Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults
Abstract
1. Introduction
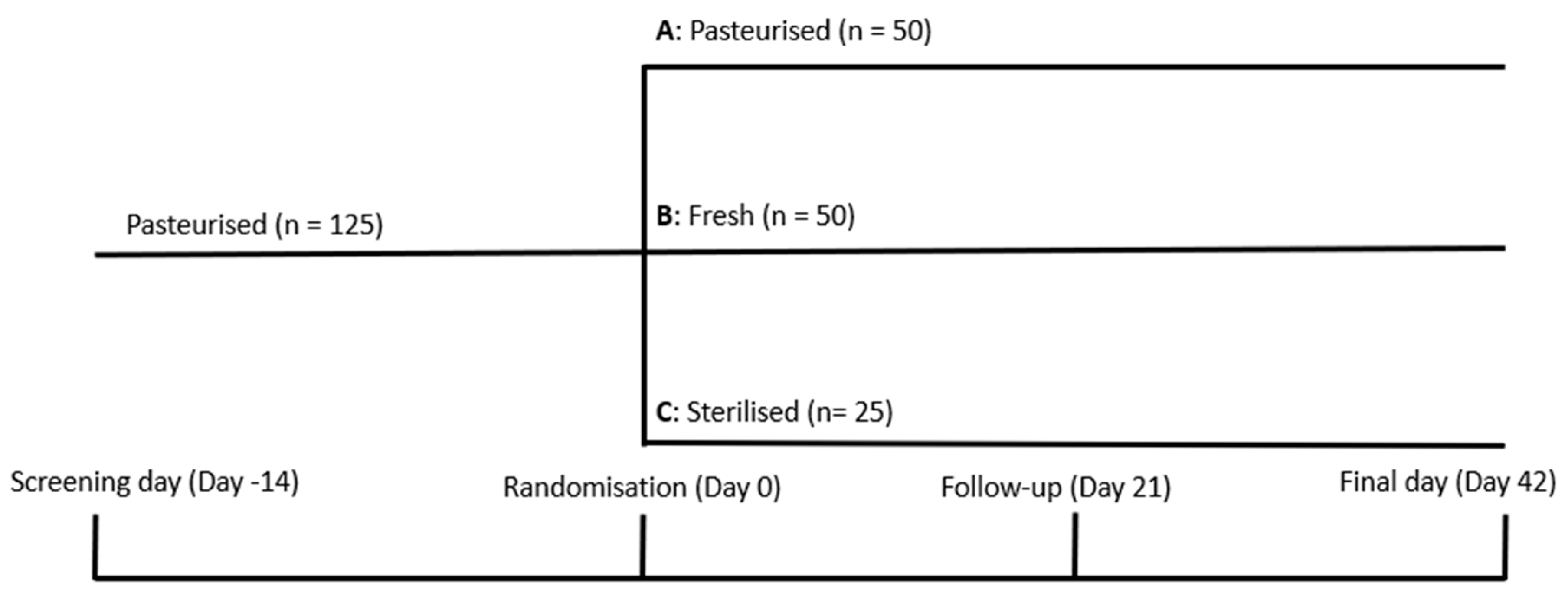
2. Materials and Methods
2.1. Diagnosis and Main Selection Criteria
2.2. Study Products
2.3. Nutritional Intervention
2.4. Measurement of Immune System Function Markers
2.4.1. Leukocyte Subpopulations
2.4.2. Immunochemistry
2.4.3. Phagocytosis and Burst
2.4.4. NK Cytotoxic Activity
2.4.5. IFN-γ Inducible Gene Expression
2.4.6. Intracellular Cytokine Profile
2.4.7. Cytokine Production
2.5. Statistical Analysis
3. Results and Discussion
3.1. Baseline
3.2. Leukocyte and Lymphocyte Subpopulations
3.3. Immunochemical Variables
3.4. Phagocytosis and Burst
3.5. NK Cytotoxic Activity
3.6. IFN-y Inducible Gene Expression
3.7. Intracellular Cytokine Profile
3.8. Cytokine Production
3.9. Limitations of the Study
- -
- The absence of a control group (white) that had not eaten yogurt of any kind for 8 weeks.
- -
- The small number of individuals in the group that consumed PMI pasteurized yogurt (group C), which resulted in notable changes in some variables not reaching statistical significance.
- -
- The absence of analysis months after having completed the dietary intervention, which would have allowed us to see if the immunomodulation observed is transitory or remains over time.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and immune function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Ferreira, R.d.S.; Mendonça, L.A.B.M.; Ribeiro, C.F.A.; Calças, N.C.; Guimarães, R.d.C.A.; Nascimento, V.A.d.; Gielow, K.d.C.F.; Carvalho, C.M.E.; Castro, A.P.d.; Franco, O.L.; et al. Relationship between intestinal microbiota, diet and biological systems: An integrated view. Crit. Rev. Food Sci. Nutr. 2022, 62, 1166–1186. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J. Role of microbiota in immunity and inflammation. Natl. Inst. Health 2011, 72, 181–204. [Google Scholar] [CrossRef]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef]
- Lisko, D.J.; Johnston, G.P.; Johnston, C.G. Effects of dietary yogurt on the healthy human gastrointestinal (Gi) microbiome. Microorganisms 2017, 5, 6. [Google Scholar] [CrossRef]
- do Carmo, M.S.; dos Santos, C.I.; Araújo, M.C.; Girón, J.A.; Fernandes, E.S.; Monteiro-Neto, V. Probiotics, mechanisms of action, and clinical perspectives for diarrhea management in children. Food Funct. 2018, 9, 5074–5095. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.d.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef]
- Briand, V.; Buffet, P.; Genty, S.; Lacombe, K.; Godineau, N.; Salomon, J.; Vandemelbrouck, E.; Ralaimazava, P.; Goujon, C.; Matheron, S.; et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: A randomized, double-blind, controlled study. Clin. Infect. Dis. 2006, 43, 1170–1175. [Google Scholar] [CrossRef]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. The influence of yogurt/lactobacillus on the innate and acquired immune response. Clin. Rev. Allergy Immunol. 2002, 22, 207–230. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- Homburger, H.A.; Singh, R.J. 96—Assessment of proteins of the immune system. In Clinical Immunology, 3rd ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M.B., Eds.; Mosby: Edinburgh, UK, 2008; pp. 1419–1434. ISBN 978-0-323-04404-2. [Google Scholar]
- Hirt, W.; Nebe, T.; Birr, C. Phagotest and Bursttest (Phagoburst), test kits for study of phagocyte functions. Wien. Klin. Wochenschr. 1994, 106, 250–252. [Google Scholar]
- Kantakamalakul, W.; Jaroenpool, J.; Pattanapanyasat, K. A novel enhanced green fluorescent protein (EGFP)-K562 flow cytometric method for measuring natural killer (NK) cell cytotoxic activity. J. Immunol. Methods 2003, 272, 189–197. [Google Scholar] [CrossRef]
- Tsutsui-Takeuchi, M.; Ushio, H.; Fukuda, M.; Yamada, T.; Niyonsaba, F.; Okumura, K.; Ogawa, H.; Ikeda, S. Roles of retinoic acid-inducible gene-I-like receptors (RLRs), Toll-like receptor (TLR) 3 and 2′-5′ oligoadenylate synthetase as viral recognition receptors on human mast cells in response to viral infection. Immunol. Res. 2015, 61, 240–249. [Google Scholar] [CrossRef][Green Version]
- Nagase, H.; Okugawa, S.; Ota, Y.; Yamaguchi, M.; Tomizawa, H.; Matsushima, K.; Ohta, K.; Yamamoto, K.; Hirai, K. Expression and Function of Toll-Like Receptors in Eosinophils: Activation by Toll-Like Receptor 7 Ligand. J. Immunol. 2003, 171, 3977–3982. [Google Scholar] [CrossRef]
- Khabar, K.S.A.; Al-Zoghaibi, F.; Al-Ahdal, M.N.; Murayama, T.; Dhalla, M.; Mukaida, N.; Taha, M.; Al-Sedairy, S.T.; Siddiqui, Y.; Kessie, G.; et al. The α chemokine, interleukin 8, inhibits the antiviral action of interferon α. J. Exp. Med. 1997, 186, 1077–1085. [Google Scholar] [CrossRef]
- Berthoud, T.K.; Manaca, M.N.; Quelhas, D.; Aguilar, R.; Guinovart, C.; Puyol, L.; Barbosa, A.; Alonso, P.L.; Dobaño, C. Comparison of commercial kits to measure cytokine responses to Plasmodium falciparum by multiplex microsphere suspension array technology. Malar. J. 2011, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Z.; Han, J.; Liu, W.; Xia, P.; Cai, X.; Liu, X.; Liu, X.; Zhang, J.; Yu, P. Multifaceted roles of T cells in obesity and obesity-related complications: A narrative review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2023, 24, e13621. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed]
- Fock, R.A.; Blatt, S.L.; Beutler, B.; Pereira, J.; Tsujita, M.; de Barros, F.E.V.; Borelli, P. Study of lymphocyte subpopulations in bone marrow in a model of protein–energy malnutrition. Nutrition 2010, 26, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.Y.; Quek, J.K.S.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.M.; Stephensen, C.B.; Kratz, M.; Bolling, B.W. Exploring the Links between Diet and Inflammation: Dairy Foods as Case Studies. Adv. Nutr. 2021, 12, 1S–13S. [Google Scholar] [CrossRef]
- Meng, H.; Ba, Z.; Lee, Y.; Peng, J.; Lin, J.; Fleming, J.A.; Furumoto, E.J.; Roberts, R.F.; Kris-Etherton, P.M.; Rogers, C.J. Consumption of Bifidobacterium animalis subsp. lactis BB-12 in yogurt reduced expression of TLR-2 on peripheral blood-derived monocytes and pro-inflammatory cytokine secretion in young adults. Eur. J. Nutr. 2017, 56, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Karawajczyk, M.; Håkansson, L.D.; Lipcsey, M.; Hultström, M.; Pauksens, K.; Frithiof, R.; Larsson, A. High expression of neutrophil and monocyte CD64 with simultaneous lack of upregulation of adhesion receptors CD11b, CD162, CD15, CD65 on neutrophils in severe COVID-19. Ther. Adv. Infect. Dis. 2021, 8, 20499361211034064. [Google Scholar] [CrossRef]
- Khan, S.R.; van der Burgh, A.C.; Peeters, R.P.; van Hagen, P.M.; Dalm, V.A.S.H.; Chaker, L. Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 664526. [Google Scholar] [CrossRef]
- Zhang, T.; Geng, S.; Cheng, T.; Mao, K.; Chitrakar, B.; Gao, J.; Sang, Y. From the past to the future: Fermented milks and their health effects against human diseases. Food Front. 2023, 4, 1747–1777. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rul, F.; Béra-Maillet, C.; Champomier-Vergès, M.C.; El-Mecherfi, K.E.; Foligné, B.; Michalski, M.C.; Milenkovic, D.; Savary-Auzeloux, I. Underlying evidence for the health benefits of fermented foods in humans. Food Funct. 2022, 13, 4804–4824. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.S.; Chen, Y.P.; Chen, M.J. The antiallergic effect of kefir lactobacilli. J. Food Sci. 2010, 75, H244–H253. [Google Scholar] [CrossRef]
- Singh, K.; Rao, A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.-Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, P.; Sharma, R. Natural Killer Cells - Their Role in Tumour Immunosurveillance. J. Clin. Diagn. Res. 2017, 11, BE01–BE05. [Google Scholar] [CrossRef]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef]
- Illikoud, N.; Mantel, M.; Rolli-Derkinderen, M.; Gagnaire, V.; Jan, G. Dairy starters and fermented dairy products modulate gut mucosal immunity. Immunol. Lett. 2022, 251–252, 91–102. [Google Scholar] [CrossRef]
- Hashemi, B.; Abdollahi, M.; Abbaspour-Aghdam, S.; Hazrati, A.; Malekpour, K.; Meshgi, S.; Kafil, H.S.; Ghazi, F.; Yousefi, M.; Roshangar, L.; et al. The effect of probiotics on immune responses and their therapeutic application: A new treatment option for multiple sclerosis. Biomed. Pharmacother. 2023, 159, 114195. [Google Scholar] [CrossRef] [PubMed]
- Koyama-Nasu, R.; Wang, Y.; Hasegawa, I.; Endo, Y.; Nakayama, T.; Kimura, M.Y. The cellular and molecular basis of CD69 function in anti-tumor immunity. Int. Immunol. 2022, 34, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Wang, J.; Huang, B.; Yin, S. Low-fat yogurt alleviates the pro-inflammatory cytokine IL-1β-induced intestinal epithelial barrier dysfunction. J. Dairy Sci. 2019, 102, 976–984. [Google Scholar] [CrossRef]
- Chang, Y.H.; Jeong, C.H.; Cheng, W.N.; Choi, Y.; Shin, D.M.; Lee, S.; Han, S.G. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 2021, 104, 7415–7425. [Google Scholar] [CrossRef]
| Day | |||||
|---|---|---|---|---|---|
| Parameter | Group | −14 | 0 | 21 | 42 |
| % CD3+ lymphocytes | A | 74.56 ± 7.05 | 75.48 ± 6.99 | 76.85 ± 6.58 | 76.24 ± 6.92 |
| B | 75.17 ± 7.07 | 75.89 ± 6.88 | |||
| C | 72.52 ± 6.69 * | 73.29 ± 7.3 * | |||
| CD3+ lymphocytes/μL | A | 1916.22 ± 583.63 | 1934.56 ± 584.23 | 1998.67 ± 669.54 | 1982.56 ± 675.94 |
| B | 1934.4 ± 536.02 | 1973.83 ± 523.42 | |||
| C | 1784.04 ± 518.14 * | 1803.02 ± 502.75 * | |||
| % CD3+ CD4+ T helper lymphocytes | A | 46.37 ± 7.51 | 47.7 ± 7.28 | 48.5 ± 7.29 | 48.09 ± 6.9 |
| B | 48.38 ± 7.73 | 49.15 ± 7.5 * | |||
| C | 45.08 ± 6.62 | 45.97 ± 7.88 * | |||
| % Cytotoxic T lymphocytes CD3+ CD8+ | A | 22.96 ± 8.68 | 22.82 ± 7.72 | 23.81 ± 8.34 | 23.88 ± 7.56 |
| B | 21.53 ± 7.52 | 21.68 ± 7.29 | |||
| C | 22.16 ± 8.02 | 22.45 ± 7.77 | |||
| Ratio of CD4+/CD8+ helper/cytotoxic T lymphocytes | A | 2.33 ± 1.02 | 2.39 ± 1.04 | 2.35 ± 1.02 | 2.28 ± 0.95 |
| B | 2.58 ± 1.17 * | 2.58 ± 1.1 * | |||
| C | 2.37 ± 1.08 | 2.38 ± 1.12 | |||
| % B lymphocytes CD19+ | A | 9.92 ± 3.14 | 10.07 ± 3.15 | 10.2 ± 3.11 | 10.41 ± 2.92 |
| B | 10.51 ± 3.39 | 10.56 ± 3.53 | |||
| C | 11.07 ± 3.38 * | 10.98 ± 3.48 * | |||
| Ratio of T/B CD3+/CD19+ lymphocytes | A | 8.44 ± 3.14 | 8.38 ± 3.33 | 8.65 ± 4.61 | 8.37 ± 4.77 |
| B | 8.01 ± 3.03 | 7.95 ± 2.59 | |||
| C | 7.22 ± 2.41 | 7.47 ± 3.03 | |||
| % NK CD3− CD56+ CD16+ lymphocytes | A | 14.97 ± 10.88 | 13.87 ± 6.71 | 12.27 ± 5.68 * | 12.65 ± 5.81 |
| B | 13.75 ± 7.36 * | 12.86 ± 7.18 | |||
| C | 15.73 ± 5.6 * | 15.14 ± 6.99 * | |||
| % Naïve T lymphocytes CD3+ CD45RA+ CD45R0− | A | 30.56 ± 11.13 | 30.53 ± 11.21 | 30.56 ± 11.17 | 31 ± 10.43 |
| B | 32.61 ± 11.55 | 33.47 ± 11.67 | |||
| C | 25.51 ± 10.5 * | 26.36 ± 10.66 * | |||
| % Memory T lymphocytes CD3+ CD45RA− CD45R0+ | A | 35.86 ± 12.86 | 34.4 ± 12.19 | 33.57 ± 12.64 * | 34.32 ± 12.43 |
| B | 32.64 ± 11.18 | 34.34 ± 11.08 | |||
| C | 36.92 ± 12.04 | 39.35 ± 14.04 | |||
| Ratio of naïve T lymphocytes to memory | A | 1.12 ± 0.71 | 1.07 ± 0.67 | 1.11 ± 0.67 | 1.17 ± 0.88 |
| B | 1.23 ± 0.81 | 1.18 ± 0.73 | |||
| C | 0.83 ± 0.55 * | 0.92 ± 0.89 * | |||
| % Naïve helper T lymphocytes CD3+ CD4+ CD45RA+ CD45R0− | A | 13.70 ± 7.63 | 13.7 ± 7.61 | 13.44 ± 7.13 | 14.04 ± 7.63 |
| B | 14.97 ± 8.1 | 16.17 ± 8.44 | |||
| C | 11.22 ± 6.38 | 11.96 ± 6.44 | |||
| %Memory helper T lymphocytes CD3+ CD4+ CD45RA-R0+ | A | 26.65 ± 9.35 | 25.51 ± 9.67 | 24.93 ± 9.12 | 25.53 ± 9.52 |
| B | 25.27 ± 8.82 | 26.75 ± 8.62 | |||
| C | 25.96 ± 9.67 | 28.12 ± 11.8 * | |||
| Ratio of naïve helper T cells to memory | A | 0.64 ± 0.40 | 0.64 ± 0.51 | 0.62 ± 0.4 | 0.66 ± 0.48 |
| B | 0.73 ± 0.57 | 0.72 ± 0.52 | |||
| C | 0.51 ± 0.36 | 0.57 ± 0.5 | |||
| % Naïve cytotoxic T lymphocytes CD3+ CD8+ CD45RA+ CD45R0− | A | 17.69 ± 6.90 | 17.82 ± 7.12 | 17.75 ± 7.1 | 17.79 ± 6.25 |
| B | 18.31 ± 7.15 | 17.96 ± 7.3 | |||
| C | 15.21 ± 6.11 * | 15.18 ± 6.74 * | |||
| MFI naïve cytotoxic T lymphocytes CD3+ CD8+ CD45RA+ CD45R0− | A | 15.73 ± 4.52 | 14.02 ± 4.42 | 12.76 ± 4.38 | 12.63 ± 3.43 |
| B | 12.5 ± 3.36 | 12.74 ± 2.7 | |||
| C | 12.28 ± 3.74 | 12.04 ± 2.84 | |||
| % Cytotoxic T lymphocytes memory CD3+ CD8+ CD45RA− CD45R0+ | A | 10.14 ± 5.89 | 9.59 ± 5.14 | 9.33 ± 5.13 | 9.72 ± 5.25 |
| B | 8.12 ± 4.04 | 8.33 ± 3.83 | |||
| C | 11.89 ± 7.87 * | 12.11 ± 7.34 * | |||
| Ratio of naïve cytotoxic T lymphocytes to memory | A | 2.79 ± 1.48 | 2.57 ± 2.04 | 2.75 ± 2.14 | 3.11 ± 3.66 |
| B | 2.95 ± 1.97 | 2.67 ± 1.69 | |||
| C | 1.88 ± 1.45 * | 1.96 ± 1.87 * | |||
| % CD11b Expression in Lymphocytes | A | 33.03 ± 9.38 | 31.75 ± 8.89 | 30.13 ± 9.54 | 31 ± 8.72 |
| B | 30.7 ± 8.23 | 30.25 ± 8.43 | |||
| C | 34.92 ± 8.35 * | 34.43 ± 11.42 * | |||
| % HLA-DR and CD11b Expression in Lymphocytes | A | 6.51 ± 3.11 | 6.46 ± 2.71 | 6.45 ± 2.23 | 6.48 ± 1.89 |
| B | 6.5 ± 2.36 | 6.6 ± 2.84 | |||
| C | 8.03 ± 3.35 * | 8.07 ± 3.2 * | |||
| % HLA-DR Expression in Monocytes | A | 53.57 ± 16.90 | 53.37 ± 17.19 | 50.83 ± 16.85 * | 49.62 ± 16.58 * |
| B | 54.24 ± 14.33 | 53.14 ± 16.06 | |||
| C | 55.02 ± 18.7 | 54.96 ± 19.41 | |||
| % CD11b Expression in Monocytes | A | 99.85 ± 0.33 | 99.9 ± 0.12 | 99.87 ± 0.16 | 99.86 ± 0.14 |
| B | 99.86 ± 0.18 | 99.85 ± 0.2 | |||
| C | 99.85 ± 0.17 | 99.91 ± 0.14 | |||
| MFI CD11b expression in monocytes | A | 96.64 ± 16.90 | 91.91 ± 21.62 | 89.69 ± 18.7 | 91.73 ± 23.01 |
| B | 89.73 ± 17.24 | 90.27 ± 22.01 | |||
| C | 84.26 ± 21.33 * | 84.84 ± 17.37 * | |||
| % CD11b Expression in Granulocytes | A | 100.00 ± 0.01 | 100.00 ± 0.01 | 100.00 ± 0.01 | 100.00 ± 0.01 |
| B | 100.00 ± 0.01 | 100.00 ± 0.01 | |||
| C | 100.00 ± 0.01 | 100.00 ± 0.01 | |||
| MFI CD11b expression in granulocytes | A | 80.96 ± 11.46 | 83.21 ± 70.11 | 74.74 ± 14.6 * | 75.63 ± 14.89 * |
| B | 79.96 ± 16.76 | 77.34 ± 19 * | |||
| C | 73.18 ± 13.65 * | 73.31 ± 16.62 * | |||
| Day | |||||
|---|---|---|---|---|---|
| Parameter | Group | −14 | 0 | 21 | 42 |
| IgG (mg/dL): | A | 1031.46 ± 162.08 | 1059.1 ± 205.53 | 1031.25 ± 148.55 | 1038.96 ± 158.06 |
| B | 1054.02 ± 246.8 | 1068.94 ± 223.82 | |||
| C | 1056.24 ± 213.66 | 1058 ± 205.65 | |||
| IgA (mg/dL) | A | 205.37 ± 69.52 | 217.89 ± 77.61 | 208.54 ± 75.74 | 213.47 ± 75.07 |
| B | 214.81 ± 78.03 | 216.4 ± 68.41 | |||
| C | 220.8 ± 75.43 | 218.99 ± 80.27 | |||
| IgM (mg/dL): | A | 121.54 ± 55.21 | 136.07 ± 68.75 | 128.67 ± 58.39 | 128.16 ± 60.72 |
| B | 141.81 ± 66.49 | 140.67 ± 66.3 | |||
| C | 109.07 ± 52.15 * | 106.09 ± 53.68 * | |||
| IgE total (KU/L): | A | 24.52 ± 8.56 | 30.43 ± 7.89 | 37.18 ± 9.95 | 42.76 ± 9.61 |
| B | 32.35 ± 9.96 | 33.79 ± 8.84 | |||
| C | 30.37 ± 8.62 | 29.04 ± 7.59 | |||
| C3c (mg/dL): | A | 121.89 ± 19.86 | 124.6 ± 25.29 | 118.15 ± 21.31 | 119.5 ± 22.9 |
| B | 121.04 ± 28.18 | 119.64 ± 27.82 | |||
| C | 121.96 ± 18.68 | 116.12 ± 16.57 | |||
| PCR (mg/L): | A | 0.81 ± 1.2 | 1.98 ± 5.8 | 1.07 ± 1.03 | 1.4 ± 1.92 |
| B | 1.49 ± 2.04 | 1.42 ± 1.77 | |||
| C | 1.83 ± 3.31 | 2.17 ± 4.13 | |||
| Day | |||||
|---|---|---|---|---|---|
| Parameter | Group | −14 | 0 | 21 | 42 |
| % and (MFI) Granulocyte phagocytosis/in the presence of E. coli | A | 91.71 ± 9.59 (476.33 ± 158.0) | 93.43 ± 6.95 (401.34 ± 147.75) | 91.21 ± 5.73 (328.41 ± 96.48 *) | 89.44 ± 9.41 (322.51 ± 92.73 *) |
| B | 91.02 ± 6.01 (345.11 ± 110.36 *) | 89.86 ± 7.41 (344.97 ± 117.8 *) | |||
| C | 91.46 ± 7.22 (334.68 ± 105.39 *) | 91.64 ± 5.58 (350.93 ± 108.21 *) | |||
| % and (MFI) Phagocytosis of monocytes in the presence of E. coli: | A | 80.16 ± 12.49 (228.32 ± 60.75) | 80.06 ± 11.93 (206.2 ± 61.02) | 72.69 ± 11.08 * (189.49 ± 40.2 *) | 74.65 ± 10.87 * (198.32 ± 46.16 *) |
| B | 70.34 ± 10.19 * (198.56 ± 48.44 *) | 73.85 ± 9.89 * (209.26 ± 48.13 *) | |||
| C | 74.08 ± 10.01 * (201.54 ± 45.23 *) | 74.64 ± 10.76 * (206.75 ± 41.35 *) | |||
| Indices % and (MFI) Phagocytosis in granulocytes | A | 57.38 ± 42.12 (16.76 ± 11.12) | 55.42 ± 35.45 (18.21 ± 9.44) | 57.19 ± 42.07 (15.74 ± 6.76) | 60.63 ± 32.69 (17.81 ± 6.04) |
| B | 56.30 ± 32.79 (14.11 ± 6.39) | 64.44 ± 28.58 (18.79 ± 10.01) | |||
| C | 56.49 ± 27.45 (15.55 ± 6.77) | 76.24 ± 42.75 * (17.96 ± 8.45) | |||
| % and (MFI) phagocytosis rates in monocytes | A | 33.43 ± 22.36 (7.91 ± 3.48) | 29.70 ± 19.18 (8.67 ± 3.55) | 32.41 ± 23.29 (8.98 ± 3.53) | 42.55 ± 50.29 * (10.11 ± 2.93 *) |
| B | 38.69 ± 37.98 * (7.66 ± 2.67) | 42.85 ± 38.90 * (10.24 ± 3.3 *) | |||
| C | 35.22 ± 27.98 (8.78 ± 3.55) | 35.43 ± 21.4 (9.51 ± 3.13 *) | |||
| Day | |||||
|---|---|---|---|---|---|
| Group | −14 | 0 | 21 | 42 | |
| % and (MFI) Granulocyte Burst in the Presence of E. coli: | A | 94.57 ± 9.10 (250.09 ± 88.75) | 96.75 ± 4.43 (228.4 ± 85.38) | 95.19 ± 6.32 (215.5 ± 67.51) | 96.58 ± 4.26 (227.51 ± 70.27) |
| B | 95.28 ± 4.41 (207.9 ± 65.38) | 96.75 ± 3.88 (228.45 ± 65.23) | |||
| C | 94.97 ± 6.42 (209.78 ± 85.4) | 96.47 ± 3.85 (213.39 ± 51.67) | |||
| % Burst and (MFI) monocytes in the presence of E. coli | A | 69.38 ± 19.22 (79.10 ± 33.88) | 69.66 ± 18.59 (67.62 ± 26.72) | 66.99 ± 18.54 (74.09 ± 36.69) | 66.72 ± 16.97 (69.35 ± 28.13) |
| B | 64.73 ± 16.76 (72.16 ± 20.41) | 67.04 ± 13.32 (68.1 ± 17.58) | |||
| C | 66.94 ± 15.91 (72.33 ± 32.92) | 63.64 ± 11.86 (83.3 ± 102.65) | |||
| % and (MFI) Burst indices in granulocytes | A | 17.11 ± 7.91 (5.23 ± 2.74) | 24.17 ± 13.18 (5.29 ± 2.33) | 23.12 ± 13.82 * (5.78 ± 2.59) | 19.25 ± 11.68 (6.63 ± 2.44) |
| B | 23.55 ± 14.84 * (5.43 ± 2.07) | 21.83 ± 12.35 * (6.31 ± 2.18) | |||
| C | 24.62 ± 15.34 * (5.54 ± 3.09) | 24.47 ± 15.79 * (5.57 ± 1.59) | |||
| % and (MFI) Burst indices in monocytes | A | 12.70 ± 6.34 (1.63 ± 0.38) | 17.88 ± 22.77 (1.58 ± 0.74) | 16.47 ± 9.59 * (1.9 ± 1.34) | 11.41 ± 5.49 (1.81 ± 0.51) |
| B | 13.35 ± 9.49 (1.8 ± 0.61) | 12.72 ± 8.77 (1.76 ± 0.27) | |||
| C | 18.77 ± 6.42 * (1.78 ± 0.47) | 11.81 ± 6.21 (2.04 ± 2.08) | |||
| Day | |||||
|---|---|---|---|---|---|
| Parameter | Group | −14 | 0 | 21 | 42 |
| % lysis of K562 cells by NK cells in a 10:1 (and 5:1) ratio | A | 22.41 ± 9.23 | 17.50 ± 8.73 (13.24 ± 6.6) | 17.71 ± 7.78 * (14.03 ± 6.49) | 17.30 ± 7.44 * (13.97 ± 5.85) |
| B | 21.13 ± 9.14 (16.08 ± 7.04) | 19.11 ± 10.32 * (16.03 ± 8.79) | |||
| C | 19.76 ± 6.73 * (16.68 ± 6.7) | 21.43 ± 8.85 (18.06 ± 8.62) | |||
| % spontaneous lysis of K562 cells | A | 4.04 ± 5.97 | 3.46 ± 2.14 | 5.09 ± 3.31 | 5.34 ± 2.85 |
| B | 5.24 ± 3.37 | 5.69 ± 3.01 * | |||
| C | 5.29 ± 3.47 | 5.39 ± 3.07 | |||
| % total K562 cell lysis | A | 93.81 ± 7.85 | 92.22 ± 13.17 | 97.18 ± 2.65 * | 96.73 ± 3.05 * |
| B | 97.34 ± 2.74 * | 96.69 ± 2.99 * | |||
| C | 97.56 ± 2.45 * | 97.05 ± 2.91 * | |||
| NK Activity 10:1 (and 5:1) corrected (%) | A | 20.84 ± 10.40 (13.64 ± 9.39) | 16.31 ± 9.68 (11.23 ± 7.4) | 14.79 ± 7.3 * (9.64 ± 7.05 *) | 14.16 ± 7.1 * (9.45 ± 6.13 *) |
| B | 17.07 ± 9.89 * (11.64 ± 7.45) | 15.89 ± 10.8 * (11.35 ± 9.61) | |||
| C | 15.58 ± 8.07 * (12.22 ± 8.03) | 17.5 ± 8.74 * (13.8 ± 8.45) | |||
| Day | |||||
|---|---|---|---|---|---|
| Group | −14 | 0 | 21 | 42 | |
| % CD69 Expression in PHA-Stimulated Cells | A | 31.37 ± 11.08 | 31.00 ± 11.14 | 28.50 ± 12.09 | 29.83 ± 13.37 |
| B | 31.47 ± 9.04 | 30.59 ± 14.07 | |||
| C | 30.21 ± 11.57 | 27.44 ± 10.21 | |||
| % IFN-gamma expression of PHA-stimulated cells | A | 17.13 ± 15.09 | 18.33 ± 14.5 | 20.62 ± 17.8 | 20.26 ± 17.57 |
| B | 17.59 ± 12.16 | 20.50 ±16.94 | |||
| C | 15.45 ± 12.12 | 24.32 ± 17.93 * | |||
| % CD69/IFN-gamma expression of PHA-stimulated cells | A | 3.27 ±3.25 | 2.21 ± 1.88 | 1.52 ± 1.45 * | 1.82 ± 1.88 * |
| B | 1.45 ± 0.96 * | 1.97 ± 1.94 | |||
| C | 1.81 ± 1.52 * | 1.74 ± 1.39 * | |||
| % MFI | A | 11.70 ± 5.41 | 11.25 ± 5.36 | 9.01 ± 3.05 * | 9.08 ± 2.99 * |
| B | 9.15 ± 3.11 * | 8.85 ± 2.86 * | |||
| C | 8.85 ± 3.06 * | 9.20 ± 2.75 * | |||
| % MFI IFN-gamma Expression of PHA-Stimulated Cells | A | 4.44 ± 3.70 | 3.63 ±3.29 | 2.45 ± 1.19 * | 2.57 ± 1.38 |
| B | 2.55 ± 1.51 | 2.55 ± 1.19 | |||
| C | 2.67 ± 1.15 | 2.47 ± 1.26 * | |||
| % CD69 expression of CD3+CD28-stimulated cells | A | 42.82 ± 19.09 | 45.58 ± 18.12 | 44.92 ± 18.93 | 45.23 ± 19.6 |
| B | 46.34 ± 19.14 | 45.98 ± 19.03 | |||
| C | 38.97 ± 19.97 * | 38.97 ± 20.09 * | |||
| % IFN-gamma expression of CD3+CD28-stimulated cells | A | 14.47 ± 12.44 | 14.15 ± 12.26 | 16.54 ± 14.14 | 16.72 ± 13.81 |
| B | 14.80 ± 10.79 | 18.27 ± 16.01 * | |||
| C | 12.28 ± 9.65 | 18.10 ± 12.97 * | |||
| % CD69/IFN-gamma expression of CD3+CD28-stimulated cells | A | 4.78 ± 4.32 | 4.61 ± 4.26 | 3.96 ± 4.12 | 4.17 ± 3.25 |
| B | 3.69 ± 3.15 | 4.45 ± 3.69 | |||
| C | 3.23 ± 3.28 * | 3.46 ± 2.85 * | |||
| % MFI CD69 Expression of CD3+CD28-Stimulated Cells | A | 10.08 ± 4.98 | 9.00 ± 4.29 | 7.55 ± 2.34 * | 8.00 ± 2.47 |
| B | 7.75 ± 2.84 | 7.80 ± 2.4 | |||
| C | 7.30 ± 2.39 * | 7.62 ± 2.55 * | |||
| % MFI IFN-gamma expression of CD3+CD28-stimulated cells | A | 4.55 ± 3.34 | 4.77 ± 4.3 | 3.30 ± 1.92 | 3.40 ± 1.76 |
| B | 3.95 ± 3.34 | 3.62 ± 1.92 | |||
| C | 3.35 ± 1.96 | 2.62 ± 1.11 * | |||
| A and C | B | A | C | A, B, C |
|---|---|---|---|---|
| ↓ IgM ↑ IL-5 ↓ CD11b MFI | ↑ IgM ↓ C3 ↑ IFN CD3/28 ↓ IL-5 ↑ CD3 ↑ CD4 ↓ CD8 ↑ CD4/CD8 ↑ CD3 RA ↑ DR MFI ↓ %CD11B lympho | ↑ IgE ↑ CD4 | ↑ NK 10:1 ↓ CD69-PHA ↓ CD3 ↓ CD4 ↑ CD19 ↓ CD3/CD19 ↑ NK% ↓ CD3RA ↑ CD3R0 ↓ RA/R0 CD3 ↑ DR ↓ MFI ↑ %CD11B lympho | ↑ Phagocytosis ↑ Burst ↑ IL-6 ↑ IL-8 ↑ IL-1beta ↑ CD16 ↓ MFI ↓ CD14 Lympho%; MFI monocyotes, MFI granulocytes ↓ CD14% ↓C D86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Pino, F.; Casquete, M.; Castro, M.J.; Redondo del Rio, P.; Gutierrez, E.; Mayo-Iscar, A.; Nocito, M.; Corell, A. Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults. Nutrients 2024, 16, 1969. https://doi.org/10.3390/nu16121969
Rivero-Pino F, Casquete M, Castro MJ, Redondo del Rio P, Gutierrez E, Mayo-Iscar A, Nocito M, Corell A. Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults. Nutrients. 2024; 16(12):1969. https://doi.org/10.3390/nu16121969
Chicago/Turabian StyleRivero-Pino, Fernando, Mar Casquete, Maria José Castro, Paz Redondo del Rio, Eloina Gutierrez, Agustín Mayo-Iscar, Mercedes Nocito, and Alfredo Corell. 2024. "Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults" Nutrients 16, no. 12: 1969. https://doi.org/10.3390/nu16121969
APA StyleRivero-Pino, F., Casquete, M., Castro, M. J., Redondo del Rio, P., Gutierrez, E., Mayo-Iscar, A., Nocito, M., & Corell, A. (2024). Prospective, Randomized, Double-Blind Parallel Group Nutritional Study to Evaluate the Effects of Routine Intake of Fresh vs. Pasteurized Yogurt on the Immune System in Healthy Adults. Nutrients, 16(12), 1969. https://doi.org/10.3390/nu16121969








