Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies
Abstract
1. Introduction
2. Materials and Methods
3. Polyunsaturated Fatty Acids (PUFAs)
- Reducing the expression of some growth factors such as HER2, EGFR, and insulin-like growth factor 1 (IGF-1R);
- Inhibiting cell proliferation by activating peroxisome proliferator-activated receptor gamma (PPARγ) or decreasing levels of fatty acid synthase (FAS) protein;
- promoting apoptosis by blocking phosphoinositide PI3K/Akt pathways, Akt phosphorylation, and NF-κB activity and reducing the B-cell lymphoma 2/B-cell lymphoma 2-like protein 4 (Bcl-2/Bax) ratio (Figure 1).
3.1. ω-3 PUFAs
3.1.1. α-Linolenic Acid
3.1.2. Eicosapentaenoic Acid
3.1.3. Docosahexaenoic Acid
3.2. ω-6 PUFAs
4. Bromelain
5. Glucosinolates
5.1. Sulforaphane
5.2. Indole-3 Carbinol
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Breast Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 13 March 2024).
- National Cancer Institute. Hormone Therapy for Breast Cancer. 2022. Available online: https://www.cancer.gov/types/breast/breast-hormone-therapy-fact-sheet (accessed on 13 March 2024).
- Arzanova, E.; Mayrovitz, H.N. The Epidemiology of Breast Cancer. In Breast Cancer, 1st ed.; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 1; pp. 1–19. [Google Scholar]
- Admoun, C.; Mayrovitz, H.N. The Etiology of Breast Cancer. In Breast Cancer, 1st ed.; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 2; pp. 21–30. [Google Scholar]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer, 1st ed.; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 3; pp. 31–42. [Google Scholar]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Baliu-Piqué, M.; Pandiella, A.; Ocana, A. Breast Cancer Heterogeneity and Response to Novel Therapeutics. Cancers 2020, 12, 3271. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting Breast Cancer: An Overlook on Current Strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.T.; Ibrahim, J.G.; Stevens, J.; Cleveland, R.; Abrahamson, P.E.; Satia, J.A.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Post diagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 2012, 23, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Marino, G.; Infusino, S.; Lanotte, L.; Gadaleta-Caldarola, G.; Gadaleta-Caldarola, G. The Mediterranean Lifestyle to Contrast Low-Grade Inflammation Behavior in Cancer. Nutrients 2023, 15, 1667. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M.; et al. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Ahmad, R. Potential phytocompounds for developing breast cancer therapeutics: Nature’s healing touch. Eur. J. Pharmacol. 2018, 827, 125–148. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.K.S.; Oz, F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured Lipid Carrier Co-loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol. Imaging Biol. 2018, 20, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. EPR Effect-Based Tumor Targeted Nanomedicine: A Promising Approach for Controlling Cancer. J. Pers. Med. 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Huang, M.; Zhai, B.T.; Fan, Y.; Sun, J.; Shi, Y.J.; Zhang, X.F.; Zou, J.B.; Wang, J.W.; Guo, D.Y. Targeted drug delivery systems for curcumin in breast cancer therapy. Int. J. Nanomed. 2023, 18, 4275–4311. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Chénais, B.; Blanckaert, V. The janus face of lipids in human breast cancer: How polyunsaturated Fatty acids affect tumor cell hallmarks. Int. J. Breast Cancer 2012, 2012, 712536. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Maheo, K.; Couet, C.; Chevalier, S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog. Lipid Res. 2010, 49, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Kuran, D.; Pogorzelska, A.; Wiktorska, K. Breast Cancer Prevention-Is there a Future for Sulforaphane and Its Analogs? Nutrients 2020, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Wei, W.; Li, X. Effects of fish oil supplementation on cardiac function in chronic heart failure: A meta-analysis of randomised controlled trials. Heart 2012, 98, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef]
- Kar, S.; Webel, R. Fish oil supplementation & coronary artery disease: Does it help? Mo Med. 2012, 109, 142. [Google Scholar]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107, S171–S184. [Google Scholar] [CrossRef] [PubMed]
- Rudkowska, I. Fish oils for cardiovascular disease: Impact on diabetes. Maturitas 2010, 67, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Kim, W.; Lupton, J.R.; McMurray, D.N. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leukot Essent Fat. Acids 2009, 81, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Hassing, M.R.; Lewandowski, P.A. Marine polyunsaturated fatty acids and cancer therapy. Br. J. Cancer 2013, 108, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.A.; Deckelbaum, R.J. Sources of the very-long-chain unsaturated omega-3 fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, S.I.; Subbaiah, P.V.; Victor, T.A.; Miller, W.M. n-3 and n-6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br. J. Cancer 1994, 70, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, P.; Zhu, L.; Zhuang, W.; Jiang, L.; Zhang, H.; Huang, H. Enhanced in vitro antitumor efficacy of a polyunsaturated fatty acid-conjugated pH-responsive self-assembled ion-pairing liposome-encapsulated prodrug. Nanotechnology 2020, 31, 155101. [Google Scholar] [CrossRef]
- Truan, J.S.; Chen, J.M.; Thompson, L.U. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol. Nutr. Food Res. 2010, 54, 1414–1421. [Google Scholar] [CrossRef]
- Saggar, J.K.; Chen, J.; Corey, P.; Thompson, L.U. Dietary flaxseed lignan or oil combined with tamoxifen treatment affects MCF-7 tumor growth through estrogen receptor-and growth factor-signalling pathways. Mol. Nutr. Food Res. 2010, 54, 415–425. [Google Scholar] [CrossRef]
- Xu, M.Q.; Hao, Y.L.; Wang, J.R.; Li, Z.Y.; Li, H.; Feng, Z.H.; Wang, H.; Wang, J.W.; Zhang, X. Antitumor activity of α-linolenic acid-paclitaxel conjugate nanoparticles: In vitro and in vivo. Int. J. Nanomed. 2021, 16, 7269–7281. [Google Scholar] [CrossRef]
- Patterson, R.E.; Flatt, S.W.; Newman, V.A.; Natarajan, L.; Rock, C.L.; Thomson, C.A.; Caan, B.J.; Parker, B.A.; Pierce, J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr. 2011, 141, 201–206. [Google Scholar] [CrossRef]
- DeGraffenried, L.A.; Friedrichs, W.E.; Fulcher, L.; Fernandes, G.; Silva, J.M.; Peralba, J.M.; Hidalgo, M. Eicosapentaenoic acid restores tamoxifen sensitivity in breast cancer cells with high Akt activity. Ann. Oncol. 2003, 14, 1051–1056. [Google Scholar] [CrossRef]
- Rose, D.P.; Connolly, J.M.; Rayburn, J.; Coleman, M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995, 87, 587–592. [Google Scholar] [CrossRef]
- Sauer, L.A.; Dauchy, R.T.; Blask, D.E.; Krause, J.A.; Davidson, L.K.; Dauchy, E.M. Eicosapentaenoic acid suppresses cell proliferation in MCF-7 human breast cancer xenografts in nude rats via a pertussis toxin–sensitive signal transduction pathway. J. Nutr. 2005, 135, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Chambrier, C.; Bastard, J.P.; Rieusset, J.; Chevillotte, E.; Bonnefont-Rousselot, D.; Therond, P.; Hainque, B.; Riou, J.P.; Laville, M.; Vidal, H. Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor γ. Obes. Res. 2002, 10, 518–525. [Google Scholar] [CrossRef]
- Merendino, N.; Costantini, L.; Manzi, L.; Molinari, R.; D’Eliseo, D.; Velotti, F. Dietary ω-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomed. Res. Int. 2013, 2013, 310186. [Google Scholar] [CrossRef] [PubMed]
- Chamras, H.; Ardashian, A.; Heber, D.; Glaspy, J.A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 2002, 13, 711–716. [Google Scholar] [CrossRef]
- Spencer, L.; Mann, C.; Metcalfe, M.; Webb, M.B.; Pollard, C.; Spencer, D.; Berry, D.; Steward, W.; Dennison, A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer 2009, 45, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Manzi, L.; Merendino, N.; Velotti, F. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J. Nutr. Biochem. 2012, 23, 452–457. [Google Scholar] [CrossRef]
- Horia, E.; Watkins, B.A. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE 2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis 2007, 28, 809–815. [Google Scholar] [CrossRef]
- Barascu, A.; Besson, P.; Le Floch, O.; Bougnoux, P.; Jourdan, M.L. CDK1-cyclin B1 mediates the inhibition of proliferation induced by omega-3 fatty acids in MDA-MB-231 breast cancer cells. Int. J. Biochem. Cell Biol. 2006, 38, 196–208. [Google Scholar] [CrossRef]
- Ravacci, G.R.; Brentani, M.M.; Tortelli, T., Jr.; Torrinhas, R.S.M.; Saldanha, T.; Torres, E.A.F.; Waitzberg, D.L. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J. Nutr. Biochem. 2013, 24, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous supplementation with ω-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22, 6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Altern. Med. Rev. 2010, 15, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.C.M.; Wong, E.Y.L.; Ooi, V.E. Docosahexaenoic Acid from a Cultured Microalga Inhibits Cell Growth and Induces Apoptosis by Upregulating Bax/Bcl-2 Ratio in Human Breast Carcinoma MCF-7 Cells. Ann. N. Y. Acad. Sci. 2004, 1030, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.L.; Mahéo, K.; Barascu, A.; Goupille, C.; De Latour, M.P.; Bougnoux, P.; Rio, P.G. Increased BRCA1 protein in mammary tumours of rats fed marine ω-3 fatty acids. Oncol. Rep. 2007, 17, 713–719. [Google Scholar] [CrossRef]
- Bernard-Gallon, D.J.; Vissac-Sabatier, C.; Antoine-Vincent, D.; Rio, P.G.; Maurizis, J.C.; Fustier, P.; Bignon, Y.J. Differential effects of n-3 and n-6 polyunsaturated fatty acids on BRCA1 and BRCA2 gene expression in breast cell lines. Br. J. Nutr. 2002, 87, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.R.; Kikawa, K.D.; Mouradian, M.; Hernandez, K.; McKinnon, K.M.; Ahwah, S.M.; Pardini, R.S. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis 2010, 31, 1523–1530. [Google Scholar] [CrossRef]
- Colas, S.; Mahéo, K.; Denis, F.; Goupille, C.; Hoinard, C.; Champeroux, P.; Tranquart, F.; Bougnoux, P. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: A role for tumor vascularization. Clin. Cancer Res. 2006, 12, 5879–5886. [Google Scholar] [CrossRef]
- Signori, C.; DuBrock, C.; Richie, J.P.; Prokopczyk, B.; Demers, L.M.; Hamilton, C.; Hartman, T.J.; Liao, J.; El-Bayoumy, K.; Manni, A. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: Interim feasibility and biomarkers analysis from a clinical trial. Eur. J. Clin. Nutr. 2012, 66, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.D.; Campos, M.M. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ghosh-Choudhury, T.; Dey, N.; Choudhury, G.G.; Ghosh-Choudhury, N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis 2012, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Russo, J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta 2012, 1826, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Mouradian, M.; Kikawa, K.D.; Dranka, B.P.; Komas, S.M.; Kalyanaraman, B.; Pardini, R.S. Docosahexaenoic acid attenuates breast cancer cell metabolism and the Warburg phenotype by targeting bioenergetic function. Mol. Carcinog. 2015, 54, 810–820. [Google Scholar] [CrossRef]
- Manzi, L.; Costantini, L.; Molinari, R.; Merendino, N. Effect of dietary ω-3 polyunsaturated fatty acid DHA on glycolytic enzymes and warburg phenotypes in cancer. Biomed. Res. Int. 2015, 2015, 137097. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lin, M. The synthesis of nano-doxorubicin and its anticancer effect. Anticancer. Agents Med. Chem. 2021, 21, 2466–2477. [Google Scholar] [CrossRef]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.; Torchilin, V.P. Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef]
- Khankari, N.K.; Bradshaw, P.T.; Steck, S.E.; He, K.; Olshan, A.F.; Shen, J.; Ahn, J.; Chen, Y.; Ahsan, H.; Terry, M.B.; et al. Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer 2015, 121, 2244–2252. [Google Scholar] [CrossRef]
- Zou, Z.; Bidu, C.; Bellenger, S.; Narce, M.; Bellenger, J. n-3 polyunsaturated fatty acids and HER2-positive breast cancer: Interest of the fat-1 transgenic mouse model over conventional dietary supplementation. Biochimie 2014, 96, 22–27. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M. Omega-3 fatty acids and cancers: A systematic update review of epidemiological studies. Br. J. Nutr. 2012, 107, S228–S239. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.; Mokbel, K. Chemoprevention of breast cancer with vitamins and micronutrients: A concise review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ren, X.L.; Fu, Y.Q.; Gao, J.L.; Li, D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: A meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
- Agrawal, P.; Nikhade, P.; Patel, A.; Mankar, N.; Sedani, S. Bromelain: A Potent Phytomedicine. Cureus 2022, 14, e27876. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Jiménez-Garcia, M.; Capó, X.; Sönmez Gürer, E.; Sharopov, F.; Rachel, T.Y.L.; Ntieche Woutouoba, D.; Rescigno, A.; Peddio, S.; Zucca, P.; et al. Anticancer properties of bromelain: State-of-the-art and recent trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef] [PubMed]
- Chobotova, K.; Vernallis, A.B.; Majid, F.A. Bromelain’s activity and potential as an anti-cancer agent: Current evidence and perspectives. Cancer Lett. 2010, 290, 148–156. [Google Scholar] [CrossRef]
- Ramli, A.N.; Aznan, T.N.; Illias, R.M. Bromelain: From production to commercialisation. J. Sci. Food Agric. 2017, 97, 1386–1395. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- De Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Shraddha Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.K.; Dunna, N.R.; Venkatabalasubramanian, S. A comprehensive overview on the anti-inflammatory, antitumor, and ferroptosis functions of bromelain: An emerging cysteine protease. Expert. Opin. Biol. Ther. 2022, 22, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mangla, B.; Javed, S.; Ahsan, W.; Kumar, P.; Garg, V.; Dureja, H. Bromelain: A review of its mechanisms, pharmacological effects and potential applications. Food Funct. 2023, 14, 8101–8128. [Google Scholar] [CrossRef] [PubMed]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef]
- Huang, J.R.; Wu, C.C.; Hou, R.C.; Jeng, K.C. Bromelain inhibits lipopolysaccharide-induced cytokine production in human THP-1 monocytes via the removal of CD14. Immunol. Investig. 2008, 37, 263–277. [Google Scholar] [CrossRef]
- Bhui, K.; Prasad, S.; George, J.; Shukla, Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009, 282, 167–176. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-B.; Lee, J.-T.; Park, H.-R.; Kim, J.-B. Medicinal Effects of Bromelain (Ananas comosus) Targeting Oral Environment as an Anti-oxidant and Anti-inflammatory Agent. J. Food Nutr. Res. 2018, 6, 773–784. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Vivithanaporn, P.; Unchern, S. Anti-inflammatory effects of bromelain in Lps-induced human U937 macrophages. Chiang Mai J. Sci. 2018, 45, 299–307. [Google Scholar]
- Raeisi, F.; Raeisi, E.; Heidarian, E.; Shahbazi-Gahroui, D.; Lemoigne, Y. Bromelain inhibitory effect on colony formation: An in vitro study on human AGS, PC3, and MCF7 cancer cells. J. Med. Signals Sens. 2019, 9, 267–273. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, S.; Sun, Y.; Matt, Y.; Colburn, N.H.; Shu, Y.; Han, X. JNK/AP-1 pathway is involved in tumor necrosis factor-alpha induced expression of vascular endothelial growth factor in MCF7 cells. Biomed. Pharmacother. 2009, 63, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Greer, P.K.; Sempowski, G.D. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin. Immunol. 2002, 104, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.R., Jr.; Singh, A.; Guernsey, L.A.; McNamara, J.T.; Zhan, L.; Maulik, N.; Thrall, R.S. Bromelain treatment reduces CD25 expression on activated CD4+ T cells in vitro. Int. Immunopharmacol. 2009, 9, 340–346. [Google Scholar] [CrossRef]
- Fouz, N.; Amid, A.; Hashim, Y.Z. Cytokinetic study of MCF-7 cells treated with commercial and recombinant bromelain. Asian Pac. J. Cancer Prev. 2014, 14, 6709–6714. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Prado, W.A.; Lavayen, V.; Büttenbender, S.L.; Beckenkamp, A.; Martins, B.S.; Lüdtke, D.S.; Campo, L.F.; Rodembusch, F.S.; Buffon, A.; et al. Bromelainfunctionalized multiple-wall lipid-core nanocapsules: Formulation, chemical structure and antiproliferative effect against human breast cancer cells (MCF-7). Pharm. Res. 2017, 34, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, E.; Aazami, M.H.; Aghamiri, S.M.; Satari, A.; Hosseinzadeh, S.; Lemoigne, Y.; Esfandiar, H. Bromelain-based chemo-herbal combination effect on human cancer cells: In-vitro study on AGS and MCF7 proliferation and apoptosis. Curr. Issues Pharm. Med. Sci. 2020, 33, 155–161. [Google Scholar] [CrossRef]
- Karimian Rad, F.; Ramezani, M.; Mohammadgholi, A. Physicochemical properties of bromelain adsorption on magnetic carbon nanoparticles and in vitro cytotoxicity on breast cancer cells. Herb. Med. J. 2020, 5, 153–162. [Google Scholar]
- Pauzi, A.Z.; Yeap, S.K.; Abu, N.; Lim, K.L.; Omar, A.R.; Aziz, S.A.; Chow, A.L.; Subramani, T.; Tan, S.G.; Alitheen, N.B. Combination of cisplatin and bromelain exerts synergistic cytotoxic effects against breast cancer cell line MDA-MB-231 in vitro. Chin. Med. 2016, 11, 46. [Google Scholar] [CrossRef]
- Bhui, K.; Tyagi, S.; Prakash, B.; Shukla, Y. Pineapple bromelain induces autophagy, facilitating apoptotic response in mammary carcinoma cells. Biofactors 2010, 36, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Patnaik, S.; Srivastava, A.K.; Mudiam, M.K.; Shukla, Y.; Panda, A.K.; Pant, A.B.; Kumar, P.; Gupta, K.C. Anti-cancer activity of bromelain nanoparticles by oral administration. J. Biomed. Nanotechnol. 2014, 10, 3558–3575. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, S.; Funing, M.; Keming, L.; Wei, S.; Guiying, X.; Rulin, Z.; Shenghe, C. Growth of breast cancer cells inhibited by bromelains extracted from the different tissues of pineapple. Folia Biol. 2020, 68, 81–88. [Google Scholar] [CrossRef]
- Dhandayuthapani, S.; Perez, H.D.; Paroulek, A.; Chinnakkannu, P.; Kandalam, U.; Jaffe, M.; Rathinavelu, A. Bromelain-induced apoptosis in GI-101A breast cancer cells. J. Med. Food 2012, 15, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mekkawy, M.H.; Fahmy, H.A.; Nada, A.S.; Ali, O.S. Radiosensitizing effect of bromelain using tumor mice model via Ki-67 and PARP-1 inhibition. Integr. Cancer Ther. 2021, 20, 15347354211060369. [Google Scholar] [CrossRef]
- Gao, L.; Loveless, J.; Shay, C.; Teng, Y. Targeting ROS-Mediated Crosstalk Between Autophagy and Apoptosis in Cancer. Adv. Exp. Med. Biol. 2020, 1260, 1–12. [Google Scholar]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Puente-Rivera, J.; Silva-Cázares, M.B. Molecular pathways related to sulforaphane as adjuvant treatment: A nanomedicine perspective in breast cancer. Medicina 2022, 58, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Khoshkbejari, M.A.; Mohammadi, M.; Abiri, A.; Mokhtarian, R.; Vazifemand, R.; Xiao, Z. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer–contradictory effects and future perspectives. Biomed. Pharmacother. 2020, 121, 109635. [Google Scholar]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble? Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, D.Y.; Cheng, L.J.; Bayrak, M.; Jegasothy, H.; Sanguansri, L.; Augustin, M.A. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil. LWT 2020, 128, 109443. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Farnham, M.W.; Stephenson, K.K.; Fahey, J.W. Glucoraphanin level in broccoli seed is largely determined by genotype. Hortic. Sci. 2005, 40, 50–53. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kwan, M.L.; Pratt, R.; Roh, J.M.; Kushi, L.H.; Danforth, K.N.; Zhang, Y.; Ambrosone, C.B.; Tang, L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci. Nutr. 2020, 8, 5673–5682. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. The impact of domestic cooking methods on myrosinase stability, glucosinolates and their hydrolysis products in different cabbage (Brassica oleracea) accessions. Foods 2021, 10, 2908. [Google Scholar] [CrossRef]
- Dosz, E.B.; Jeffery, E.H. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J. Funct. Foods 2013, 5, 987–990. [Google Scholar] [CrossRef]
- Bricker, G.V.; Riedl, K.M.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.M.; Schwartz, S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Ho, E. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Treasure, K.; Harris, J.; Williamson, G. Exploring the anti-inflammatory activity of sulforaphane. Immunol. Cell Biol. 2023, 101, 805–828. [Google Scholar] [CrossRef]
- Licznerska, B.; Szaefer, H.; Krajka-Kuźniak, V. R-sulforaphane modulates the expression profile of AhR, ERα, Nrf2, NQO1, and GSTP in human breast cell lines. Mol. Cell Biochem. 2021, 476, 525–533. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.J.; Moon, D.O. Sulforaphane sensitizes human breast cancer cells to paclitaxel-induced apoptosis by downregulating the NF-κB signaling pathway. Oncol. Lett. 2017, 13, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Hunakova, L.; Sedlakova, O.; Cholujova, D.; Gronesova, P.; Duraj, J.; Sedlak, J. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma 2009, 56, 548. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, X.; Li, Y.; Min, H.; Zhao, X.; Zhang, Y.; Qi, Y.; Shi, J.; Qi, S.; Bao, Y.; et al. Sulforaphane Mediates Glutathione Depletion via Polymeric Nanoparticles to Restore Cisplatin Chemosensitivity. ACS Nano. 2019, 13, 13445–13455. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Huang, L.; Yi, K.; Chen, H.; Liu, S.; Zhang, W.; Wang, F. Co-administration of sulforaphane and doxorubicin attenuates breast cancer growth by preventing the accumulation of myeloid-derived suppressor cells. Cancer Lett. 2020, 493, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Keshandehghan, A.; Nikkhah, S.; Tahermansouri, H.; Heidari-Keshel, S.; Gardaneh, M. Co-treatment with sulforaphane and nano-metformin molecules accelerates apoptosis in HER2+ breast cancer cells by inhibiting key molecules. Nutr. Cancer 2020, 72, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelska, A.; Mazur, M.; Świtalska, M.; Wietrzyk, J.; Sigorski, D.; Fronczyk, K.; Wiktorska, K. Anticancer effect and safety of doxorubicin and nutraceutical sulforaphane liposomal formulation in triple-negative breast cancer (TNBC) animal model. Biomed. Pharmacother. 2023, 161, 114490. [Google Scholar] [CrossRef]
- Kanematsu, S.; Uehara, N.; Miki, H.; Yoshizawa, K.; Kawanaka, A.; Yuri, T.; Tsubura, A. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer. Res. 2010, 30, 3381–3390. [Google Scholar] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence are accompanied by DNA Hypomethylation and Changes in microRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef]
- Cao, W.; Lu, X.; Zhong, C.; Wu, J. Sulforaphane suppresses MCF-7 breast cancer cells growth via miR-19/PTEN axis to antagonize the effect of butyl benzyl phthalate. Nutr. Cancer 2023, 75, 980–991. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxid. Med. Cell Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Tollefsbol, T.O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 2010, 5, e11457. [Google Scholar] [CrossRef]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer Prev. Res. 2018, 11, 451–464. [Google Scholar] [CrossRef]
- Amarakoon, D.; Lee, W.J.; Tamia, G.; Lee, S.H. Indole-3-Carbinol: Occurrence, Health-Beneficial Properties, and Cellular/Molecular Mechanisms. Annu. Rev. Food Sci. Technol. 2023, 14, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Lachnitt, N.; Rudzitis-Auth, J.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. Indole-3-carbinol is a potent inhibitor of ischemia-reperfusion-induced inflammation. J. Surg. Res. 2017, 215, 34–46. [Google Scholar] [CrossRef]
- Khan, A.S.; Langmann, T. Indole-3-carbinol regulates microglia homeostasis and protects the retina from degeneration. J. Neuroinflamm. 2020, 17, 327. [Google Scholar] [CrossRef]
- Prado, N.J.; Ramirez, D.; Mazzei, L.; Parra, M.; Casarotto, M.; Calvo, J.P.; Cuello Carrión, D.; Ponce Zumino, A.Z.; Diez, E.R.; Camargo, A.; et al. Anti-inflammatory, antioxidant, anti-hypertensive, and antiarrhythmic effect of indole-3-carbinol, a phytochemical derived from cruciferous vegetables. Heliyon 2022, 8, e08989. [Google Scholar] [CrossRef]
- Peng, C.; Wu, C.; Xu, X.; Pan, L.; Lou, Z.; Zhao, Y.; Jiang, H.; He, Z.; Ruan, B. Indole-3-carbinol ameliorates necroptosis and inflammation of intestinal epithelial cells in mice with ulcerative colitis by activating aryl hydrocarbon receptor. Exp. Cell Res. 2021, 404, 112638. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Yang, C.; Lu, M.; Bao, J.; Shen, H.; Deng, B.; Li, S.; Li, W.; Zhang, M.; Cao, C. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous Aβ catabolic enzyme neprilysin. Theranostics 2021, 11, 8797–8812. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, K.A.; El-Naga, R.N.; Wahdan, S.A. Neuroprotective effects of indole-3-carbinol on the rotenone rat model of Parkinson’s disease: Impact of the SIRT1-AMPK signaling pathway. Toxicol. Appl. Pharmacol. 2022, 435, 115853. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Akhtar, A.; Chauhan, M.; Dhingra, N.; Pilkhwal Sah, S. Protective effect of indole-3-carbinol, an NF-κB inhibitor in experimental paradigm of Parkinson’s disease: In silico and in vivo studies. Brain Behav. Immun. 2020, 90, 108–137. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.P.; Word, B.; Taylor, S.; Hammons, G.J.; Lyn-Cook, B.D. Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention: Effects of indole-3-carbinol (I3C) and genistein. Anticancer. Res. 2004, 24, 133–137. [Google Scholar] [PubMed]
- Adler, S.; Rashid, G.; Klein, A. Indole-3-carbinol inhibits telomerase activity and gene expression in prostate cancer cell lines. Anticancer. Res. 2011, 31, 3733–3737. [Google Scholar] [PubMed]
- Megna, B.W.; Carney, P.R.; Nukaya, M.; Geiger, P.; Kennedy, G.D. Indole-3-carbinol induces tumor cell death: Function follows form. J. Surg. Res. 2016, 204, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, J.; Nam, M.J.; Park, S.H. Indole-3-carbinol induces apoptosis in human osteosarcoma MG-63 and U2OS cells. Biomed. Res. Int. 2018, 2018, 7970618. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Park, S.H.; Nam, M.J. Anticarcinogenic effect of indole-3-carbinol (I3C) on human hepatocellular carcinoma SNU449 cells. Hum. Exp. Toxicol. 2019, 38, 136–147. [Google Scholar] [CrossRef]
- Shertzer, H.G.; Berger, M.L.; Tabor, M.W. Intervention in free radical mediated hepatotoxicity and lipid peroxidation by indole-3-carbinol. Biochem. Pharmacol. 1988, 37, 333–338. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.M.; Park, S.H.; Nam, M.J. Induction of apoptosis in indole-3-carbinol-treated lung cancer H1299 cells via ROS level elevation. Hum. Exp. Toxicol. 2021, 40, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Baez-Gonzalez, A.S.; Carrazco-Carrillo, J.A.; Figueroa-Gonzalez, G.; Quintas-Granados, L.I.; Padilla-Benavides, T.; Reyes-Hernandez, O.D. Functional effect of indole-3 carbinol in the viability and invasive properties of cultured cancer cells. Biochem. Biophys. Rep. 2023, 35, 101492. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Li, Y.; Sarkar, F.H. Inactivation of akt and NF-kappaB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr. Cancer 2004, 48, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Gallacher-Horley, B.; Houghton, C.E.; Manson, M.M.; Hudson, E.A. Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol. Cancer Ther. 2002, 1, 1161–1172. [Google Scholar]
- Cover, C.M.; Hsieh, S.J.; Tran, S.H.; Hallden, G.; Kim, G.S.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J. Biol. Chem. 1998, 273, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Cram, E.J.; Liu, B.D.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J. Biol. Chem. 2001, 276, 22332–22340. [Google Scholar] [CrossRef] [PubMed]
- Firestone, G.L.; Bjeldanes, L.F. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J. Nutr. 2003, 133 (Suppl. 7), 2448S–2455S. [Google Scholar] [CrossRef]
- Cover, C.M.; Hsieh, S.J.; Cram, E.J.; Hong, C.; Riby, J.E.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res. 1999, 59, 1244–1251. [Google Scholar]
- Hajra, S.; Patra, A.R.; Basu, A.; Saha, P.; Bhattacharya, S. Indole-3-Carbinol (I3C) enhances the sensitivity of murine breast adenocarcinoma cells to doxorubicin (DOX) through inhibition of NF-κβ, blocking angiogenesis and regulation of mitochondrial apoptotic pathway. Chem. Biol. Interact. 2018, 290, 19–36. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Dai, Q.; Si, H.; Zhang, L.; Eltom, S.E.; Si, H. Combined Luteolin and Indole-3-Carbinol Synergistically Constrains ERα-Positive Breast Cancer by Dual Inhibiting Estrogen Receptor Alpha and Cyclin-Dependent Kinase 4/6 Pathway in Cultured Cells and Xenograft Mice. Cancers 2021, 13, 2116. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Aranha, O.; Sarkar, F.H. Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr. Cancer 2003, 45, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.A.; Campana, R.; Wei, C.; Su, C.H.; Hanks, A.M.; Bornmann, W.G.; Keyomarsi, K. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERα-positive breast cancer cells. Cell Cycle 2014, 13, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Safe, S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol. Lett. 2001, 120, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Tsai, C.H.; Kulp, S.K.; Chen, C.S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kanno, Y.; Yamashita, N.; Degawa, M.; Yoshinari, K.; Nemoto, K. The Differential Selectivity of Aryl Hydrocarbon Receptor (AHR) Agonists towards AHR-Dependent Suppression of Mammosphere Formation and Gene Transcription in Human Breast Cancer Cells. Biol. Pharm. Bull. 2021, 44, 571–578. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology studies of indole-3-carbinol in F344/N rats and B6C3F1/N mice and toxicology and carcinogenesis studies of indole-3-carbinol in Harlan Sprague Dawley rats and B6C3F1/N mice (gavage studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2017, 584, NTP-TR-584. [Google Scholar]
- Hargraves, K.G.; He, L.; Firestone, G.L. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol. Carcinog. 2016, 55, 486–498. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Gamal-Eldeen, A.M.; Gouhar, S.A.; Abo-Elfadl, M.T.; El-Saeed, G. Modulatory Effect of Indoles on the Expression of miRNAs Regulating G1/S Cell Cycle Phase in Breast Cancer Cells. Appl. Biochem. Biotechnol. 2020, 192, 1208–1223. [Google Scholar] [CrossRef] [PubMed]
- Nouri Emamzadeh, F.; Word, B.; Cotton, E.; Hawkins, A.; Littlejohn, K.; Moore, R.; Miranda-Carbon, G.; Orish, C.N.; Lyn-Cook, B. Modulation of Estrogen α and Progesterone Receptors in Triple Negative Breast Cancer Cell Lines: The Effects of Vorinostat and Indole-3-Carbinol In Vitro. Anticancer. Res. 2020, 40, 3669–3683. [Google Scholar] [CrossRef] [PubMed]
- Grose, K.R.; Bjeldanes, L.F. Oligomerization of indole-3-carbinol in aqueous acid. Chem. Res. Toxicol. 1992, 5, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, T.T.; Teng, Z.; Chen, P.; Sun, J.; Wang, Q. Encapsulation of indole-3-carbinol and 3,3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013, 139, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Gehrcke, M.; Giuliani, L.M.; Ferreira, L.M.; Barbieri, A.V.; Sari, M.H.M.; da Silveira, E.F.; Azambuja, J.H.; Nogueira, C.W.; Braganhol, E.; Cruz, L. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mat. Sci. Eng. C-Mater 2017, 74, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.A.; Arneson, D.W.; Putnam, W.C.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethan. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yannai, S.; Rennert, G.; Gruener, N.; Fares, F.A. 3,3′-Diindolylmethane induces apoptosis in human cancer cells. Biochem. Biophys. Res. Commun. 1996, 228, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Kim, H.A.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis 2002, 23, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Firestone, G.L.; Bjeldanes, L.F. Bcl-2 family-mediated apoptotic effects of 3,3′-diindolylmethane (DIM) in human breast cancer cells. Biochem. Pharmacol. 2002, 63, 1085–1097. [Google Scholar] [CrossRef]
- Rahman, K.W.; Sarkar, F.H. Inhibition of nuclear translocation of nuclear factor-{kappa}B contributes to 3,3′-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res. 2005, 65, 364–371. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, S.; Wang, Z.; Ali, A.S.; Sethi, S.; Sakr, W.A.; Raz, A.; Rahman, K.M. 3,3′-Diindolylmethane enhances taxotere-induced growth inhibition of breast cancer cells through downregulation of FoxM1. Int. J. Cancer 2011, 129, 1781–1791. [Google Scholar] [CrossRef]
- Hsu, E.L.; Chen, N.; Westbrook, A.; Wang, F.; Zhang, R.; Taylor, R.T.; Hankinson, O. CXCR4 and CXCL12 down-regulation: A novel mechanism for the chemoprotection of 3,3′-diindolylmethane for breast and ovarian cancers. Cancer Lett. 2008, 265, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. 3,3′-Diindolylmethane Inhibits TNF-α- and TGF-β-Induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. Nutr. Cancer 2019, 71, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Sohn, H.; Xue, L.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane is a novel mitochondrial H(+)-ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006, 66, 4880–4887. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Firestone, G.L.; Bjeldanes, L.F. DIM stimulates IFNgamma gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene 2005, 24, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol. Cancer 2015, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y. 3,3′-Diindolylmethane inhibits breast cancer cell growth via miR-21-mediated Cdc25A degradation. Mol. Cell Biochem. 2011, 358, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, S.V.; Alekseev, B.Y.; Sergeeva, N.S.; Karalkin, P.A.; Nezhurina, E.K.; Kirsanova, V.A.; Sviridova, I.K.; Akhmedova, S.A.; Volchenko, N.N.; Bolotina, L.V.; et al. Breast cancer organoid model allowed to reveal potentially beneficial combinations of 3,3′-diindolylmethane and chemotherapy drugs. Biochimie 2020, 179, 217–227. [Google Scholar] [CrossRef]
- Harakeh, S.; Akefe, I.O.; Saber, S.H.; Alamri, T.; Al-Raddadi, R.; Al-Jaouni, S.; Tashkandi, H.; Qari, M.; Moulay, M.; Aldahlawi, A.; et al. Nanoformulated 3′-diindolylmethane modulates apoptosis, migration, and angiogenesis in breast cancer cells. Heliyon 2023, 10, e23553. [Google Scholar] [CrossRef]
- Isabella, S.; Mirunalini, S. 3,3′-Diindolylmethane-encapsulated chitosan nanoparticles accelerate molecular events during chemical carcinogen-induced mammary cancer in Sprague Dawley rats. Breast Cancer 2019, 26, 499–509. [Google Scholar] [CrossRef]
 ) cell proliferation by reducing (
) cell proliferation by reducing ( ) the expression of some growth factors, including human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1R), by either activating (
) the expression of some growth factors, including human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1R), by either activating ( ) peroxisome proliferator-activated receptor gamma (PPARγ) or decreasing levels of fatty acid synthase (FAS) protein. Furthermore, PUFAs promote cell apoptosis by blocking phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathways, downregulating phosphorylated Akt, inhibiting nuclear factor-kappa B (NF-κB) activity, and lowering B-cell lymphoma 2/B-cell lymphoma 2-like protein 4 (Bcl-2/Bax ratio). Created with BioRender.com (https://app.biorender.com/illustrations/65fc51a4a6668e33e3bf3d89 (accessed on 9 April 2024)).
) peroxisome proliferator-activated receptor gamma (PPARγ) or decreasing levels of fatty acid synthase (FAS) protein. Furthermore, PUFAs promote cell apoptosis by blocking phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathways, downregulating phosphorylated Akt, inhibiting nuclear factor-kappa B (NF-κB) activity, and lowering B-cell lymphoma 2/B-cell lymphoma 2-like protein 4 (Bcl-2/Bax ratio). Created with BioRender.com (https://app.biorender.com/illustrations/65fc51a4a6668e33e3bf3d89 (accessed on 9 April 2024)).
 ) cell proliferation by reducing (
) cell proliferation by reducing ( ) the expression of some growth factors, including human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1R), by either activating (
) the expression of some growth factors, including human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1R), by either activating ( ) peroxisome proliferator-activated receptor gamma (PPARγ) or decreasing levels of fatty acid synthase (FAS) protein. Furthermore, PUFAs promote cell apoptosis by blocking phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathways, downregulating phosphorylated Akt, inhibiting nuclear factor-kappa B (NF-κB) activity, and lowering B-cell lymphoma 2/B-cell lymphoma 2-like protein 4 (Bcl-2/Bax ratio). Created with BioRender.com (https://app.biorender.com/illustrations/65fc51a4a6668e33e3bf3d89 (accessed on 9 April 2024)).
) peroxisome proliferator-activated receptor gamma (PPARγ) or decreasing levels of fatty acid synthase (FAS) protein. Furthermore, PUFAs promote cell apoptosis by blocking phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathways, downregulating phosphorylated Akt, inhibiting nuclear factor-kappa B (NF-κB) activity, and lowering B-cell lymphoma 2/B-cell lymphoma 2-like protein 4 (Bcl-2/Bax ratio). Created with BioRender.com (https://app.biorender.com/illustrations/65fc51a4a6668e33e3bf3d89 (accessed on 9 April 2024)).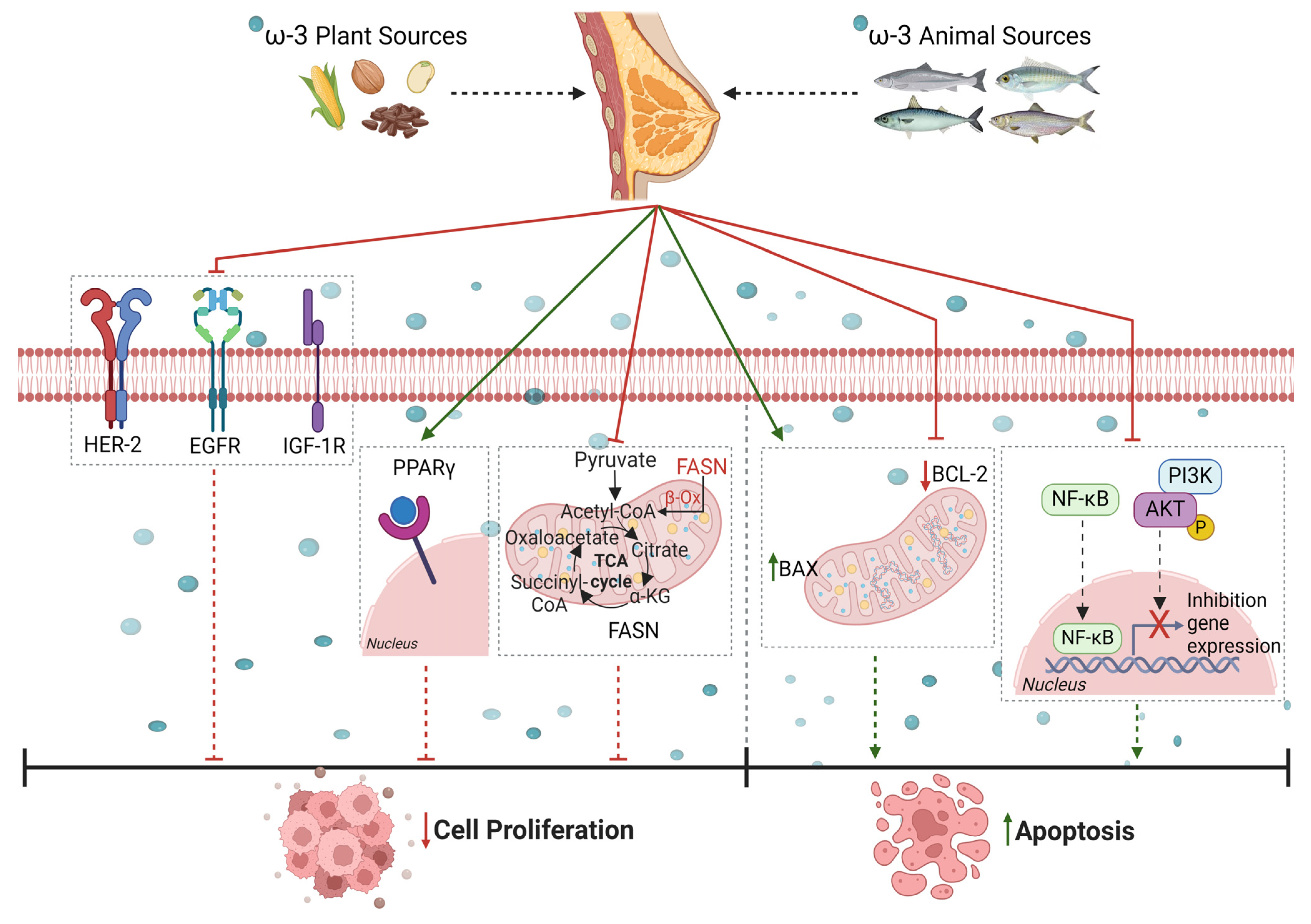
 ) cytotoxicity and reduces (
) cytotoxicity and reduces ( ) cancer cell proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the mitogen-activated protein kinase (MAPK) signaling pathway, extracellular signal-related kinase (ERK), nuclear factor-kappa B (NF-κB), c-Jun N-terminal kinase (JNK), serine/threonine protein kinase (Akt), microtubule-associated protein B-light chain 3 (LC3), and p38; (II) inducing cell death by apoptosis/autophagy via Bcl-2-associated X (BAX), B-cell lymphoma 2 (Bcl-2), caspases-3 and -9, reactive oxygen species (ROS), p53, LC3, and BECLIN 1; (III) blocking (
) cancer cell proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the mitogen-activated protein kinase (MAPK) signaling pathway, extracellular signal-related kinase (ERK), nuclear factor-kappa B (NF-κB), c-Jun N-terminal kinase (JNK), serine/threonine protein kinase (Akt), microtubule-associated protein B-light chain 3 (LC3), and p38; (II) inducing cell death by apoptosis/autophagy via Bcl-2-associated X (BAX), B-cell lymphoma 2 (Bcl-2), caspases-3 and -9, reactive oxygen species (ROS), p53, LC3, and BECLIN 1; (III) blocking ( ) the cell cycle by inhibiting cyclins B and E through the activation of p21; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-2, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) by stimulating T helper cells (LyTh), and via the proteolytic cleavage of clusters of differentiation 44 (CD44) and CD45RA; and (V) reducing inflammation by influencing cycloox-ygenase-2 (COX-2). Created with BioRender.com. https://app.biorender.com/illustrations/6613c5688fba6222e90aa178 (accessed on 10 April 2024).
) the cell cycle by inhibiting cyclins B and E through the activation of p21; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-2, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) by stimulating T helper cells (LyTh), and via the proteolytic cleavage of clusters of differentiation 44 (CD44) and CD45RA; and (V) reducing inflammation by influencing cycloox-ygenase-2 (COX-2). Created with BioRender.com. https://app.biorender.com/illustrations/6613c5688fba6222e90aa178 (accessed on 10 April 2024).
 ) cytotoxicity and reduces (
) cytotoxicity and reduces ( ) cancer cell proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the mitogen-activated protein kinase (MAPK) signaling pathway, extracellular signal-related kinase (ERK), nuclear factor-kappa B (NF-κB), c-Jun N-terminal kinase (JNK), serine/threonine protein kinase (Akt), microtubule-associated protein B-light chain 3 (LC3), and p38; (II) inducing cell death by apoptosis/autophagy via Bcl-2-associated X (BAX), B-cell lymphoma 2 (Bcl-2), caspases-3 and -9, reactive oxygen species (ROS), p53, LC3, and BECLIN 1; (III) blocking (
) cancer cell proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the mitogen-activated protein kinase (MAPK) signaling pathway, extracellular signal-related kinase (ERK), nuclear factor-kappa B (NF-κB), c-Jun N-terminal kinase (JNK), serine/threonine protein kinase (Akt), microtubule-associated protein B-light chain 3 (LC3), and p38; (II) inducing cell death by apoptosis/autophagy via Bcl-2-associated X (BAX), B-cell lymphoma 2 (Bcl-2), caspases-3 and -9, reactive oxygen species (ROS), p53, LC3, and BECLIN 1; (III) blocking ( ) the cell cycle by inhibiting cyclins B and E through the activation of p21; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-2, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) by stimulating T helper cells (LyTh), and via the proteolytic cleavage of clusters of differentiation 44 (CD44) and CD45RA; and (V) reducing inflammation by influencing cycloox-ygenase-2 (COX-2). Created with BioRender.com. https://app.biorender.com/illustrations/6613c5688fba6222e90aa178 (accessed on 10 April 2024).
) the cell cycle by inhibiting cyclins B and E through the activation of p21; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-2, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) by stimulating T helper cells (LyTh), and via the proteolytic cleavage of clusters of differentiation 44 (CD44) and CD45RA; and (V) reducing inflammation by influencing cycloox-ygenase-2 (COX-2). Created with BioRender.com. https://app.biorender.com/illustrations/6613c5688fba6222e90aa178 (accessed on 10 April 2024).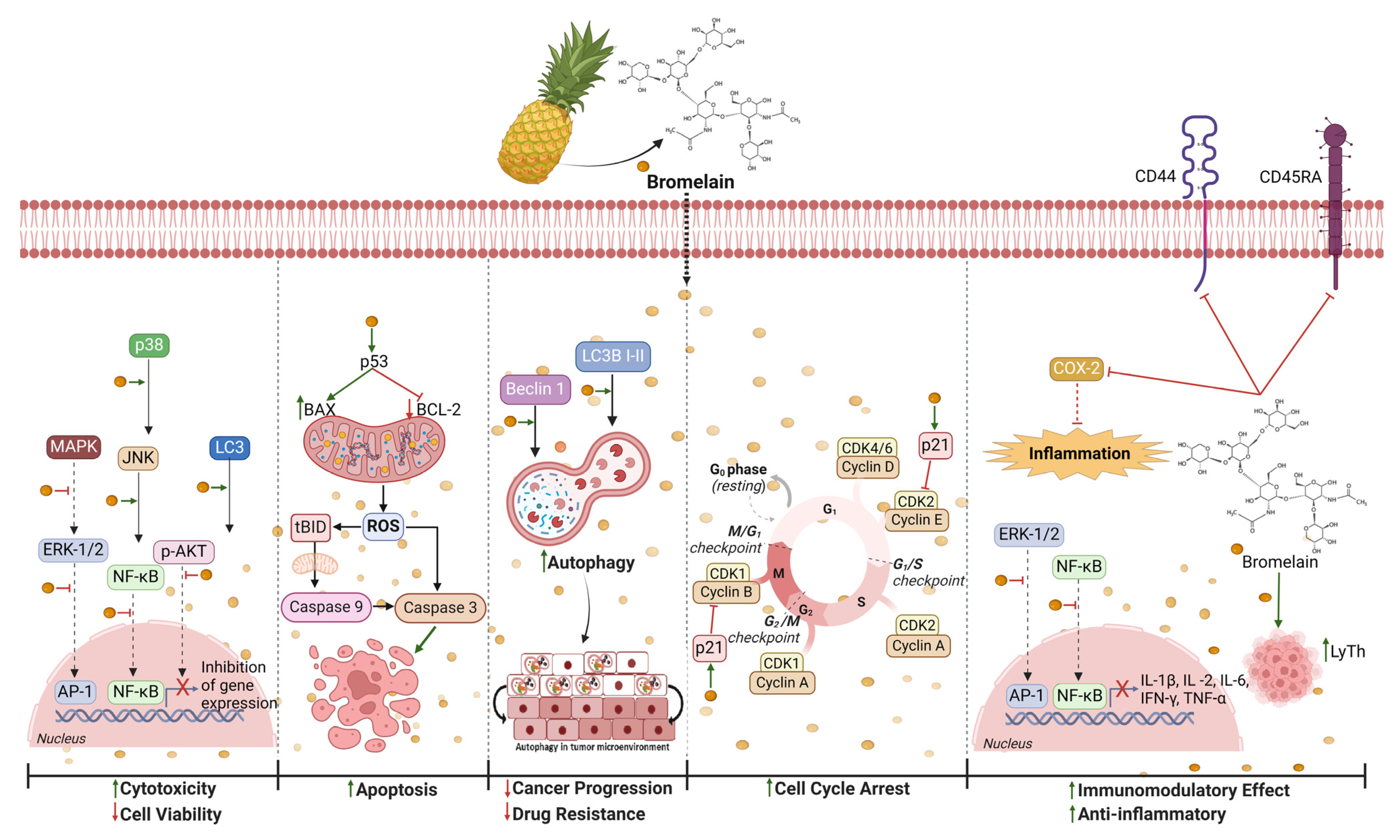
 ) cytotoxicity and reduces (
) cytotoxicity and reduces ( ) cancer cells proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the phosphatase and tensin homolog (PTEN), phosphoinositide 3-kinases (PI3K), and serine/threonine protein kinase (AKT) signaling pathways; via inhibitors of nuclear factor-kappa-B kinase subunit alpha and beta (IKK-α,β) and nuclear factor-kappa-B (NF-κB), inhibiting (
) cancer cells proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the phosphatase and tensin homolog (PTEN), phosphoinositide 3-kinases (PI3K), and serine/threonine protein kinase (AKT) signaling pathways; via inhibitors of nuclear factor-kappa-B kinase subunit alpha and beta (IKK-α,β) and nuclear factor-kappa-B (NF-κB), inhibiting ( ) DNA methyltransferases (DNMTs) and histone deacetylases (HDACs); and via nuclear factor erythroid 2-related factor 2 (NRF2) by binding antioxidant response elements (AREs); (II) inducing cell death by apoptosis via Bcl-2-associated X (Bax); B-cell lymphoma 2 (Bcl-2); cytochrome complex (Cyt C); caspases 8, 9, and 3; and by autophagy via microtubule-associated protein light chain 3 I,II (LC3 I,II); (III) blocking the cell cycle by inhibiting cyclins D, A, and B through the activation of p21 and p27; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-4, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) and via the proteolytic cleavage of clusters of differentiation 44 (CD44); and (V) reducing inflammation by inhibiting cycloox-ygenase-2 (COX-2), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Created with BioRender.com. “https://app.biorender.com/illustrations/661e36e12bb8f001aab26646 (accessed on 16 April 2024)”.
) DNA methyltransferases (DNMTs) and histone deacetylases (HDACs); and via nuclear factor erythroid 2-related factor 2 (NRF2) by binding antioxidant response elements (AREs); (II) inducing cell death by apoptosis via Bcl-2-associated X (Bax); B-cell lymphoma 2 (Bcl-2); cytochrome complex (Cyt C); caspases 8, 9, and 3; and by autophagy via microtubule-associated protein light chain 3 I,II (LC3 I,II); (III) blocking the cell cycle by inhibiting cyclins D, A, and B through the activation of p21 and p27; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-4, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) and via the proteolytic cleavage of clusters of differentiation 44 (CD44); and (V) reducing inflammation by inhibiting cycloox-ygenase-2 (COX-2), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Created with BioRender.com. “https://app.biorender.com/illustrations/661e36e12bb8f001aab26646 (accessed on 16 April 2024)”.
 ) cytotoxicity and reduces (
) cytotoxicity and reduces ( ) cancer cells proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the phosphatase and tensin homolog (PTEN), phosphoinositide 3-kinases (PI3K), and serine/threonine protein kinase (AKT) signaling pathways; via inhibitors of nuclear factor-kappa-B kinase subunit alpha and beta (IKK-α,β) and nuclear factor-kappa-B (NF-κB), inhibiting (
) cancer cells proliferation primarily by (I) modulating the expression of genes crucial for cell differentiation and proliferation via the phosphatase and tensin homolog (PTEN), phosphoinositide 3-kinases (PI3K), and serine/threonine protein kinase (AKT) signaling pathways; via inhibitors of nuclear factor-kappa-B kinase subunit alpha and beta (IKK-α,β) and nuclear factor-kappa-B (NF-κB), inhibiting ( ) DNA methyltransferases (DNMTs) and histone deacetylases (HDACs); and via nuclear factor erythroid 2-related factor 2 (NRF2) by binding antioxidant response elements (AREs); (II) inducing cell death by apoptosis via Bcl-2-associated X (Bax); B-cell lymphoma 2 (Bcl-2); cytochrome complex (Cyt C); caspases 8, 9, and 3; and by autophagy via microtubule-associated protein light chain 3 I,II (LC3 I,II); (III) blocking the cell cycle by inhibiting cyclins D, A, and B through the activation of p21 and p27; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-4, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) and via the proteolytic cleavage of clusters of differentiation 44 (CD44); and (V) reducing inflammation by inhibiting cycloox-ygenase-2 (COX-2), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Created with BioRender.com. “https://app.biorender.com/illustrations/661e36e12bb8f001aab26646 (accessed on 16 April 2024)”.
) DNA methyltransferases (DNMTs) and histone deacetylases (HDACs); and via nuclear factor erythroid 2-related factor 2 (NRF2) by binding antioxidant response elements (AREs); (II) inducing cell death by apoptosis via Bcl-2-associated X (Bax); B-cell lymphoma 2 (Bcl-2); cytochrome complex (Cyt C); caspases 8, 9, and 3; and by autophagy via microtubule-associated protein light chain 3 I,II (LC3 I,II); (III) blocking the cell cycle by inhibiting cyclins D, A, and B through the activation of p21 and p27; (IV) having an immunomodulatory effect by reducing the production of IL-1β, IL-4, IL-6 (interleukin), interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α) and via the proteolytic cleavage of clusters of differentiation 44 (CD44); and (V) reducing inflammation by inhibiting cycloox-ygenase-2 (COX-2), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). Created with BioRender.com. “https://app.biorender.com/illustrations/661e36e12bb8f001aab26646 (accessed on 16 April 2024)”.
| Cell Lines | Organism | Immunoprofile | Characteristics |
|---|---|---|---|
| BT−474 | Human | ER−, PR+, HER2+ | Epithelial cell line from ductal carcinoma |
| BT−549 | Human | ER−, PR−, HER2− | Epithelial cell line from ductal carcinoma |
| GI−101A | Human | ER−, PR−, HER2+ enriched | Epithelial cell line from a metastatic breast tumor |
| HCC70 | Human | ER−, PR−, HER2− | Epithelial cell line from ductal carcinoma |
| HCC1806 | Human | ER−, PR−, HER2− | Epithelial cell line from mammary gland |
| MCF−10A | Human | ER−, PR−, HER2− and EGFR+ | Non-tumorigenic epithelial cell line from mammary gland |
| MCF−10F | Human | ER−, PR−, HER2− and EGFR+ | Non-tumorigenic epithelial cell line from mammary gland |
| MCF−7 | Human | ER+, PR+, HER2− | Epithelial cell line from mammary adenocarcinoma |
| MDA−MB−231 | Human | ER−, PR−, HER2− and EGFR+ | Epithelial cell line from mammary adenocarcinoma |
| MDA−MB−453 | Human | ER−, PR−, HER2+ enriched and AR+ | Epithelial cell line from metastatic mammary carcinoma |
| MDA−MB−468 | Human | ER−, PR−, HER2− and EGFR+ | Epithelial cell line from mammary adenocarcinoma |
| SK−BR−3 | Human | ER−, PR−, HER2+ enriched | Epithelial cell line from mammary adenocarcinoma |
| SUM−159PT | Human | ER−, PR−, HER2− | Epithelial cell line from mammary carcinoma |
| T−47D | Human | ER+, PR+, HER2− | Epithelial cell line from infiltrating ductal carcinoma |
| ZR−75−1 | Human | ER+, PR+, HER2− | Epithelial cell line from ductal carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mecca, M.; Sichetti, M.; Giuseffi, M.; Giglio, E.; Sabato, C.; Sanseverino, F.; Marino, G. Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies. Nutrients 2024, 16, 1883. https://doi.org/10.3390/nu16121883
Mecca M, Sichetti M, Giuseffi M, Giglio E, Sabato C, Sanseverino F, Marino G. Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies. Nutrients. 2024; 16(12):1883. https://doi.org/10.3390/nu16121883
Chicago/Turabian StyleMecca, Marisabel, Marzia Sichetti, Martina Giuseffi, Eugenia Giglio, Claudia Sabato, Francesca Sanseverino, and Graziella Marino. 2024. "Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies" Nutrients 16, no. 12: 1883. https://doi.org/10.3390/nu16121883
APA StyleMecca, M., Sichetti, M., Giuseffi, M., Giglio, E., Sabato, C., Sanseverino, F., & Marino, G. (2024). Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies. Nutrients, 16(12), 1883. https://doi.org/10.3390/nu16121883







