Digital Biohacking Approach to Dietary Interventions: A Comprehensive Strategy for Healthy and Sustainable Weight Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
- -
- Food consumption data: Our team developed a web-based application (ArMOnIA, https://www.apparmonia.com, accessed on 25 January 2024) which allowed us to retrieve a comprehensive list of all the food consumed over a period of more than 1 year for each participant. This application facilitated the comprehensive tracking of all foods consumed by each participant over a period exceeding one year. Participants could input their dietary intake during the monitoring period directly into the application, with the data stored in a NoSQL database. This list includes detailed information about the macronutrient composition, calorie intake, and food category of each food item. Furthermore, the foods have been categorized into six meals for each day: the main ones (Breakfast, Lunch, and Dinner) and snacks between them.
- -
- Carbon footprint assessment: We conducted a thorough analysis of the carbon footprint impact associated with each food item. To calculate this impact, we utilized a classification system that corresponds to the My Emission-free Food Carbon Footprint Calculator database (https://myemissions.green/food-carbon-footprint-calculator/, accessed on 25 January 2024). Each food was assigned to the respective food class, enabling us to accurately determine its carbon footprint impact.
- Daily calorie intake and macronutrient composition were obtained from ArMOnIA, where users input their dietary information into a structured NoSQL database.
- Daily weight and Resting Metabolic Rate (RMR) were obtained from the Mi Body Composition Scale 2 [20]. Users weighed themselves each morning before breakfast, with this data accessed via an API integrated into ArMOnIA through an Amazfit Developer Account.
- Daily energy expenditure for Physical Activities (PA) was collected from MiBand 6 [21]. MiBand 6 was worn 24/7 for the duration of the study, allowing it to be recharged for one hour approximately once a week. These activities were accurately recorded and retrieved through dedicated APIs, as outlined in the previous point.
2.3. Digital Biohacking
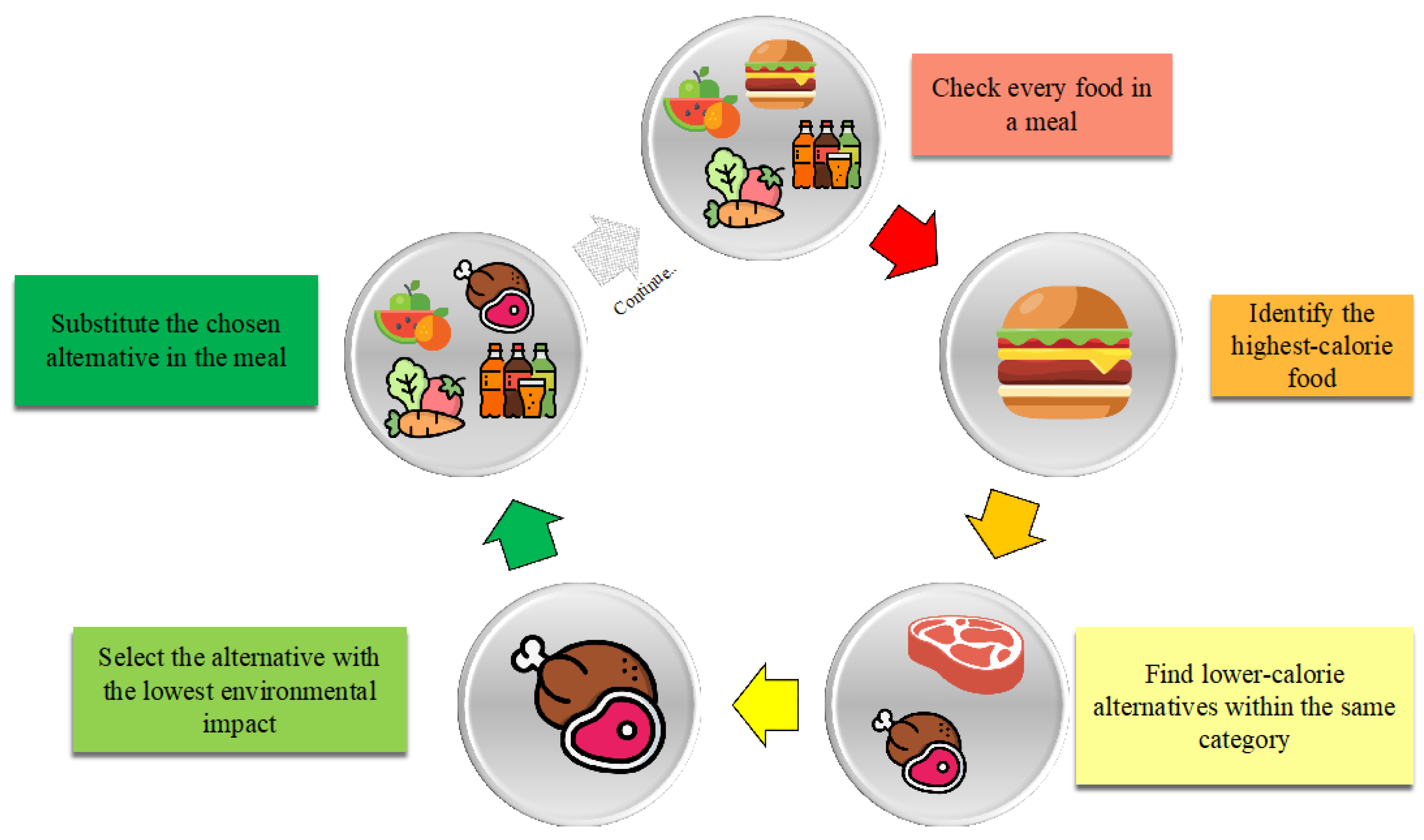
- Initialization: The algorithm begins by copying a dataset related to dietary habits (diet_week) into another variable (bh). Additionally, empty lists are initialized to store information about replacements (indexes, switches, meals, new, calories_reduction, impact_reduction, quantity), and an empty dictionary (dictionary) is created to track correspondences between replaced and new food items.
- Data iteration: For each unique date-meal combination, the algorithm evaluates the total calorie intake. If it is below a threshold (e.g., 100 kcal), no replacement is made. Otherwise, the food item with the highest caloric intake (excluding condiments and spices) is identified for replacement. The algorithm searches for alternative foods within the same meal category, aiming to reduce the caloric intake by 100–200 kcal while considering the carbon footprint impact.
- Food replacement: Suitable alternative food options are selected randomly from a pre-defined list, ensuring they belong to the same macro-category and have similar nutritional properties but lower caloric content and environmental impact. The replacements are recorded in lists, and a DataFrame summarizing these changes is created.
- Simulation using the PMA: The PMA simulates the effects of the proposed dietary interventions by optimizing parameters to minimize RMSE in GRU models. These parameters are tailored to each participant’s metabolic profile, enabling accurate predictions of weight changes and metabolic outcomes. Techniques such as Walk-Forward Validation (WFV) and Walk-Forward Simulation (WFS) are employed to validate the model’s predictions.
- Handling missing data: To maintain data integrity, missing values are addressed using methods from previous studies, ensuring that the dataset remains robust and reliable for simulation purposes.
2.4. Validation
2.4.1. Simulations
- -
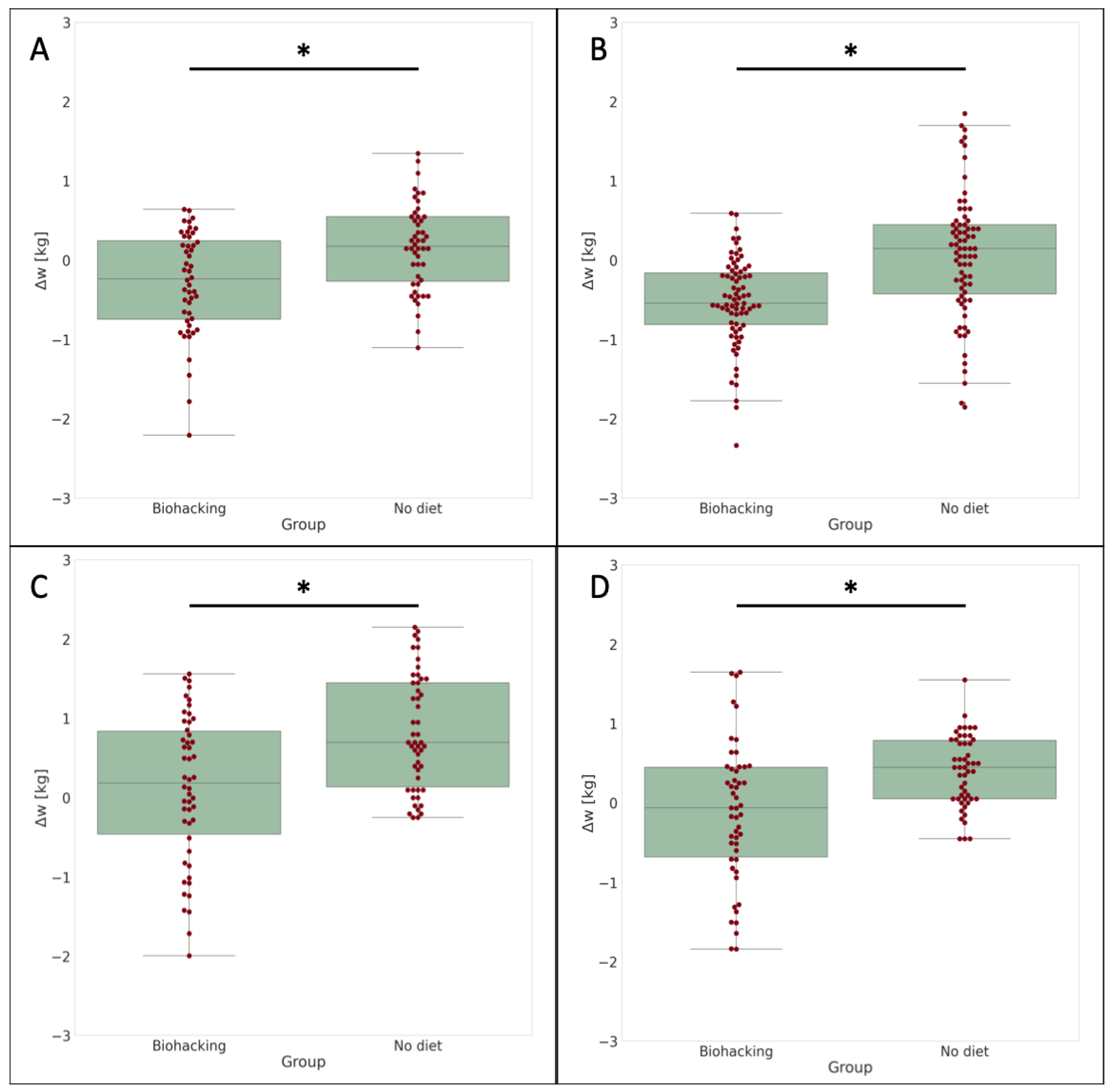
- Firstly, it evaluates whether there is a statistically significant difference between the mean weight changes of the two distributions. If the p-value resulting from the t-test is below the chosen significance level () we can conclude that there is a significant difference between the distributions.
- -
- Secondly, the t-test also allows us to investigate the directionality of the difference. By checking the sign of the t-statistic, we can determine if the simulated weight changes tend to be lower (statistically negative) or higher than the actual weight changes. A negative t-statistic indicates that, on average, the simulated weight changes are lower than the actual weight changes.
2.4.2. Real Data
2.5. Computational Requirements and Python Libraries
3. Results
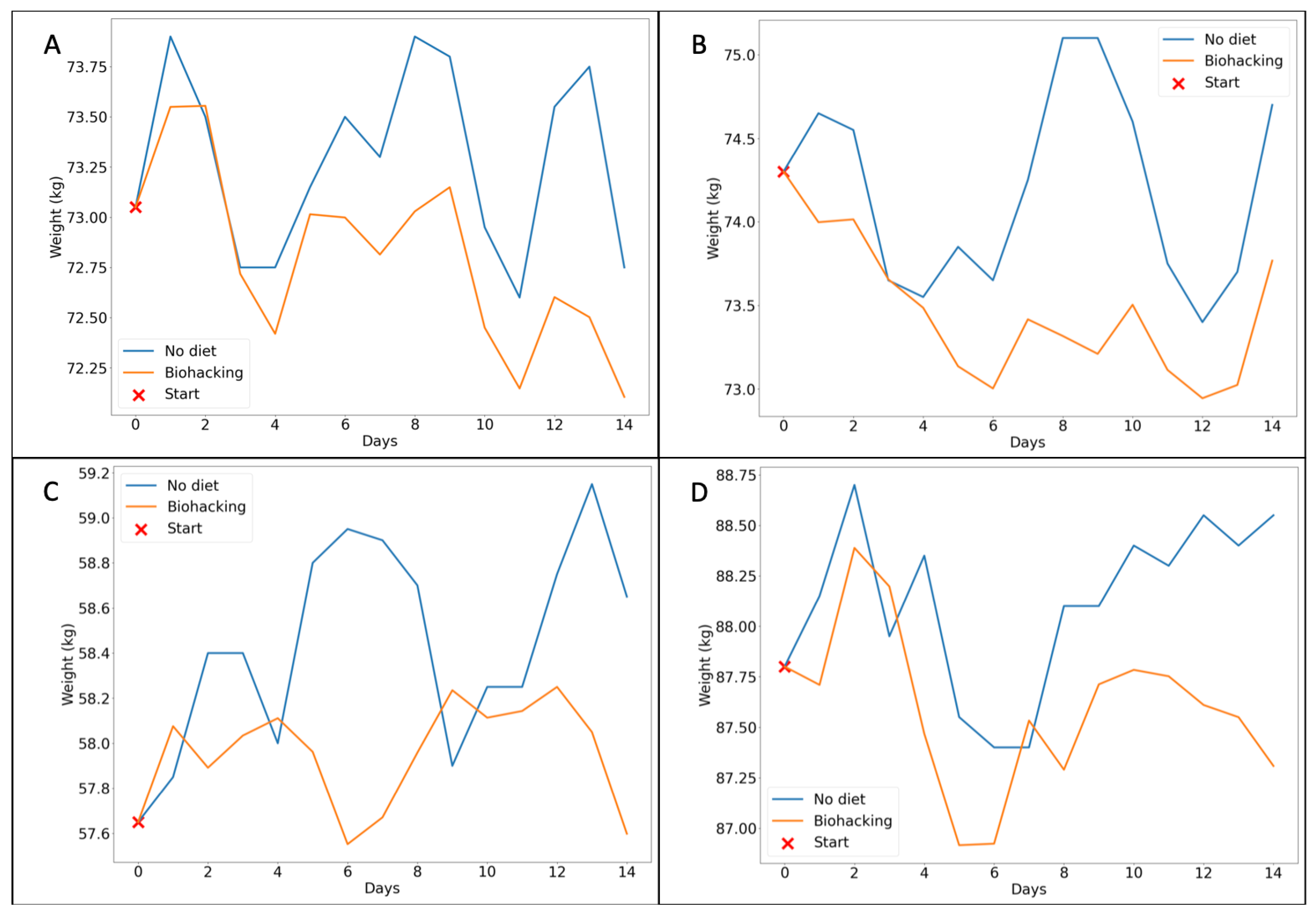
3.1. Simulations
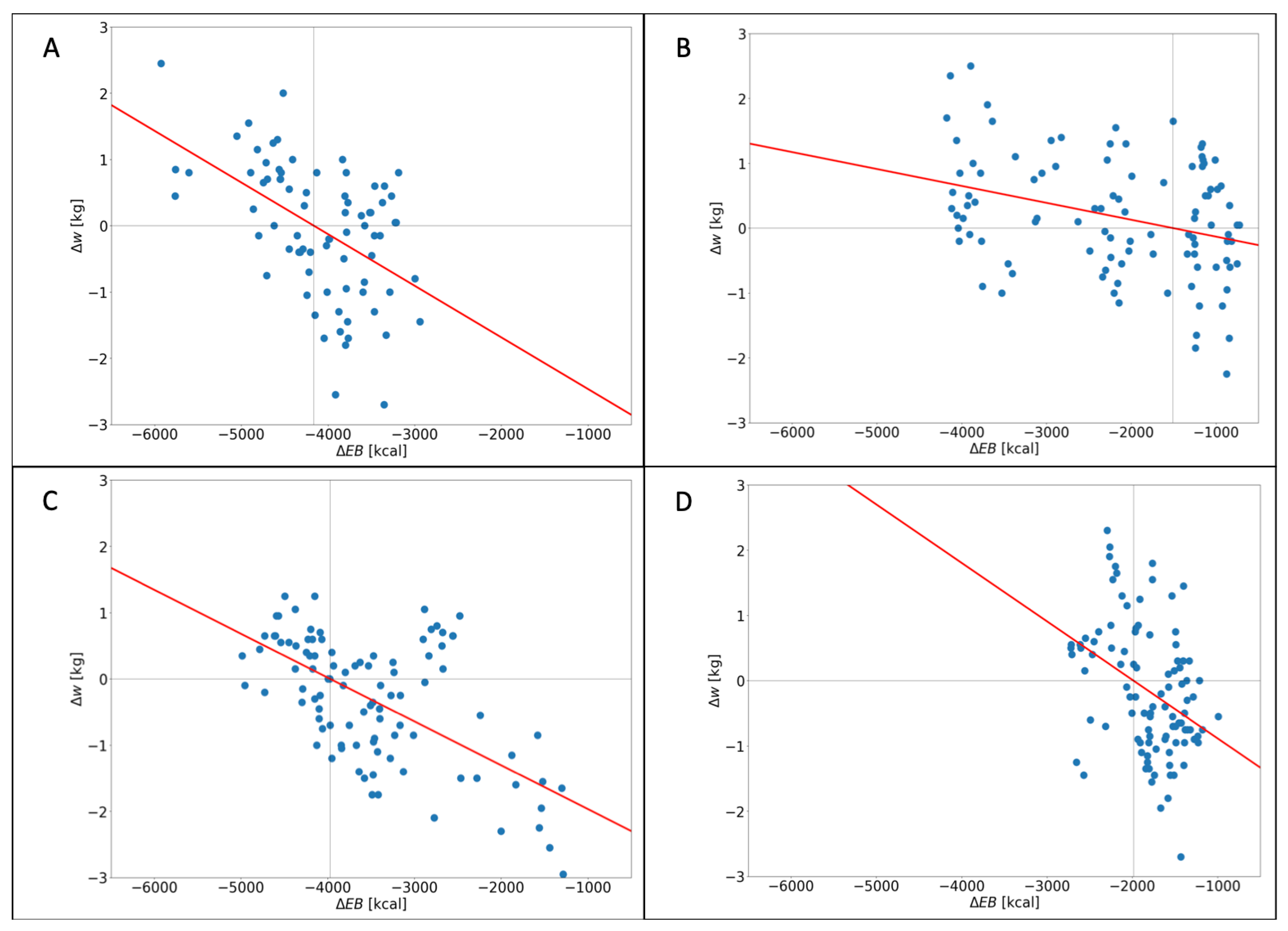
3.2. Linear Regression Analysis
4. Discussion
5. Limitations and Future Trends
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.; Cardel, M.; Donahoo, W.T. Social and Environmental Factors Influencing Obesity; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Allison, D.B.; Downey, M.; Atkinson, R.L.; Billington, C.J.; Bray, G.A.; Eckel, R.H.; Finkelstein, E.A.; Jensen, M.D.; Tremblay, A. Obesity as a Disease: A White Paper on Evidence and Arguments Commissioned by the Council of The Obesity Society. Obesity 2008, 16, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2010, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; VanHeest, J.L.; Forsythe, C.E. Diet and Exercise for Weight Loss. Sports Med. 2005, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Grunwald, G.; Melanson, E.; Saris, W.; Hill, J. The Role of Low-Fat Diets in Body Weight Control: A Meta-Analysis of Ad Libitum Dietary Intervention Studies. Int. J. Obes. 2000, 24, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- McCann, B.S.; Retzlaff, B.M.; Dowdy, A.A.; Walden, C.E.; Knopp, R.H. Promoting Adherence to Low-Fat, Low-Cholesterol Diets: Review and Recommendations. J. Am. Diet. Assoc. 1990, 90, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Swink, T.D.; Vining, E.P.; Freeman, J.M. The Ketogenic Diet: 1997. Adv. Pediatr. 1997, 44, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; McGrogan, J.R. Worldwide Use of the Ketogenic Diet. Epilepsia 2005, 46, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Montani, J.; Dulloo, A.G. Low-carbohydrate Ketogenic Diets in Body Weight Control: A Recurrent Plaguing Issue of Fad Diets? Obes. Rev. 2021, 22, e13195. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- Ganiyu, A.B.; Mabuza, L.H.; Malete, N.H.; Govender, I.; Ogunbanjo, G.A. Non-Adherence to Diet and Exercise Recommendations amongst Patients with Type 2 Diabetes Mellitus Attending Extension II Clinic in Botswana. Afr. J. Prim. Health Care Fam. Med. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Castellani, V.; Sala, S. Environmental Impacts of Food Consumption in Europe. J. Clean. Prod. 2017, 140, 753–765. [Google Scholar] [CrossRef]
- Donati, M.; Menozzi, D.; Zighetti, C.; Rosi, A.; Zinetti, A.; Scazzina, F. Towards a Sustainable Diet Combining Economic, Environmental and Nutritional Objectives. Appetite 2016, 106, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nagata, J.M.; Brown, T.A.; Lavender, J.M.; Murray, S.B. Emerging Trends in Eating Disorders among Adolescent Boys: Muscles, Macronutrients, and Biohacking. Lancet Child Adolesc. Health 2019, 3, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Gangadharbatla, H. Biohacking: An Exploratory Study to Understand the Factors Influencing the Adoption of Embedded Technologies within the Human Body. Heliyon 2020, 6, e03931. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Abeltino, A.; Serantoni, C.; Ardito, F.; Malta, D.; De Spirito, M.; Maulucci, G. Personalized Self-Monitoring of Energy Balance through Integration in a Web-Application of Dietary, Anthropometric, and Physical Activity Data. J. Pers. Med. 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Abeltino, A.; Bianchetti, G.; Serantoni, C.; Ardito, C.F.; Malta, D.; De Spirito, M.; Maulucci, G. Personalized Metabolic Avatar: A Data Driven Model of Metabolism for Weight Variation Forecasting and Diet Plan Evaluation. Nutrients 2022, 14, 3520. [Google Scholar] [CrossRef]
- Abeltino, A.; Bianchetti, G.; Serantoni, C.; Riente, A.; De Spirito, M.; Maulucci, G. Putting the Personalized Metabolic Avatar into Production: A Comparison between Deep-Learning and Statistical Models for Weight Prediction. Nutrients 2023, 15, 1199. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, D.-W.; Zeng, X.-A.; Liu, D.; Pu, H. Research Developments in Methods to Reduce the Carbon Footprint of the Food System: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1270–1286. [Google Scholar] [CrossRef] [PubMed]
- Lubis, J.; Thongdaeng, N.; Haqiyah, A.; Sukur, A.; Abidin, D.; Irawan, A.A.; Sumartiningsih, S.; Hanief, Y.N. The Effect of Five-Week Aerobic Interval Training on the Body Composition of Pencak Silat Elite Athletes. Int. J. Kinesiol. Sports Sci. 2022, 10, 16–24. [Google Scholar] [CrossRef]
- Pino-Ortega, J.; Gómez-Carmona, C.D.; Rico-González, M. Accuracy of Xiaomi Mi Band 2.0, 3.0 and 4.0 to Measure Step Count and Distance for Physical Activity and Healthcare in Adults over 65 Years. Gait Posture 2021, 87, 6–10. [Google Scholar] [CrossRef]
- Saenz, C.; Hooper, S.; Orange, T.; Knight, A.; Barragan, M.; Lynch, T.; Remenapp, A.; Coyle, K.; Winters, C.; Hausenblas, H. Effect of a Free-Living Ketogenic Diet on Feasibility, Satiety, Body Composition, and Metabolic Health in Women: The Grading Level of Optimal Carbohydrate for Women (GLOW) Study. J. Am. Coll. Nutr. 2021, 40, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Pêgo-Fernandes, P.M.; Bibas, B.J.; Deboni, M. Obesity: The Greatest Epidemic of the 21st Century? Sao Paulo Med. J. 2011, 129, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Peralta, M.; Naia, A.; Loureiro, N.; de Matos, M.G. Prevalence of Adult Overweight and Obesity in 20 European Countries, 2014. Eur. J. Public Health 2018, 28, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M. Childhood Obesity in Europe: A Growing Concern. Public Health Nutr. 2001, 4, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting Obesity in Children from European World Health Organization Member States. Epidemiological Data, Medical-Social Aspects, and Prevention Programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar] [CrossRef]
- Krzysztoszek, J.; Laudańska-Krzemińska, I.; Bronikowski, M. Assessment of Epidemiological Obesity among Adults in EU Countries. Ann. Agric. Environ. Med. 2019, 26, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. COVID-19 and Obesity: Overlapping of Two Pandemics. Obes. Facts 2021, 14, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Grazio, S.; Balen, D. Obesity: Risk Factor and Predictor of Osteoarthritis. Lijec. Vjesn. 2009, 131, 22–26. [Google Scholar]
- Alshehri, F.; Muhammad, G. A Comprehensive Survey of the Internet of Things (IoT) and AI-Based Smart Healthcare. IEEE Access 2021, 9, 3660–3678. [Google Scholar] [CrossRef]
- Ahmad, W.; Rasool, A.; Javed, A.R.; Baker, T.; Jalil, Z. Cyber Security in IoT-Based Cloud Computing: A Comprehensive Survey. Electronics 2022, 11, 16. [Google Scholar] [CrossRef]
- Durojaye, O.; Laseinde, T.; Oluwafemi, I. A Descriptive Review of Carbon Footprint. In Human Systems Engineering and Design II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 960–968. [Google Scholar] [CrossRef]
- Gillings, S.; Harris, S.J. Estimating the Carbon Footprint of Citizen Science Biodiversity Monitoring. People Nat. 2022, 4, 996–1006. [Google Scholar] [CrossRef]
- Sazonov, E.S.; Fontana, J.M. A Sensor System for Automatic Detection of Food Intake through Non-Invasive Monitoring of Chewing. IEEE Sens. J. 2012, 12, 1340–1348. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Izadi, V.; Azadbakht, L. The Effect of Low Calorie Diet on Adiponectin Concentration: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2015, 47, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative Adversarial Networks: An Overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef]
- Galloway-Peña, J.; Hanson, B. Tools for Analysis of the Microbiome. Dig. Dis. Sci. 2020, 65, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; De Maio, F.; Abeltino, A.; Serantoni, C.; Riente, A.; Santarelli, G.; Sanguinetti, M.; Delogu, G.; Martinoli, R.; Barbaresi, S.; et al. Unraveling the Gut Microbiome–Diet Connection: Exploring the Impact of Digital Precision and Personalized Nutrition on Microbiota Composition and Host Physiology. Nutrients 2023, 15, 3931. [Google Scholar] [CrossRef]
- Suckling, R.J.; Swift, P.A. The Health Impacts of Dietary Sodium and a Low-Salt Diet. Clin. Med. 2015, 15, 585–588. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef]
- Serantoni, C.; Zimatore, G.; Bianchetti, G.; Abeltino, A.; De Spirito, M.; Maulucci, G. Unsupervised Clustering of Heartbeat Dynamics Allows for Real Time and Personalized Improvement in Cardiovascular Fitness. Sensors 2022, 22, 3974. [Google Scholar] [CrossRef]
- Muoio, D.M.; Leddy, J.J.; Horvath, P.J.; Awad, A.B.; Pendergast, D.R. Effect of Dietary Fat on Metabolic Adjustments to Maximal VO2 and Endurance in Runners. Med. Sci. Sports Exerc. 1994, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Azzolina, D.; Folino, F.; Valentini, R.; Bendinelli, C.; Gafare, C.E.; Cainelli, E.; Vedovelli, L.; Iliceto, S.; Gregori, D.; et al. Dietary and Lifestyle Patterns Are Associated with Heart Rate Variability. J. Clin. Med. 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Rizzo, G.E.; Serantoni, C.; Abeltino, A.; Rizzi, A.; Tartaglione, L.; Caputo, S.; Flex, A.; De Spirito, M.; Pitocco, D.; et al. Spatial Reorganization of Liquid Crystalline Domains of Red Blood Cells in Type 2 Diabetic Patients with Peripheral Artery Disease. Int. J. Mol. Sci. 2022, 23, 11126. [Google Scholar] [CrossRef]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Investigation of DHA-Induced Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells through the Combination of Metabolic Imaging and Molecular Biology. Antioxidants 2022, 11, 1072. [Google Scholar] [CrossRef]
| Parameter | Average Daily Variation | Decrease Percentage |
|---|---|---|
| Average daily intake reduction | −236.78 ± 50.65 kcal | 14.24 ± 3.1% |
| Average daily carbon footprint impact reduction | 15.12 ± 1.13% |
| Participant | Actual Delta Weight | Simulated Delta Weight | Delta Weight Loss 2 | p-Value 3 | t-Statistic 4 |
|---|---|---|---|---|---|
| 0 | 0.17 ± 0.54 kg | −0.29 ± 0.64 kg | −0.48 ± 0.54 kg | −5.81 | |
| 1 | −0.02 ± 0.86 kg | −0.70 ± 0.56 kg | −0.68 ± 0.77 kg | −6.24 | |
| 2 | 0.82 ± 0.72 kg | 0.12 ± 0.92 kg | −0.70 ±1.06 kg | −4.64 | |
| 3 | 0.40 ± 0.44 kg | −0.12 ± 0.87 kg | −0.52 ± 1.01 kg | −3.62 |
| Participant | p-Value 2 | Slope (Kg/Kcal) 3 | 4 | Pearson Coefficient 5 |
|---|---|---|---|---|
| 0 | −0.0008 | 0.25 | −0.5 | |
| 1 | −0.0003 | 0.11 | −0.33 | |
| 2 | −0.0009 | 0.14 | −0.37 | |
| 3 | −0.0007 | 0.38 | −0.62 |
| Generic Diets | Digital Biohacking | |
|---|---|---|
| Personalization | Limited | Highly Customized |
| User-specific data consideration | Minimal | Comprehensive |
| Incorporation of taste preferences | Limited | Extensive |
| Sustainability (emission reduction) | Not addressed | Addressed |
| Long-term adherence potential | Challenging | Promising |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeltino, A.; Bianchetti, G.; Serantoni, C.; Riente, A.; De Spirito, M.; Maulucci, G. Digital Biohacking Approach to Dietary Interventions: A Comprehensive Strategy for Healthy and Sustainable Weight Loss. Nutrients 2024, 16, 2021. https://doi.org/10.3390/nu16132021
Abeltino A, Bianchetti G, Serantoni C, Riente A, De Spirito M, Maulucci G. Digital Biohacking Approach to Dietary Interventions: A Comprehensive Strategy for Healthy and Sustainable Weight Loss. Nutrients. 2024; 16(13):2021. https://doi.org/10.3390/nu16132021
Chicago/Turabian StyleAbeltino, Alessio, Giada Bianchetti, Cassandra Serantoni, Alessia Riente, Marco De Spirito, and Giuseppe Maulucci. 2024. "Digital Biohacking Approach to Dietary Interventions: A Comprehensive Strategy for Healthy and Sustainable Weight Loss" Nutrients 16, no. 13: 2021. https://doi.org/10.3390/nu16132021
APA StyleAbeltino, A., Bianchetti, G., Serantoni, C., Riente, A., De Spirito, M., & Maulucci, G. (2024). Digital Biohacking Approach to Dietary Interventions: A Comprehensive Strategy for Healthy and Sustainable Weight Loss. Nutrients, 16(13), 2021. https://doi.org/10.3390/nu16132021










