Abstract
Caffeine is a well-described ergogenic aid used to enhance athletic performance. Using animal models can greatly increase our understanding of caffeine’s mechanisms in performance. Here, we adapted an animal weight-lifting exercise model to demonstrate caffeine’s ergogenic effect in rats. Male Wistar rats (315 ± 35 g) were randomly divided into two groups: one group received 5 mg·kg−1 of caffeine (0.5 mL; CEx; n = 5) and the other 0.9% NaCl (0.5 mL; PEx; n = 4) through an orogastric probe (gavage) one hour before exercise. Weight-lifting exercise sessions were performed over three subsequent days, and the number of complete squats performed was counted. Analyses of the area under the curve in all three experiments showed that the CEx group responded more to stimuli, performing more squats (1.7-, 2.0-, and 1.6-fold; p < 0.05) than the control group did. These three days’ data were analyzed to better understand the cumulative effect of this exercise, and a hyperbolic curve was fitted to these data. Data fitting from the caffeine-supplemented group, CEx, also showed larger Smax and Kd (2.3-fold and 1.6-fold, respectively) than the PEx group did. Our study demonstrated an acute ergogenic effect of caffeine in an animal weight-lifting exercise model for the first time, suggesting potential avenues for future research.
1. Introduction
Caffeine (1,3,7–trimethylxanthine) is among the most widely consumed psychoactive molecules. Xanthine is found in different plants’ seeds, leaves, and fruit, such as coffee, cocoa, mate, and guarana. It has been consumed for centuries worldwide in many dietary products, such as beverages, infusions, soft drinks, chocolate, and, more recently, energy drinks [1,2]. Coffee is the primary nutritional caffeine source in Western Europe and the United States, with about 89% of the adult US population consuming it daily [3]. In South America, in addition to coffee and carbonated soda, infusions using yerba mate (Ilex paraguariensis leaves and branches) are daily sources of caffeine for a broad population [4,5,6]. In Eastern societies, particularly in China and Southeast Asia, caffeine intake is also high, with an increasing trend in the last decades, mainly due to the high consumption of traditional infusions, such as black and green teas [6,7,8]. Still, xanthine ingestion might be underestimated, as sometimes caffeine is not declared on food or beverage labels [2].
In humans, caffeine absorption varies with gastric emptying, with a half-life of four to six hours and a peak of absorption of one hour [9,10,11]. Methylxanthine increases circulating catecholamines, raising blood pressure and heart rate [12]. Due to its hydrophobic character and poor albumin binding (10–30%), caffeine can easily pass through cellular membranes and the blood–brain barrier, freely entering and leaving tissues [13]. Caffeine’s ergogenic, motor coordination, and anti-fatigue effects are due to its action as an antagonist of adenosine A2A receptors blocking adenosine on it, which causes more dopamine to be released in the striatum. This also suggests a significant impact on improving caffeine’s ergogenic effects in the forebrain [14,15,16]. Caffeine-induced stimulation provides performance benefits such as endurance, motivation, fatigue reduction, and increased mental alertness [8,17].
Human studies have used 3–6 mg·kg−1 body mass doses to achieve caffeine’s ergogenic role [9,10,11,18,19,20]. Acute caffeine ingestion promotes training-induced adaptations in power and speed. Previous bench press experiments indicated chronic pre-exercise caffeine ingestion improves velocity-load and power-load curves in upper limb exercises [21]. Caffeine intake increases the total amount of work performed during strength training sessions due to improvements in power production [21,22]. Caffeine also improves recovery between strenuous exercise sessions due to its hypoalgesic effect on delayed-onset muscle soreness [23,24]. Caffeine’s effects depend on doses and individual sensitivity [9,10,18,25]. Caffeine habituation—a desensitization of caffeine’s stimulatory effects—may result in an increased amount of caffeine needed to have the same antagonist activity on receptors [18,25]. Although caffeine’s ergogenic effects do not entirely diminish in chronic conditions, regular repeated exposure increases tolerance [26].
Xanthine was added to the list of banned substances by the International Olympic Committee in 1984 and the World Anti-Doping Agency (WADA) in 2000, but removed from the list in 2004, which led to athletes’ increased consumption of caffeine [27,28,29]. Over the past few decades, use of caffeine-based ergogenic aids, particularly among athletes and physically active people, has become widespread, especially through energy drink brands’ sponsorships of events in different sports [30,31,32]. However, caffeine remains on a WADA substance-monitoring list [33]. Therefore, understanding caffeine’s effects on performance and exercise is crucial to various fields of science.
The administration of paraxanthine also promotes increased nitric oxide availability and vascular function [34], which are related to decline with age [35]. Furthermore, findings from a study carried out in mice indicate the administration of paraxanthine promotes significantly enhanced aerobic endurance and muscular hypertrophy [35].
Animal models can help comprehend caffeine’s tissue and cellular effects, and our group used animals under stress induced by different types of exercise to study general principles and compare results with those obtained in humans [36,37]. Different animal models have been developed to study exercise. Rats have been the most used animals due to their muscle response being similar to that of humans [38]. To date, few studies have evaluated strength enhancement in rats after acute caffeine ingestion compared to the large amount of data obtained during rats’ swimming or running. In this investigation, with the hypothesis that caffeine can enhance the performance of rats similarly to that of humans during weight-lifting exercise, we adapted an exercise model to assess the caffeine ergogenic effect in rats during weight-lifting exercise [39].
2. Materials and Methods
2.1. Animals
In an initial allocation, ten adult male Wistar rats (315 ± 35 g) were divided into two groups of five animals using GraphPad QuickCalcs (GraphPad Software LLC., Boston, MA, USA). However, one rat presented locomotion problems and was removed from the experiment. Thus, groups were divided as follows: control (PEx: n = 4) and caffeine (CEx: n = 5). The small sample size was defined considering that this was the first study evaluating caffeine’s ergogenic effect in a repeated-movement weight-lifting animal model. Therefore, the initial intention was to demonstrate primary evidence regarding this effect in our exercise protocol. A scientist not related to this study was asked to administer an orogastric probe (gavage) of 0.9% NaCl (0.5 mL; PEx) or 5 mg·kg−1 caffeine (0.5 mL; CEx) one hour before the exercise session in a separate room. He was the only individual who knew the animals’ identities and relationship with treatment.
Rats were kept in individual cages; each rat was marked with a permanent marker on its tail with the letter of its group and its respective number in that group. Rats were in an environment with a controlled temperature of 22 ± 2 °C, with a relative humidity of 40 ± 5%, receiving a cycle of 12 h of light and 12 h of darkness per day. They were fed balanced feed and water ad libitum. This study strictly followed the Normative Resolution MTCI #57/2022 of the Brazilian National Council for Animal Experimentation Control (CONCEA). This study was approved by the Ethics Committee in Research of Tiradentes University (UNIT/SE/031005).
2.2. The Squat Machine
The exercise was performed in a system adapted from Tamaki et al., which is described in detail elsewhere [39]. Specific modifications to the Tamaki system are described as follows:
- Using a platform-type grid electrode with conductor gel, the stimuli site was transferred from the tail to the rat’s hind paw, allowing more freedom of movement.
- We installed a control circuit to measure the complete squat movement.
- Red and yellow LEDs were added to the electric control system, allowing the most accurate protocol evaluation. A red LED indicated when a pulse was administered, and a yellow LED was connected to a sensor on the machine’s main lever (activated when the rat performed an entire squat) (Figure 1).
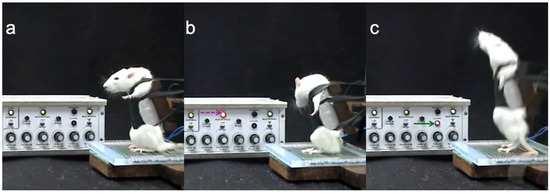 Figure 1. Three moments were captured from rat performance videos. (a) The rat is at rest, without stimulus. (b) Setting the stimulus, as indicated by the red LED (dashed magenta arrow). (c) The rat achieved complete movement after stimulation, as indicated by the flashing yellow LED (solid green arrow).
Figure 1. Three moments were captured from rat performance videos. (a) The rat is at rest, without stimulus. (b) Setting the stimulus, as indicated by the red LED (dashed magenta arrow). (c) The rat achieved complete movement after stimulation, as indicated by the flashing yellow LED (solid green arrow).
Exercise sessions were filmed (Supplementary Materials Videos S1 and S2). Two independent researchers analyzed the videos in slow motion.
Electrostimulation occurred every 2 s for 1 s (1 Hz) using a transcutaneous electrical nerve stimulator (TENS) Quark, Dualpex 961 (São Paulo, Brazil), which was previously calibrated by the National Institute of Metrology, Quality, and Technology (INMETRO, Duque de Caxias, Rio de Janeiro, Brazil). The short pulse duration promoted adequate stimulation, inducing rats to perform voluntary movements and avoiding tissue damage.
2.3. The Exercise Protocol
Rats were adapted to the squatting machine for a week. During adaptation, neither load nor electrical stimuli were used. After this period, rats were tested daily for three days to determine the ideal electrical charge and frequency for the stimulation (also without additional load other than the machine and animal weight). The one repetition maximum (1RM) test was performed to establish the individual load. Although some rats supported loads greater than 300% of their body mass, we set 80% of the load obtained on the 1RM test as the exercise load. On the fourth day, we performed 20 stimuli (one second on; two seconds off) intervals, according to the Tamaki et al. model, until exhaustion [39].
The rats followed the same protocol for three consecutive days.
2.4. Statistical Analysis
Data normality was analyzed using the Shapiro–Wilk test (p > 0.05). The average number of squats performed by rats of the same group after each stimulus (AVG) was calculated. The area under the curve (AUC) was calculated for AVG ± SEM after stimuli. AUCs were compared using the Student’s t-test for independent samples. We calculated Cohen’s d effect size (d) when using the t-test and r effect size for the Mann–Whitney test. For non-normal distributions, we used the Mann–Whitney test.
The cumulative mean of the three days’ AVGs was also calculated for each group. This value is mathematically defined by the successive sum of the corresponding three days’ mean AVGs after a determined stimulus, along with the previous ones, until the last stimulus.
A nonlinear regression equation was used to fit the curve of cumulative values as follows:
is the maximum value of , and is the number of stimuli necessary to reach half of . Statistics and fitting were performed using GraphPad Prism 10.2.3 (GraphPad Software LLC.).
3. Results
Caffeine is a well-known ergogenic agent in long-term exercise. To achieve the effect of caffeine in an acute animal model of weight-lifting exercise, we measured the number of squats per stimulus in rats. During all three protocol days, the caffeine group (CEx) had a greater response to the stimuli than the control group (PEx) did (p < 0.05). The three calculated mean AUCs of the CEx group were consistently larger (60–106%) than those of the PEx group. Also, the CEx group exercised longer than the PEx group did on two of the three protocol days (Figure 2).
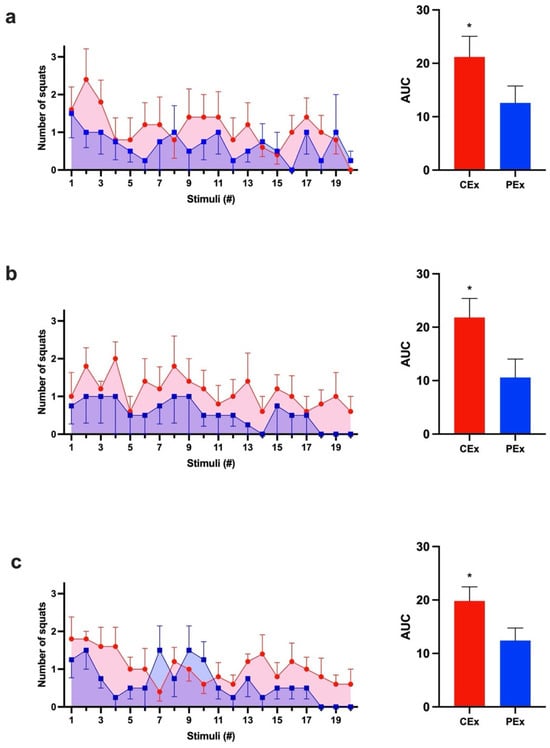
Figure 2.
The caffeine group presented a greater response to stimuli during the three consecutive days. CEx (Red, ●); PEx (Blue, ■). (a) Day 1 graph of AVG ± SEM after each stimulus and Student’s t-test comparison between CEx AUC (21.2 ± 3.9) and PEx AUC (12.6 ± 3.2) (p < 0.05; d = 2.3; CI: 9.8 to 7.4). (b) Day 2 graph of AVG ± SEM after each stimulus and Student’s t-test comparison between CEx AUC (21.8 ± 3.6) and PEx AUC (10.6 ± 3.4) (p < 0.05; d = 3.1; CI: 12.4 to 10.0). (c) Day 3 graph of AVG ± SEM after each stimulus and Student’s t-test comparison between CEx AUC (19.8 ± 2.7) and PEx (12.4 ± 2.4) (p < 0.05; d = 2.9; CI: 8.2 to 6.5). * Significant (p < 0.05).
To better understand these data, data were plotted as the accumulated mean AVG of the three experiments against the number of stimuli. A hyperbola better fits these data with fewer variables and constraints. The Smax in the supplemented group (CEx) was 2.3 times bigger than that of the control (PEx). The Kd was also 1.6 times bigger in the caffeine group (Figure 3).
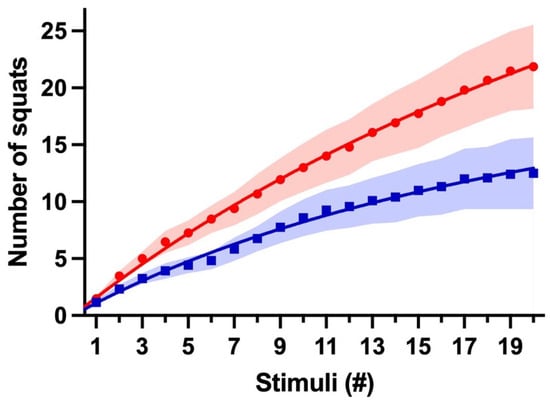
Figure 3.
Caffeine-induced ergogenic response. This graph shows the cumulative mean AVGs of three days for CEx (Red, ●) and PEx (Blue, ■) groups. Lines represent CEx fitting hyperbola Smax = 69.5 and Kd = 43.2, and PEx fitting hyperbola Smax = 30.4 and Kd = 27.0; shading represents SEM variation.
To highlight the fatigue resistance induced by caffeine, we compared the AVGs of CEx and PEx groups during the first and last five stimuli in the three experiments. The CEx group demonstrated a higher response to stimuli than the control group (PEx) did during the first five and last five stimuli (Figure 4).
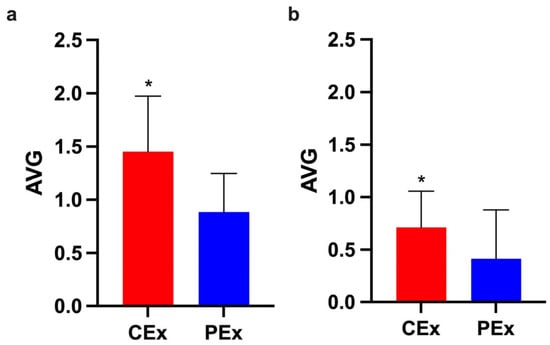
Figure 4.
The CEx group had a higher response at the beginning and end of the exercise. These graphs present the mean ± SD of CEx (Red) and PEx (Blue) groups. (a) The CEx group demonstrated a greater response during the first five stimuli (p < 0.05; d = 1.3; CI: 0.2 to 0.9). (b) The CEx group demonstrated a greater response during the last five stimuli (p < 0.05; r = 0.4; U = 61.5). * Significant (p < 0.05).
4. Discussion
Caffeine is a well-studied ergogenic resource with a well-described and accepted enhancement of muscle endurance, strength, and power in different exercise models [29,40,41]. As a psychoactive drug, caffeine can also lead to an additional placebo effect in humans [42,43,44]. Here, we used a modified animal model to study caffeine in weight-lifting exercises, where the placebo interference was annulated. In addition, an animal model can permit different pharmacological or biochemical studies. Due to the acute use of caffeine (a single dose once a day) and this study’s duration (three days), there were no other measurable effects on the rat’s physiology (blood pressure, cardiac frequency, or sleeping).
Animal models are crucial for better understanding biological processes, including exercise. For methodological reasons, rodent exercise protocols are usually used in running or swimming studies, while studies based on strength training are rarely adopted. In the early 1990s, Tamaki et al. proposed an animal model analogous to human weight-lifting training, allowing a better understanding of molecular and cellular mechanisms related to weight-lifting exercise [39,45,46,47,48]. Here, we adapted Tamaki’s apparatus to study acute caffeine ingestion’s effect on exercise performance. We introduced two new control circuits, which allowed us to evaluate the performance by quantifying complete squats performed by rats.
Our model used operant conditioning to make rats perform repetitive movements voluntarily. We used a grid electrode to evoke discomfort in rats’ hind limbs, inducing rats to perform squats. As an electrical stimulation, TENS avoids involuntary muscle contraction [49]. Inducing voluntary muscular contraction is essential to mimicking human exercise practice and making more reliable inferences from animal models to human models.
Several studies have used animal models to analyze the molecular mechanisms underlying the caffeine ergogenic effect [14,15,16,50]. As we used a small sample size for this pilot study and only evaluated performance, we proposed a first-step investigation. We highlighted this weight-lifting model as a promising method to expand the understanding of caffeine mechanisms of action in future studies. Also, this weight-lifting model can be adapted to other species, such as mice, allowing the study of differences and similarities across species. This model can be used to understand the effects of weight-lifting exercise physiology under several clinical conditions and interventions in future studies [51,52,53].
In this study, caffeine improved the rats’ performance during weight-lifting exercises. We used the AUC as a summary measure to analyze performance through 20 stimuli. Comparing AUCs from three consecutive days, the caffeine-treated group responded more (1.7-, 2.0-, and 1.6-fold) to stimuli than the control group did (Figure 2). Also, our data show that the treated group exhibited a Smax ~2.3-fold bigger than that of the PEx group (Figure 3). Furthermore, the higher response of the CEx group during the first five and last five stimuli might demonstrate a consistently greater response to stimuli caused by caffeine during the exercise (Figure 4). Our data concurs with what was previously described: rats’ acute caffeine administration decreased fatigue during swimming or treadmill-running exercise protocols [50,54,55,56,57].
To the best of our knowledge, this is the first study demonstrating caffeine’s ergogenic effect in rats during a weight-lifting exercise with repeated movements. Although this is an exciting finding, more studies are needed to determine the cause of the increase in squat repetitions. Performance improvements precede muscle hypertrophy in animal models, as there is a synchronization between muscle fiber number and performance gains independent of alterations in muscle mass [45,58]. As the rats performed squats against the same weight during the three days and muscle hypertrophy was not assessed, we cannot elucidate for force gain or increased resistance. Previous studies have demonstrated opposite results after larger doses of caffeine (19.7 mg·kg−1; 15 mg·kg−1; and 6 mg·kg−1) with only increased strength or enhanced resistance in the grip of rats’ front paws while rats were pulled by the tail [14,15,59].
Absorption and bioavailability of caffeine are generally similar between humans, dogs, rabbits, rats, and mice, with interspecies differences in the route of metabolism and enzymes involved in this process (for a comprehensive study of similarities and differences among species, we strongly suggest reading Arnaud 2011 [60]). It was not our goal to compare or extrapolate the totality of results in rat models for humans, but to advocate that an animal model can be useful for understanding systemic caffeine effects. In this study, we used an intermediate caffeine dose (5 mg·kg−1) as described for humans (3–10 mg·kg−1), considering the same absorption window peak (one hour) [40,41,60].
It was widely discussed that one of caffeine’s mechanisms of action could be increased Ca2+ release from the sarcoplasmic reticulum (SR), enhancing muscular contraction [61,62,63], including an uneven effect on different muscular fibers [64,65]. These results were used by previous studies to support the more prominent effect of caffeine increasing repetitions in the absence of strength enhancement in humans [66]. It is crucial to say that most of these studies were performed under supraphysiological caffeine concentrations. Caffeine concentrations in vivo are in the 10–50 μM range, and it is feasible to say that caffeine does not directly affect muscle fibers at physiological concentrations [40]. Caffeine antagonizes adenosine receptors, which causes more dopamine to be released in the striatum. This also suggests a significant impact on improving caffeine’s ergogenic effects in the forebrain [14,15,16].
The Tamaki apparatus privileges rats’ lower limb muscles, mainly the ones predominantly composed of fast-twitch fibers, such as the extensor digitorum longus and plantaris [39,67]. During Tamaki’s experiment, these muscles presented bigger hypertrophy compared to muscles composed of slow-twitch fibers, such as the gastrocnemius and soleus, which presented discreet hypertrophy [39]. Our hypothesis is supported by previous studies that proposed that the caffeine ergogenic effect might not be related to enhancing Ca2+ release from SR, as it was described only when caffeine was in high and non-physiological concentrations [40,68]. Therefore, more studies are needed to understand the mechanisms underlying the weight-lifting performance enhancement found in our study. However, our study certainly showed that this adaptation of Tamaki’s apparatus can be useful for understanding the effects of exercise and weight-lifting training on physiology.
Different human studies and reviews showed a caffeine-enhancement effect on endurance and strength during several exercise protocols [66,69,70,71,72,73,74,75]. Also, some reviews proposed that caffeine is more likely to increase repetitions than enhance strength during human weight-lifting exercises [66,72,76]. Thus, further studies are required to determine our protocol’s cause of endurance gain.
5. Conclusions
Our study demonstrated the caffeine ergogenic effect in an animal model analogous to human weight-lifting exercise. Although this effect is well described for humans, this is the first study to demonstrate it in animals.
Our protocol can be useful in understanding the molecular mechanisms underlying weight-lifting exercise performance. Adapting this model to mice can also allow the study of strength exercise in other physiopathological models and help determine shared vs. unique mechanisms across species. Therefore, the animal model used in our study can expand the understanding of weight-lifting exercise physiology and exercise effects in physiopathology.
6. Study Limitations
This was a pilot study with a small number of animals. Investigating the effects of caffeine in an animal model seemed interesting, given that our group has been researching xanthine for nearly two decades. Our main goal was to enhance an existing proposed system with a new platform and electronics, thus making the experimentation easier to follow.
We now aim to adapt the model for larger studies using mice to investigate the effects of strength exercise on different diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16132022/s1, Video S1: a control animal performance test session; Video S2: a caffeine-supplemented animal performance test session.
Author Contributions
Conceptualization, L.C.C. and A.M.; writing—original draft preparation, L.C.C., E.P.-A., J.M.-P., R.C.-C. and E.P.; writing—review and editing, L.C.C., V.C. and I.J.; supervision, L.C.C.; project administration, L.C.C.; funding acquisition, L.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Brazil Olympic Committee (BOC); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Financiadora de Estudos e Projetos (FINEP); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ); INCT-TeraNano (CNPq #403193/2022-2, -FAPEMIG CBB # APQ-03613-17). Merck-Sigma-Aldrich; SISCAPA; Universidade Federal do Estado do Rio de Janeiro (UNIRIO); and the Waters Corporation. Also, it was supported in part by funding from the Natural Sciences Research Council (NSERC #203475), the Canada Foundation for Innovation (CFI #225404, #30865), the Ontario Research Fund (RDI #34876, RE010-020), IBM, and the Ian Lawson van Toch Fund. V.C. is supported by a Clinician Scientist Salary Support Award from the Department of Medicine, University of Toronto, Toronto, Canada. The funders had no role in this study’s design, data collection, analysis, publication decisions, or manuscript preparation.
Institutional Review Board Statement
The use of animals for these experiments strictly followed the Normative Resolution MTCI #57/2022 of the Brazilian National Council for Animal Experimentation Control (CONCEA).
Informed Consent Statement
Not applicable.
Data Availability Statement
Original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are deeply grateful for the work of Erilo Aragão Prado, who helped extensively in building the equipment proposed earlier by Tamaki. Erilo Aragão was essential to its development.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barone, J.J.; Roberts, H. Human Consumption of Caffeine; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Bailey, R.L.; Saldanha, L.G.; Dwyer, J.T. Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutr. Rev. 2014, 72 (Suppl. 1), 9–13. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., 3rd; Keast, D.R.; Lieberman, H.R. Trends in intake and sources of caffeine in the diets of US adults: 2001-2010. Am. J. Clin. Nutr. 2015, 101, 1081–1087. [Google Scholar] [CrossRef]
- Mesquita, M.; Santos, E.; Kassuya, C.A.; Salvador, M.J. Chimarrão, terere and mate-tea in legitimate technology modes of preparation and consume: A comparative study of chemical composition, antioxidant, anti-inflammatory and anti-anxiety properties of the mostly consumed beverages of Ilex paraguariensis St. Hil. J. Ethnopharmacol. 2021, 279, 114401. [Google Scholar] [CrossRef]
- Heck, C.I.; de Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Xiao, X.; Sui, H.; Yang, D.; Yong, L.; Song, Y. Trends of caffeine intake from food and beverage among Chinese adults: 2004–2018. Food Chem. Toxicol. 2023, 173, 113629. [Google Scholar] [CrossRef]
- Clark, I.; Landolt, H.P. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep. Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Keisler, B.D.; Armsey, T.D., 2nd. Caffeine as an ergogenic aid. Curr. Sports Med. Rep. 2006, 5, 215–219. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Higaki, K.; Choe, S.Y.; Löbenberg, R.; Welage, L.S.; Amidon, G.L. Mechanistic understanding of time-dependent oral absorption based on gastric motor activity in humans. Eur. J. Pharm. Biopharm. 2008, 70, 313–325. [Google Scholar] [CrossRef]
- Han, J.Y.; Moon, Y.J.; Han, J.H.; Kim, J.H.; Woo, J.H.; Yoo, H.S.; Hong, J.T.; Ahn, H.Y.; Hong, J.M.; Oh, K.W. (-)-Epigallocatechin-3-O-gallate (EGCG) attenuates the hemodynamics stimulated by caffeine through decrease of catecholamines release. Arch. Pharm. Res. 2016, 39, 1307–1312. [Google Scholar] [CrossRef]
- Bonati, M.; Latini, R.; Tognoni, G.; Young, J.F.; Garattini, S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab. Rev. 1984, 15, 1355–1383. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.B.; Santos, N.S.; Santos, A.P.T.; da Panatta, G.; Speck, A.E.; Cunha, R.A.; Aguiar, A.S., Jr. Adenosine A(2A) and dopamine D(2) receptor interaction controls fatigue resistance. Front. Pharmacol. 2024, 15, 1390187. [Google Scholar] [CrossRef] [PubMed]
- de Bem Alves, A.C.; Speck, A.E.; Farias, H.R.; Martins, L.M.; Dos Santos, N.S.; Pannata, G.; Tavares, A.P.; de Oliveira, J.; Tomé, Â.R.; Cunha, R.A.; et al. The striatum drives the ergogenic effects of caffeine. Purinergic Signal 2023, 19, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S., Jr.; Speck, A.E.; Canas, P.M.; Cunha, R.A. Neuronal adenosine A(2A) receptors signal ergogenic effects of caffeine. Sci. Rep. 2020, 10, 13414. [Google Scholar] [CrossRef] [PubMed]
- Yanik, G.; Glaum, S.; Radulovacki, M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987, 403, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ganio, M.S.; Klau, J.F.; Casa, D.J.; Armstrong, L.E.; Maresh, C.M. Effect of caffeine on sport-specific endurance performance: A systematic review. J. Strength Cond. Res. 2009, 23, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ding, L.; Qin, Q.; Lei, T.H.; Girard, O.; Cao, Y. Effect of caffeine ingestion on time trial performance in cyclists: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2024, 21, 2363789. [Google Scholar] [CrossRef] [PubMed]
- Saavedra Velásquez, N.; Cuadrado Peñafiel, V.; de la Vega Marcos, R. Can caffeine improve your performance? Psychophysiological effects—A systematic review. Nutr. Hosp. 2024, 41, 677–685. [Google Scholar] [CrossRef]
- Giráldez-Costas, V.; Ruíz-Moreno, C.; González-García, J.; Lara, B.; Del Coso, J.; Salinero, J. Pre-exercise Caffeine Intake Enhances Bench Press Strength Training Adaptations. Front. Nutr. 2021, 8, 622564. [Google Scholar] [CrossRef]
- Giráldez-Costas, V.; González-García, J.; Lara, B.; Coso, J.D.; Wilk, M.; Salinero, J.J. Caffeine Increases Muscle Performance During a Bench Press Training Session. J. Hum. Kinet. 2020, 74, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.; Tucker, M.; Butts, C.; McDermott, B.; Vingren, J.; Kunces, L.; Lee, E.; Munoz, C.; Williamson, K.; Armstrong, L.; et al. Effect of Caffeine on Perceived Soreness and Functionality Following an Endurance Cycling Event. J. Strength Cond. Res. 2017, 31, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Guimaraes-Ferreira, L.; Clarke, N. Not Another Caffeine Effect on Sports Performance Study-Nothing New or More to Do? Nutrients 2022, 14, 4696. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J. What Should We Do About Habitual Caffeine Use in Athletes? Sports Med. 2019, 49, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.L.; Díaz-Lara, J.; Pareja-Galeano, H.; Del Coso, J. Caffeinated Drinks and Physical Performance in Sport: A Systematic Review. Nutrients 2021, 13, 2944. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- RB Leipzig: How Did Red Bull Build a Champions League Side from Scratch? Available online: https://www.bbc.com/sport/football/51475532 (accessed on 19 May 2024).
- McLaren Racing Announces Monster Energy As an Official Partner of McLaren Formula 1 Team. Available online: https://www.mclaren.com/racing/formula-1/2023/mclaren-racing-announces-monster-energy-as-an-official-partner-of-mclaren-formula-1-team/ (accessed on 19 May 2024).
- Monster Energy Continuing to Sponsor UFC Fighter Conor McGregor. Available online: https://www.miamiherald.com/sports/fighting/article167315472.html (accessed on 19 May 2024).
- WADA. World Anti-Doping Code International Standard Prohibited List. 2024. Available online: https://www.wada-ama.org/sites/default/files/2023-09/2024list_en_final_22_september_2023.pdf (accessed on 25 June 2024).
- Ferré, S.; Orrú, M.; Guitart, X. Paraxanthine: Connecting Caffeine to Nitric Oxide Neurotransmission. J. Caffeine Res. 2013, 3, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine Supplementation Increases Muscle Mass, Strength, and Endurance in Mice. Nutrients 2022, 14, 893. [Google Scholar] [CrossRef]
- de Almeida, R.D.; Prado, E.S.; Llosa, C.D.; Magalhães-Neto, A.; Cameron, L.C. Acute supplementation with keto analogues and amino acids in rats during resistance exercise. Br. J. Nutr. 2010, 104, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.T.; Gonçalves, S.C.; Pedrosa, M.L.; Silva, M.E.; Bassini, A.; Coelho, W.S.; de Magalhães-Neto, A.M.; Prado, E.S.; Cameron, L.C. Keto analogues and amino acid supplementation and its effects on ammonaemia during extenuating endurance exercise in ketogenic diet-fed rats. Br. J. Nutr. 2018, 120, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Timson, B.F. Evaluation of animal models for the study of exercise-induced muscle enlargement. J. Appl. Physiol. 1990, 69, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Uchiyama, S.; Nakano, S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med. Sci. Sports Exerc. 1992, 24, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Rush, J.W.; van Soeren, M.H. Caffeine and exercise: Metabolism and performance. Can. J. Appl. Physiol. 1994, 19, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Beedie, C.J.; Stuart, E.M.; Coleman, D.A.; Foad, A.J. Placebo effects of caffeine on cycling performance. Med. Sci. Sports Exerc. 2006, 38, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Pollo, A.; Carlino, E.; Benedetti, F. The top-down influence of ergogenic placebos on muscle work and fatigue. Eur. J. Neurosci. 2008, 28, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Lyons, M.; Hankey, J. Placebo effects of caffeine on short-term resistance exercise to failure. Int. J. Sports Physiol. Perform. 2009, 4, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Uchiyama, S.; Uchiyama, Y.; Akatsuka, A.; Roy, R.R.; Edgerton, V.R. Anabolic steroids increase exercise tolerance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E973–E981. [Google Scholar] [CrossRef]
- Tamaki, T.; Uchiyama, S.; Uchiyama, Y.; Akatsuka, A.; Yoshimura, S.; Roy, R.R.; Edgerton, V.R. Limited myogenic response to a single bout of weight-lifting exercise in old rats. Am. J. Physiol. Cell Physiol. 2000, 278, C1143–C1152. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Akatsuka, A.; Tokunaga, M.; Ishige, K.; Uchiyama, S.; Shiraishi, T. Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am. J. Physiol. 1997, 273, C246–C256. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Tsukamoto, H.; Yoshimura, S.; Tamaki, T. Relationship between oxidative stress in muscle tissue and weight-lifting-induced muscle damage. Pflug. Arch. 2006, 452, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Jung, J.H.; Cho, H.Y.; In, T.S. Effects of Transcutaneous Electrical Nerve Stimulation with Taping on Wrist Spasticity, Strength, and Upper Extremity Function in Patients with Stroke: A Randomized Control Trial. J. Clin. Med. 2024, 13, 2229. [Google Scholar] [CrossRef]
- Davis, J.M.; Zhao, Z.; Stock, H.S.; Mehl, K.A.; Buggy, J.; Hand, G.A. Central nervous system effects of caffeine and adenosine on fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R399–R404. [Google Scholar] [CrossRef]
- Weitz, J.E.; Ritchlin, C.T. Mechanistic insights from animal models of psoriasis and psoriatic arthritis. Curr. Rheumatol. Rep. 2013, 15, 377. [Google Scholar] [CrossRef]
- Kenney, H.M.; Wood, R.W.; Ramirez, G.; Bell, R.D.; Chen, K.L.; Schnur, L.; Rahimi, H.; Korman, B.D.; Xing, L.; Ritchlin, C.T.; et al. Implementation of automated behavior metrics to evaluate voluntary wheel running effects on inflammatory-erosive arthritis and interstitial lung disease in TNF-Tg mice. Arthritis Res. Ther. 2023, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Gudjonsson, J.E.; Le, S.; Maverakis, E.; Plazyo, O.; Ritchlin, C.; Scher, J.U.; Singh, R.; Ward, N.L.; Bell, S.; et al. New Frontiers in Psoriatic Disease Research, Part I: Genetics, Environmental Triggers, Immunology, Pathophysiology, and Precision Medicine. J. Investig. Dermatol. 2021, 141, 2112–2122.e3. [Google Scholar] [CrossRef]
- Zheng, X.; Hasegawa, H. Administration of caffeine inhibited adenosine receptor agonist-induced decreases in motor performance, thermoregulation, and brain neurotransmitter release in exercising rats. Pharmacol. Biochem. Behav. 2016, 140, 82–89. [Google Scholar] [CrossRef]
- Zheng, X.; Takatsu, S.; Wang, H.; Hasegawa, H. Acute intraperitoneal injection of caffeine improves endurance exercise performance in association with increasing brain dopamine release during exercise. Pharmacol. Biochem. Behav. 2014, 122, 136–143. [Google Scholar] [CrossRef]
- Boyer, M.; Rees, S.; Quinn, J.; Grattan-Miscio, K.; McCallum, M.; Saari, M.J. Caffeine as a performance-enhancing drug in rats: Sex, dose, housing, and task considerations. Percept. Mot. Ski. 2003, 97, 259–270. [Google Scholar] [CrossRef]
- Ryu, S.; Choi, S.-K.; Joung, S.-S.; Suh, H.; Cha, Y.-S.; Lee, S.; Lim, K. Caffeine as a Lipolytic Food Component Increases Endurance Performance in Rats and Athletes. J. Nutr. Sci. Vitaminol. 2001, 47, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rader, E.P.; Miller, G.R.; Chetlin, R.D.; Wirth, O.; Baker, B.A. Volitional Weight-Lifting in Rats Promotes Adaptation via Performance and Muscle Morphology prior to Gains in Muscle Mass. Environ. Health Insights 2014, 8, EHI-S15257. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, H.; Yan, Y.; Yang, W.; Chen, S.; Song, G.; Li, X.; Gu, Y.; Yun, H.; Li, Y. Synergistic Effect of Rhodiola rosea and Caffeine Supplementation on the Improvement of Muscle Strength and Muscular Endurance: A Pilot Study for Rats, Resistance Exercise-Untrained and -Trained Volunteers. Nutrients 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–91. [Google Scholar] [CrossRef]
- Rousseau, E.; Ladine, J.; Liu, Q.Y.; Meissner, G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch. Biochem. Biophys. 1988, 267, 75–86. [Google Scholar] [CrossRef]
- Weber, A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J. Gen. Physiol. 1968, 52, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Herz, R. The Relationship between Caffeine Contracture of Intact Muscle and the Effect of Caffeine on Reticulum. J. General. Physiol. 1968, 52, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Pagala, M.K.; Taylor, S.R. Imaging caffeine-induced Ca2+ transients in individual fast-twitch and slow-twitch rat skeletal muscle fibers. Am. J. Physiol. 1998, 274, C623–C632. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; DeBoer, G.E.; Bunge, G.; Andrish, J.T.; Tetzlaff, J.E.; Cruse, R.P. Fiber-Type Specific Caffeine Sensitivities in Normal Human Skinned Muscle Fibers. Anesthesiology 1990, 72, 50–54. [Google Scholar] [CrossRef]
- Polito, M.D.; Souza, D.B.; Casonatto, J.; Farinatti, P. Acute effect of caffeine consumption on isotonic muscular strength and endurance: A systematic review and meta-analysis. Sci. Sports 2016, 31, 119–128. [Google Scholar] [CrossRef]
- Eng, C.M.; Smallwood, L.H.; Rainiero, M.P.; Lahey, M.; Ward, S.R.; Lieber, R.L. Scaling of muscle architecture and fiber types in the rat hindlimb. J. Exp. Biol. 2008, 211, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Oxford, S.W. Acute caffeine ingestion enhances performance and dampens muscle pain following resistance exercise to failure. J. Sports Med. Phys. Fit. 2012, 52, 280–285. [Google Scholar]
- Duncan, M.J.; Stanley, M.; Parkhouse, N.; Cook, K.; Smith, M. Acute caffeine ingestion enhances strength performance and reduces perceived exertion and muscle pain perception during resistance exercise. Eur. J. Sport. Sci. 2013, 13, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.L.; Messias, F.R.; Zanchi, N.E.; Gerlinger-Romero, F.; Duncan, M.J.; Guimarães-Ferreira, L. Effects of acute caffeine ingestion on resistance training performance and perceptual responses during repeated sets to failure. J. Sports Med. Phys. Fit. 2015, 55, 383–389. [Google Scholar]
- Warren, G.L.; Park, N.D.; Maresca, R.D.; McKibans, K.I.; Millard-Stafford, M.L. Effect of caffeine ingestion on muscular strength and endurance: A meta-analysis. Med. Sci. Sports Exerc. 2010, 42, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiu, B.; Gao, J.; Del Coso, J. Effects of Caffeine Intake on Endurance Running Performance and Time to Exhaustion: A Systematic Review and Meta-Analysis. Nutrients 2022, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.L.; Clarke, N.D. Effect of Coffee and Caffeine Ingestion on Resistance Exercise Performance. J. Strength Cond. Res. 2016, 30, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Smirmaul, B.P.; de Moraes, A.C.; Angius, L.; Marcora, S.M. Effects of caffeine on neuromuscular fatigue and performance during high-intensity cycling exercise in moderate hypoxia. Eur. J. Appl. Physiol. 2017, 117, 27–38. [Google Scholar] [CrossRef]
- Astorino, T.A.; Roberson, D.W. Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: A systematic review. J. Strength Cond. Res. 2010, 24, 257–265. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).