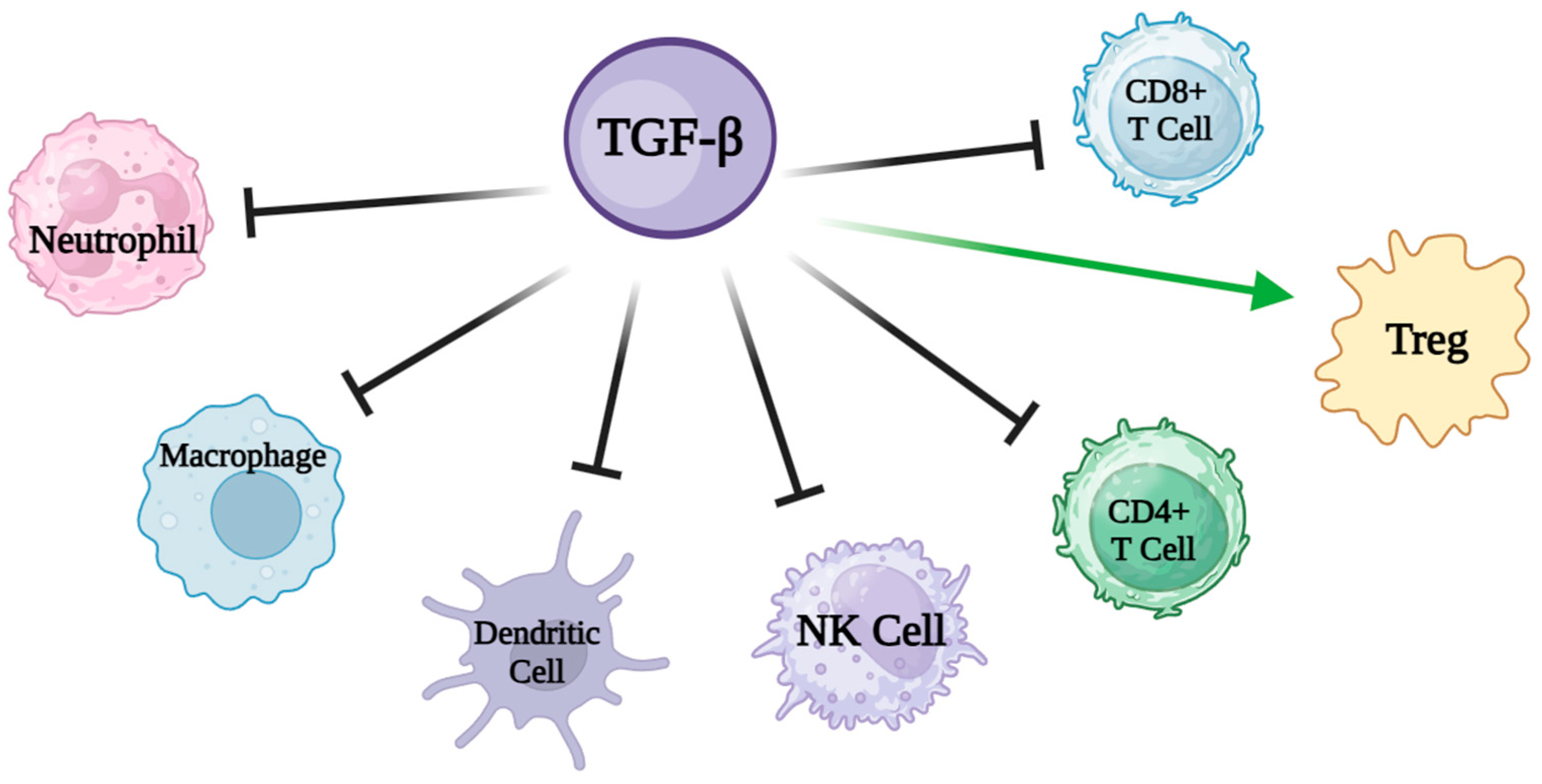
4. Oral Nutritional Supplements Enriched with Transforming Growth Factor-Beta 2 (TGF-Beta)
5. Immunonutrition and Oral Nutritional Supplements
5.1. Arginine
5.2. Omega 3
5.3. Nucleotides
6. Potential Bioactive Candidates Added to ONSs
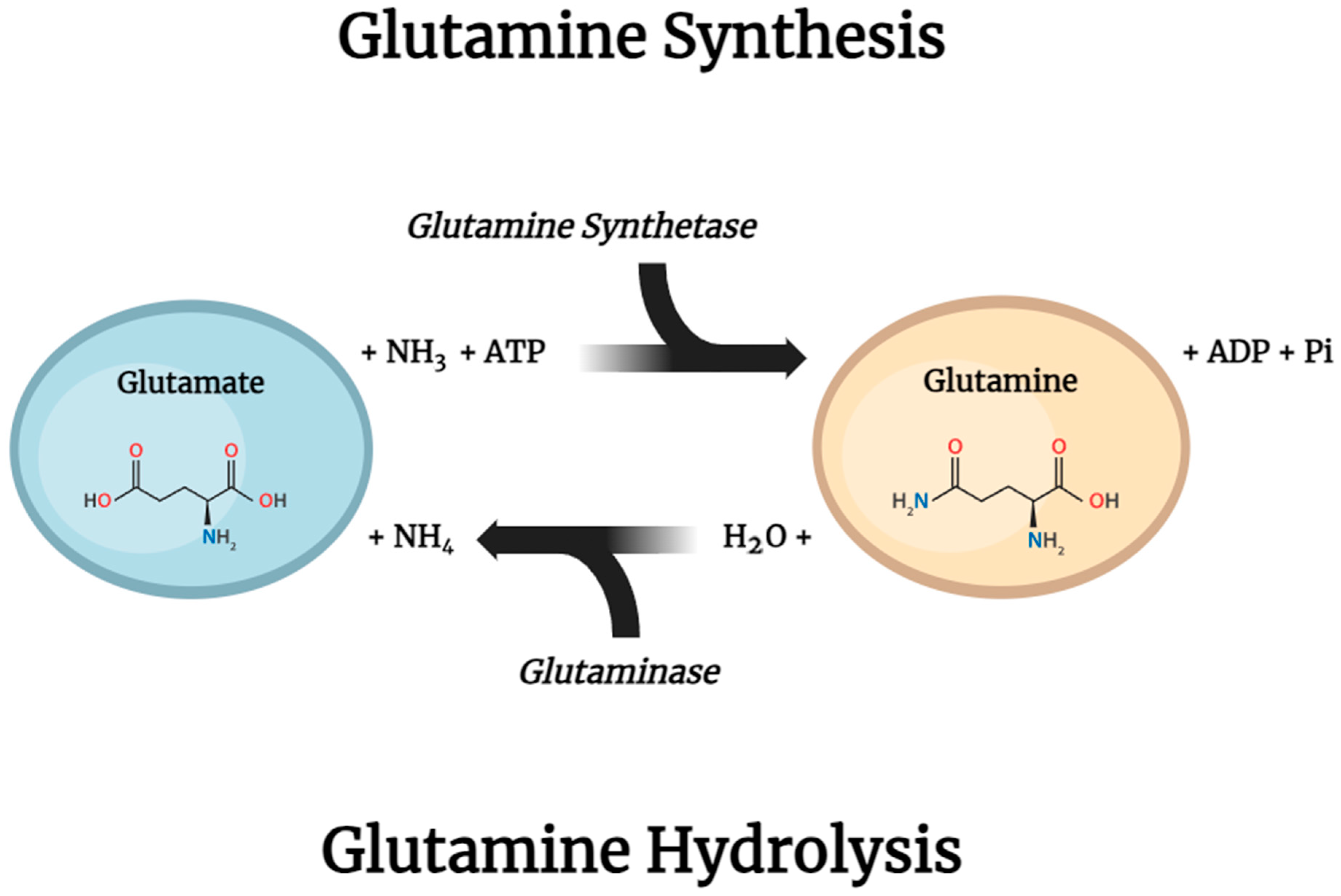
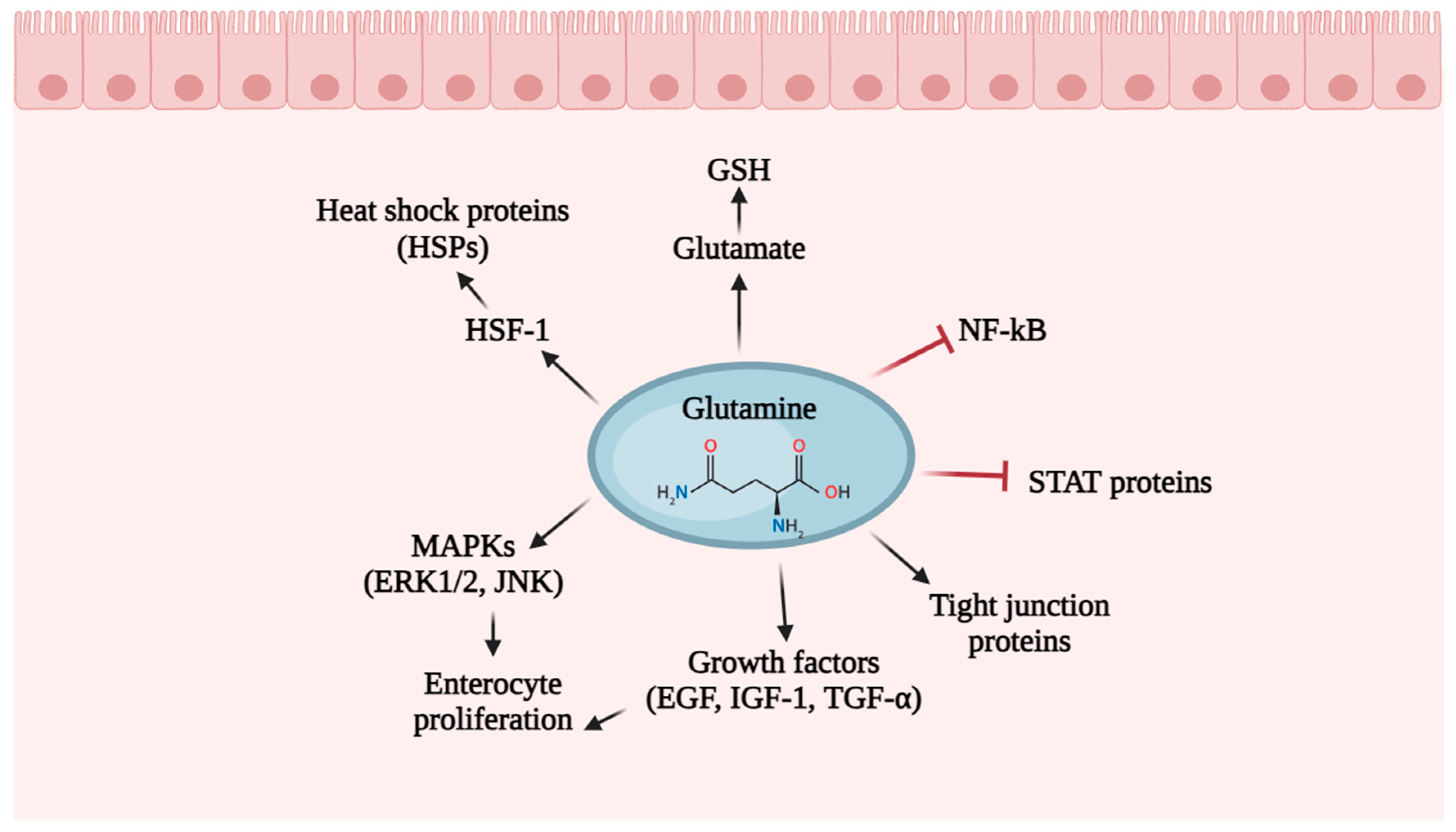
6.1. Glutamine
6.2. Lactoferrin
6.3. Butyrate
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Susan, M. Hill, Oral nutritional supplementation: A user’s guide. Paediatr. Child Health 2017, 27, 378–382. [Google Scholar] [CrossRef]
- Philipson, T.J.; Snider, J.T.; Lakdawalla, D.N.; Stryckman, B.; Goldman, D.P. Impact of oral nutritional supplementation on hospital outcomes. Am. J. Manag. Care 2013, 19, 121–128. [Google Scholar] [CrossRef]
- Lim, S.L.; Ong, K.C.B.; Chan, Y.H.; Loke, W.C.; Ferguson, M.; Daniels, L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin. Nutr. 2012, 31, 345–350. [Google Scholar] [CrossRef]
- Schoonhoven, L.; Grobbee, D.E.; Donders, A.R.T.; Algra, A.; Grypdonck, M.H.; Bousema, M.T.; Schrijvers, A.J.P.; Buskens, E. Prediction of pressure ulcer development in hospitalized patients: A tool for risk assessment. Qual. Saf. Health Care 2006, 15, 65–70. [Google Scholar] [CrossRef]
- Brown, B.; Roehl, K.; Betz, M. Enteral nutrition formula selection: Current evidence and implications for practice. Nutr. Clin. Pract. 2015, 30, 72–85. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hojsak, I.; Benninga, M.A.; Hauser, B.; Kansu, A.; Kelly, V.B.; Stephen, A.M.; Lopez, A.M.; Slavin, J.; Tuohy, K. Benefits of dietary fibre for children in health and disease. Arch. Dis. Child. 2022, 107, 973–979. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Mei, Z.; Yuan, J.; Li, D. Biological activity of galacto-oligosaccharides: A review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef]
- François, I.E.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Hamer, H.; Windey, K.; Welling, G.W.; Delcour, J.A.; Courtin, C.M.; et al. Effects of wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal parameters in healthy preadolescent children. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; DiLorenzo, C.; Berger, Y.M.; Faure, C.; Langendam, W.M.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, A.M. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef]
- Axelrod, C.H.; Saps, M. The Role of Fiber in the Treatment of Functional Gastrointestinal Disorders in Children. Nutrients 2018, 10, 1650. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposal classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Xu, B.; Cao, J.; Fu, J.; Li, Z.; Jin, M.; Wang, X.; Wang, Y. The effects of nondigestible fermentable carbohydrates on adults with overweight or obesity: A meta-analysis of randomized controlled trials. Nutr. Rev. 2022, 80, 165–177. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Salvatore, S.; Battigaglia, M.S.; Murone, E.; Dozio, E.; Pensabene, L.; Agosti, M. Dietary Fibers in Healthy Children and in Pediatric Gastrointestinal Disorders: A Practical Guide. Nutrients 2023, 15, 2208. [Google Scholar] [CrossRef]
- Coppa, G.V.; Bruni, S.; Morelli, L.; Soldi, S.; Gabrielli, O. The first prebiotics in humans: Human milk oligosaccharides. J. Clin. Gastroenterol. 2004, 38 (Suppl. S6), S80–S83. [Google Scholar] [CrossRef]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharmacol. 2021, 12, 712531. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Connor, F.; Salvatore, S.; D’Auria, E.; Baldassarre, M.E.; Acunzo, M.; Di Bella, G.; Farella, I.; Sestito, S.; Pensabene, L. Cows’ Milk Allergy-Associated Constipation: When to Look for It? A Narrative Review. Nutrients 2022, 14, 1317. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Baaleman, D.F.; Tabbers, M.M.; Smidt, H.; Benninga, M.A. Nonpharmacologic Treatment for Children with Functional Constipation: A Systematic Review and Meta-analysis. J. Pediatr. 2022, 240, 136–149.e5. [Google Scholar] [CrossRef] [PubMed]
- Toporovski, M.S.; de Morais, M.B.; Abuhab, A.; Crippa, J.M.A. Effect of Polydextrose/Fructooligosaccharide Mixture on Constipation Symptoms in Children Aged 4 to 8 Years. Nutrients 2021, 13, 1634. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.R.; Celiberto, L.S.; Lee, S.M.; Jacobson, K. Fiber and Prebiotic Interventions in Pediatric Inflammatory Bowel Disease: What Role Does the Gut Microbiome Play? Nutrients 2020, 12, 3204. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, Y.; Zhou, Z.; Liu, N.; Jung, S.; Lee, M.S.; Li, S. Preventive Prebiotic Effect of α-Galacto-Oligosaccharide against Dextran Sodium Sulfate-Induced Colitis Gut Microbiota Dysbiosis in Mice. J. Agric. Food Chem. 2021, 69, 9597–9607. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.C.; van den Heuvel, E.G.H.M. Galactooligosaccharides improve mineral absorption bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Q.; Guang, C.; Zhang, W.; Mu, W. An overview on biological production of functional lactose derivatives. Appl. Microbiol. Biotechnol. 2019, 103, 3683–3691. [Google Scholar] [CrossRef]
- Sims, I.M.; Tannock, G.W. Galacto- and Fructo-oligosaccharides Utilized for Growth by Cocultures of Bifidobacterial Species Characteristic of the Infant Gut. Appl. Environ. Microbiol. 2020, 86, e00214-20. [Google Scholar] [CrossRef]
- O’Sullivan, M.; O’Morain, C. Nutritional therapy in inflammatory bowel disease. Curr. Treat. Options Gastroenterol. 2004, 7, 191–198. [Google Scholar] [CrossRef]
- Verma, S.; Kirkwood, B.; Brown, S.; Giaffer, M.H. Oral nutritional supplementation is effective in the maintenance of remission in Crohn’s disease. Dig. Liver Dis. 2000, 32, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Sheppard, D. TGF-β signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Lagna, G.; Hata, A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008, 454, 56–61. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Stamataki, A.; Gikas, A.; Malgarinos, G. Maintenance treatment of Crohn’s disease with a polymeric feed rich in TGF-β. Ann. Gastroenterol. 2010, 23, 113–118. [Google Scholar]
- Strainic, M.; Shevach, E.; An, F.; Lin, F.; Medof, M.E. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat. Immunol. 2013, 14, 162–171. [Google Scholar] [CrossRef]
- Brown, C.C.; Rudensky, A.Y. Spatiotemporal regulation of peripheral T cell tolerance. Science 2023, 380, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.G.; Gao, S.; Wang, X.; Li, M.O. TGFb control of immune responses in cancer: A holistic immuno-oncology perspective. Nat. Rev. Immunol. 2023, 23, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Mulsow, J.J.; O’Keane, C.; Docherty, N.G.; Watson, R.W.G.; O’connell, P.R. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-tissue organization of the fibroblast lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, R.B.; Menache, C.C.; Megerian, J.T.; Baird, A.E. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 8–15. [Google Scholar] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019, 157, 440–450. [Google Scholar] [CrossRef]
- Agin, M.; Yucel, A.; Gumus, M.; Yuksekkaya, H.A.; Tumgor, G. The Effect of Enteral Nutrition Support Rich in TGF-β in the Treatment of Inflammatory Bowel Disease in Childhood. Medicina 2019, 55, 620. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Concept of Immuno-Nutrition. J. Nutr. Food Sci. 2016, 6, 1000500. [Google Scholar] [CrossRef]
- Calder, P.C. Immunonutrition may have beneficial effects in surgical patients. BMJ 2003, 327, 117–118. [Google Scholar] [CrossRef]
- Grimble, R. Basics in clinical nutrition: Immunonutrition—Nutrients which influence immunity: Effect and mechanism of action. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2009, 4, e10–e13. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.P.; Yin, Y.L.; Huang, R.L.; Li, T.; Hou, R.; Liu, Y.; Wu, X.; Liu, Z.; Wang, W.; Xiong, H.; et al. Rice protein concentrate partially replaces dried whey in the diet for early-weaned piglets and improves their growth performance. J. Sci. Food Agric. 2008, 88, 1187–1193. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Arginine synthesis. In Cellular and Molecular Biology of Nitric Oxide; Laskin, J.D., Laskin, D.L., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 57–85. [Google Scholar]
- Brosnan, M.E.; Brosnan, J.T. Renal arginine metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef] [PubMed]
- Szefel, J.; Danielak, A.; Kruszewski, W.J. Metabolic pathways of L-arginine and therapeutic consequences in tumors. Adv. Med. Sci. 2019, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Förstermann, U.; Kleinert, H. Nitric oxide synthase: Expression and expressional control of the three isoforms. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995, 352, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Poulos, T.L. Structure-function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Channon, K.M. Synthesis and recycling of tetrahydrobi-opterin in endothelial function and vascular disease. Nitric Oxide 2011, 25, 81–88. [Google Scholar] [CrossRef]
- Predonzani, A.; Calì, B.; Agnellini, A.H.; Molon, B. Spotlights on immunological effects of reactive nitrogen species: When inflammation says nitric oxide. World J. Exp. Med. 2015, 5, 64–76. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Prast, H.; Philippu, A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 2001, 64, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Fakler, C.R.; Kaftan, H.A.; Nelin, L.D. Two cases suggesting a role for the L-arginine nitric oxide pathway in neonatal blood pressure regulation. Acta Paediatr. 1995, 84, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.C.; Barbul, A. Cellular and physiological effects of arginine. Mini Rev. Med. Chem. 2004, 4, 823–832. [Google Scholar] [CrossRef]
- Flynn, N.E.; Meininger, C.J.; Haynes, T.E.; Wu, G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427. [Google Scholar] [CrossRef]
- Orlando, G.F.; Wolf, G.; Engelmann, M. Role of neuronal nitric oxide synthase in the regulation of the neuroendocrine stress response in rodents: Insights from mutant mice. Amino Acids 2008, 35, 17–27. [Google Scholar] [CrossRef]
- Langkamp-Henken, B.; Johnson, L.R.; Viar, M.J.; Geller, A.M.; Kotb, M. Differential effect on polyamine metabolism in mitogen- and superantigen-activated human T-cells. Biochim. Biophys. Acta 1998, 425, 337–347. [Google Scholar] [CrossRef]
- Reitano, G.; Grasso, S.; Distefano, G.; Messina, A. The serum insulin and growth hormone response to arginine and to arginine with glucose in the premature infant. J. Clin. Endocrinol. 1971, 33, 924–928. [Google Scholar] [CrossRef]
- Lewis, B.; Langkamp-Henken, B.J. Arginine enhances in vivo immune responses in young, adult and aged mice. J. Nutr. 2000, 130, 1827–1830. [Google Scholar] [CrossRef]
- Waugh, W.H.; Daeschner, C.W., 3rd; Files, B.A.; McConnell, M.E.; Strandjord, S.E. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: Early phase two results. J. Natl. Med. Assoc. 2001, 93, 363–371. [Google Scholar]
- Marin, V.B.; Rodriguez-Osiac, L.; Schlessinger, L.; Villegas, J.; Lopez, M.; Castillo-Duran, C. Controlled study of enteral arginine supplementation in burned children: Impact on immunologic and metabolic status. Nutrition 2006, 22, 705–712. [Google Scholar] [CrossRef]
- Martí I Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M. The suckling rat as a model for immunonutrition studies in early life. Clin. Dev. Immunol. 2012, 2012, 537310. [Google Scholar] [CrossRef]
- Rashid, J.; Kumar, S.S.; Job, K.M.; Liu, X.; Fike, C.D.; Sherwin, C.M. Therapeutic Potential of Citrulline as an Arginine Supplement: A Clinical Pharmacology Review. Pediatr. Drugs 2020, 22, 279–293. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Bishop, K.S.; Erdrich, S.; Karunasinghe, N.; Han, D.Y.; Zhu, S.; Jesuthasan, A.; Ferguson, L.R. An investigation into the association between DNA damage and dietary fatty acid in men with prostate cancer. Nutrients 2015, 7, 405–422. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Lipid rafts—Composition, characterization and controversies. J. Nutr. 2007, 137, 545–547. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Modulation of immune function by dietary fatty acids. Proc. Nutr. Soc. 1998, 57, 277–292. [Google Scholar] [CrossRef]
- Calder, P.C. The relationship between the fatty acid composition of immune cells and their function. Prost. Leuk. Essent. Fatty Acids 2008, 79, 101–108. [Google Scholar] [CrossRef]
- Uauy, R.D.; Birch, D.G.; Birch, E.E.; Tyson, J.E.; Hoffman, D.R. Effect of dietary n-3 fatty acids on retinal function of very low birthweight neonates. Pediatr. Res. 1990, 28, 485–492. [Google Scholar] [CrossRef]
- Carlson, S.E.; Werkman, S.H.; Rhodes, P.G.; Tolley, E. Visualacuity development in healthy preterm infants: Effect of marine-oil supplementation. Am. J. Clin. Nutr. 1993, 58, 35–42. [Google Scholar] [CrossRef]
- Richardson, A.J. Clinical trials of fatty acid treatment in ADHD, dyslexia, dyspraxia and the autistic spectrum. Prost. Leuk. Essent. Fatty Acids 2004, 70, 383–390. [Google Scholar] [CrossRef]
- Sanders, T.A.; Naismith, D.J. A comparison of the influence of breast-feeding and bottle-feeding on the fatty acid composition of the erythrocytes. Br. J. Nutr. 1979, 41, 619–623. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Vassiliou, E.; Adhikary, S.; Toscano, M.G.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010, 9, 12. [Google Scholar] [CrossRef]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; DiazDelfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma): RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef]
- De Caterina, R.; Libby, P. Control of endothelial leukocyte adhesion molecules by fatty acids. Lipids 1996, 31, S57–S63. [Google Scholar] [CrossRef]
- Babcock, T.A.; Novak, T.; Ong, E.; Jho, D.H.; Helton, W.S.; Espat, N.J. Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-a production by v-3 fatty acid is associated with differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J. Surg. Res. 2002, 107, 135–139. [Google Scholar]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Draper, E.; Reynolds, C.M.; Canavan, M.; Mills, K.H.; Loscher, C.E.; Roche, H.M. Omega-3 fatty acids attenuate dendritic cell function via NF-kB independent of PPARg. J. Nutr. Biochem. 2011, 22, 784–790. [Google Scholar] [CrossRef]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, S1505–S1519. [Google Scholar] [CrossRef]
- Podpeskar, A.; Crazzolara, R.; Kropshofer, G.; Hetzer, B.; Meister, B.; Müller, T.; Salvador, C. Omega-3 Fatty Acids and Their Role in Pediatric Cancer. Nutrients 2021, 13, 1800. [Google Scholar] [CrossRef]
- Suchner, U.; Kuhn, K.S.; Furst, P. The scientific basis of immunonutrition. Proc. Nutr. Soc. 2000, 59, 553–563. [Google Scholar] [CrossRef]
- Schloerb, P.R. Immune-enhancing diets: Products, components, and their rationales. JPEN J. Parenter. Enter. Nutr. 2001, 25, S3–S7. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenberg, N.A. The role of nucleotides in the immune and gastrointestinal systems: Potential clinical applications. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Rudolph, F.B.; Kulkarni, A.D.; Fanslow, W.C.; Pizzini, R.P.; Kumar, S.; Van Buren, C.T. Role of RNA as a dietary source of pyrimidines and purines in immune function. Nutrition 1990, 6, 45–52. [Google Scholar]
- Jyonouchi, H.; Lei, Z.-S.; Tomita, Y.; Yokoyama, H. Nucleotide-free diet impairs T-helper cell functions in antibody production in response to T-dependent antigens in normal C57BL/6 mice. J. Nutr. 1994, 124, 475–484. [Google Scholar] [CrossRef]
- Hasko, G. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000, 14, 2065–2074. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Carver, J.D.; Pimentel, B.; Cox, W.I.; Barness, L.A. Dietary nucleotide effects upon immune function in infants. Pediatrics 1991, 88, 359–363. [Google Scholar] [CrossRef]
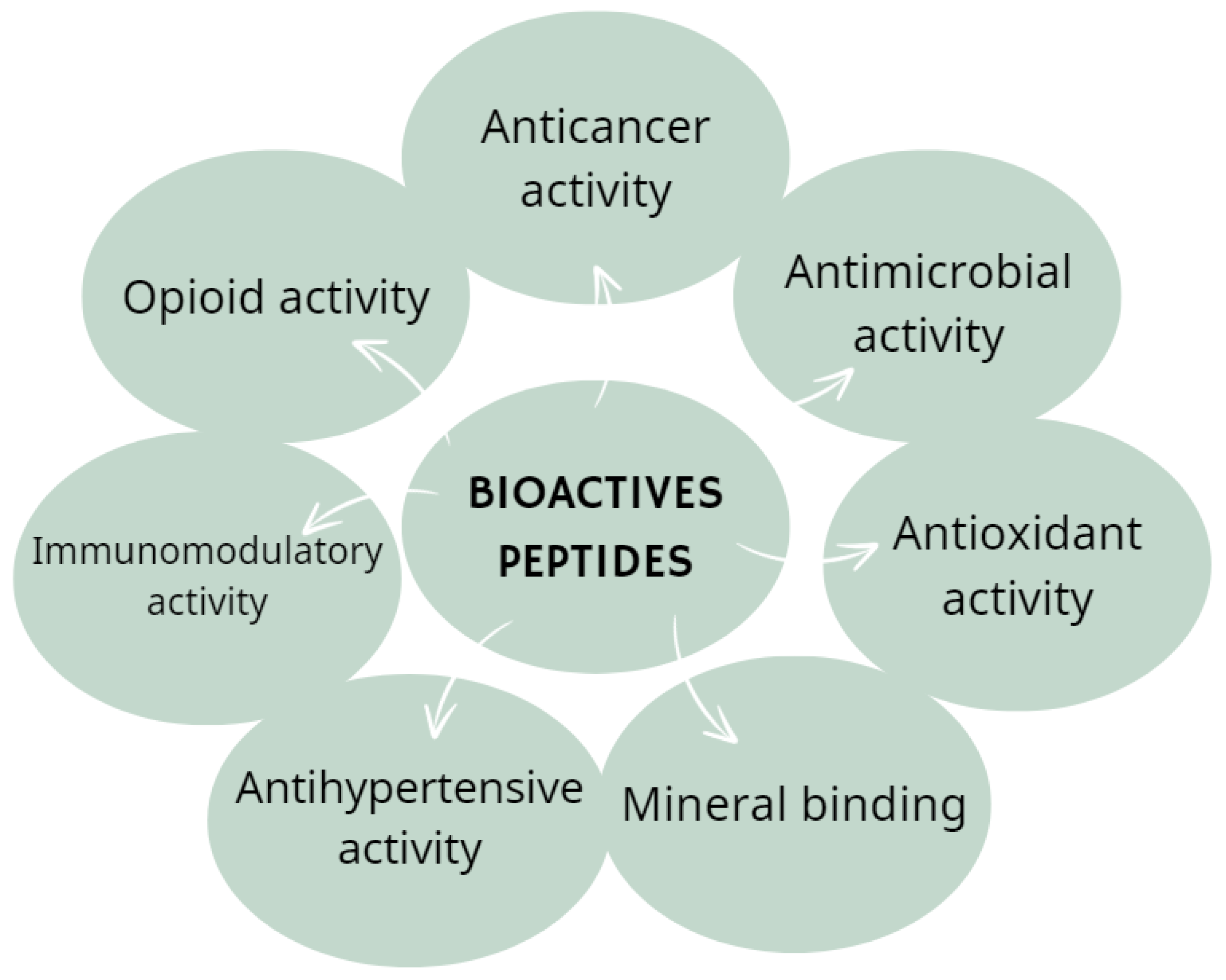
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Mizock, B. Immunonutrition and critical illness: An update. Nutrition 2010, 26, 701–707. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Pantaleao, L.C.; Donato, J., Jr.; de Bittencourt, P.I.H., Jr.; Tirapegui, J. Oral supplementations with free and dipeptide forms of l-glutamine in endotoxemic mice: Effects on muscle glutamine-glutathione axis and heat shock proteins. J. Nutr. Biochem. 2014, 25, 345–352. [Google Scholar] [CrossRef]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, A.; Wernerman, J. Glutamine and glutathione at icu admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Rogero, M.M.; Borges, M.C.; Pires, I.S.D.; Borelli, P.; Tirapegui, J. Ffect of glutamine supplementation and in vivo infection with mycobacterium bovis (bacillus calmette-guerin) in the function of peritoneal macrophages in early weaned mice. Ann. Nutr. Metab. 2007, 51, 173–174. [Google Scholar]
- Flaring, U.B.; Rooyackers, O.E.; Wernerman, J.; Hammarqvist, F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin. Sci. 2003, 104, 275–282. [Google Scholar] [CrossRef]
- Leite, J.S.; Raizel, R.; Hypolito, T.M.; Rosa, T.D.; Cruzat, V.F.; Tirapegui, J. L-glutamine and l-alanine supplementation increase glutamine-glutathione axis and muscle hsp-27 in rats trained using a progressive high-intensity resistance exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 842–849. [Google Scholar] [CrossRef]
- Marino, L.V.; Pathan, N.; Meyer, R.; Wright, V.; Habibi, P. Glutamine depletion and heat shock protein 70 (HSP70) in children with meningococcal disease. Clin. Nutr. 2014, 33, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I.H., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.R.; Hu, Z.; Shi, X.; Jiang, L.; Boroughs, L.K.; Kovacs, Z.; Boriack, R.; Rakheja, D.; Sullivan, L.B.; Linehan, W.M.; et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014, 7, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.F.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Newsholme, P.; Diniz, V.L.S.; Dodd, G.T.; Cruzat, V. Glutamine metabolism and optimal immune and CNS function. Proc. Nutr. Soci. 2023, 82, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Parry-Billings, M. Properties of glutamine release from muscle and its importance for the immune system. JPEN J. Parenter. Enter. Nutr. 1990, 14, 63S–67S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Prabhu, R.; Balasubramanian, K.A. Surgical manipulation of the intestine and distant organ damage—Protection by oral glutamine supplementation. Surgery 2005, 137, 48–55. [Google Scholar] [CrossRef]
- Deters, B.J.; Saleem, M. The role of glutamine in supporting gut health and neuropsychiatric factors. Food Sci. Hum. Wellness 2021, 10, 149–154. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Wu, Z. Glutamine and intestinal barrier function. Amino Acids 2014, 47, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lewis, P.; Samuelson, D.; Liboni, K.; Neu, J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liv. Physiol. 2004, 287, G726–G733. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Neu, J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J. Nutr. 2009, 139, 710–714. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 2009, 37, 111–122. [Google Scholar] [CrossRef]
- Kaplan, M.H. STAT signaling in inflammation. JAK-STAT 2013, 2, e24198. [Google Scholar] [CrossRef]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Ropeleski, M.J.; Riehm, J.; Baer, K.A.; Musch, M.W.; Chang, E.B. Anti-apoptotic effects of L-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 2005, 129, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.R.; Dias, T.B.; Natov, P.S.; Zachara, N.E. Stress-induced O-GlcNAcylation: An adaptive process of injured cells. Biochem. Soc. Trans. 2017, 45, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin Induces Concentration-Dependent Functional Modulation of Intestinal Proliferation and Differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Grover, M.; Giouzeppos, O.; Schnagl, R.D.; May, J.T. Effect of human milk prostaglandins and lactoferrin on respiratory syncytial virus and rotavirus. Acta Paediatr. 1997, 86, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Ammendolia, M.G.; Superti, F. Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection. Biochem. Cell Biol. 2012, 90, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.; Abo-Elela, M.G.M.; El-Baseer, K.A.A.; Ahmed, A.E.; Ahmad, F.A.; Tawfeek, M.S.K.; Houfey, A.A.E.; Aboul_Khair, M.D.; Abdel-Salam, A.M.; Abo-Elgheit, A.; et al. Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine 2016, 95, e4560. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARSCoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, N.A.; Hamidah, A.; Purnomo, M.T.; Ardianah, E. Effect of lactoferrin in oral nutrition supplement (ONS) towards IL-6 and IL-10 in failure to thrive children with infection. F1000Research 2023, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Motoki, N.; Mizuki, M.; Tsukahara, T.; Miyakawa, M.; Kubo, S.; Oda, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Nomiyama, T. Effects of Lactoferrin-Fortified Formula on Acute Gastrointestinal Symptoms in Children Aged 12-32 Months: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pediatr. 2020, 8, 233. [Google Scholar] [CrossRef]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Byrne, C.; Chambers, E.; Morrison, D.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Kumar, A.; Alrefai, W.A.; Borthakur, A.; Dudeja, P.K. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G602–G607. [Google Scholar] [CrossRef]
- Takebe, K.; Nio, J.; Morimatsu, M.; Karaki, S.-I.; Kuwahara, A.; Kato, I.; Iwanaga, T. Histochemical demonstration of a Na+ coupled transporter for short-chain fatty acids (slc5a8) in the intestine and kidney of the mouse. Biomed. Res. 2005, 26, 213–221. [Google Scholar] [CrossRef][Green Version]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein–coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bush, K.; Nagy, L.E. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.; Wagenaar, G.T.; Garssen, J.; Folkerts, G.; Henricks, P.A. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.D.; Lupton, J.R. Dietary fiber. Adv. Nutr. 2011, 2, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; de Giovanni di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef]

| Formula Type | Summary of Characteristics | Recommendations for Use |
|---|---|---|
| Polymeric | Comprise macronutrients such as unhydrolyzed proteins, fats, and carbohydrates. Varies in concentration within the range of 1–2 kcal/mL. Include essential vitamins and minerals. Might be tailored for specific diseases and/or incorporate prebiotics and probiotics. | Designed for patients without severe malabsorptive disorders. |
| Fiber-containing | The inclusion of fiber is meant to enhance the well-being of the gastrointestinal tract by regulating the frequency and/or consistency of stool through the maintenance of a healthy GI flora. The fiber content generally falls below the recommended total daily fiber intake. These products may include prebiotics in the form of fructooligosaccharides, oligofructose, or inulin. May incorporate probiotics. | Suggested for patients experiencing diarrhea and/or to support or sustain the gut microbiota. |
| Whole food/blenderized | Whole foods blended to create a texture suitable for enteral consumption, enabling patients to obtain nutritional qualities not present in typical enteral formulas, such as phytochemicals. | Only deemed suitable for application in medically stable patients with a healed feeding tube site and no indications of infection. Should be administered as bolus feeds to uphold safe food practices. Involvement of a dietitian is crucial in formulating the feeding composition to ensure sufficient nutrient delivery. |
| Diabetes/glucose intolerance | Designed to alleviate hyperglycemia by incorporating a macronutrient composition of 40% carbohydrates, 40% fats, and 20% protein. The presence of fats and soluble fibers in the diet may impede gastric emptying, thereby averting an increase in blood glucose levels. | Current research does not strongly support the use of enteral formulas specifically designed for individuals with diabetes mellitus (DM). Instead, emphasis should be placed on avoiding overfeeding. |
| Renal | Fluid restricted. Designed with reduced levels of electrolytes, particularly potassium and phosphorus, to avoid excessive supply in patients with renal insufficiency. Protein content may vary. | The initial choice for patients with renal insufficiency should be a standard enteral formula. If there are notable electrolyte imbalances or they arise, contemplation of a renal formula is warranted until electrolyte levels stabilize. |
| Hepatic | Formulated with reduced protein content featuring a higher proportion of branched-chain amino acids and lower aromatic amino acids to mitigate the risk of hepatic encephalopathy. The lower protein content, however, may lead to insufficient protein delivery. Designed with restrictions on fluid and sodium to lessen the impact of ascites. | As the initial approach, the standard enteral nutrition (EN) formula is recommended for patients with hepatic encephalopathy. Reserve only for individuals with encephalopathy where conventional treatment with luminal-acting antibiotics and lactulose fails to improve the condition. |
| Elemental/semielemental | Macronutrients are hydrolyzed to maximize absorption. | The objective is to limit enteral delivery to 60–70% of the target energy requirements while ensuring sufficient protein intake. Designed for individuals with malabsorptive disorders; not recommended for regular use. |
| Pulmonary/fish oil | In efforts to reduce carbon dioxide production, these formulas contain >50% total calories from fat, with lower carbohydrate (<30%) and similar protein content (16–18%). Typically, they also contain ω-3 fatty acids derived from fish oil to increase the delivery of the anti-inflammatory properties of EPA/DHA. | Implement measures to avoid excessive enteral nutrition (EN) delivery, aiming to minimize complications linked to overfeeding. Exercise caution when considering the use of pulmonary formulas in septic and critically ill patients. |
| Immunonutrition/ immunomodulating | Contain pharmacologically active substances, such as arginine, glutamine, ω-3 fatty acids, γlinolenic acid, nucleotides, and/or antioxidants in efforts to modulate immune function. | Providing immune-modulating substances as part of enteral nutrition (EN) may offer potential benefits for patients undergoing elective surgery. However, the existing research is insufficient to endorse the routine use of immune-modulating formulas for critically ill patients. |
| Prebiotic | Health Benefits |
|---|---|
| FOS | Enhances gut health by fostering the proliferation and function of beneficial gut bacteria; aids in regulating blood sugar levels by delaying carbohydrate digestion; potentially lowers the risk of specific cancers; may contribute to better bone health through enhanced calcium absorption; and could diminish the risk of heart disease by lowering cholesterol levels and inflammation. |
| GOS | Enhances gut health by stimulating the growth and function of beneficial gut bacteria; alleviates the risk of constipation and related digestive problems by promoting regular bowel movements; boosts the immune system through heightened production of beneficial bacteria; lowers the likelihood of infections and inflammatory bowel conditions; and may aid in weight management by enhancing satiety and reducing calorie consumption. |
| ONSs Supplemented with TGF-β | Disease | Functions |
| IBD | Immunomodulatory roles especially relevant to the GI tract. | |
| Healing of the intestinal mucosa and in the development of fibrosis and stenosis through the regulation of fibroblast activity. |
| Oral Nutrition Supplements | Characteristics | Where Is It? | Functions |
|---|---|---|---|
| Arginine | Conditionally essential amino acid in situations such as developmental stages and certain pathological conditions (infection or inflammation, or under conditions of impaired renal and/or intestinal metabolic functions), where endogenous production is insufficient. | Seafood, watermelon juice, nuts, seeds, algae, meats, rice protein concentrate, and soy protein isolate. |
|
| Omega-3 | Are polyunsaturated fatty acids (PUFAs) characterized by a double bond at the n-3 position. These PUFAs include eicosapentaenoic acid (EPA), alpha-linolenic acid (ALA), and docosahexaenoic acid (DHA). Alpha-linolenic acid is essential (ALA). | EPA and DHA are mainly found in the marine ecosystem, fish and seafood, but also in leafy vegetables and nuts, which are famous for their high n-3 FA content, making them the main source of ALA. Leafy vegetables and nuts are also famous for their high n-3 FA content, making them the main source of ALA. |
|
| Nucleotides | Consist of a sugar molecule (ribose in RNA or deoxyribose in DNA), a phosphate group, and a nitrogen-containing base. The bases found in DNA are adenine (A), cytosine (C), guanine (G), and thymine (T), while RNA substitutes uracil (U) for thymine. Both DNA and RNA molecules are polymers composed of extended chains of nucleotides. | Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Naturally found in all foods of both animal and plant origin (healthy individuals typically consume 1–2 g of nucleotides daily through their diet), or through endogenous synthesis, which serves as the primary source of nucleotides. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchi, N.; Romanelli, R.; Ricevuti, F.; Carbone, M.G.; Dinardo, M.; Cesarano, E.; De Michele, A.; Messere, G.; Morra, S.; Scognamiglio, A.; et al. Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View. Nutrients 2024, 16, 2067. https://doi.org/10.3390/nu16132067
Cecchi N, Romanelli R, Ricevuti F, Carbone MG, Dinardo M, Cesarano E, De Michele A, Messere G, Morra S, Scognamiglio A, et al. Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View. Nutrients. 2024; 16(13):2067. https://doi.org/10.3390/nu16132067
Chicago/Turabian StyleCecchi, Nicola, Roberta Romanelli, Flavia Ricevuti, Maria Grazia Carbone, Michele Dinardo, Elisabetta Cesarano, Alfredo De Michele, Giovanni Messere, Salvatore Morra, Armando Scognamiglio, and et al. 2024. "Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View" Nutrients 16, no. 13: 2067. https://doi.org/10.3390/nu16132067
APA StyleCecchi, N., Romanelli, R., Ricevuti, F., Carbone, M. G., Dinardo, M., Cesarano, E., De Michele, A., Messere, G., Morra, S., Scognamiglio, A., & Spagnuolo, M. I. (2024). Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View. Nutrients, 16(13), 2067. https://doi.org/10.3390/nu16132067








