Evaluation of 12-Week Standardized Beetroot Extract Supplementation in Older Participants: A Preliminary Study of Human Health Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
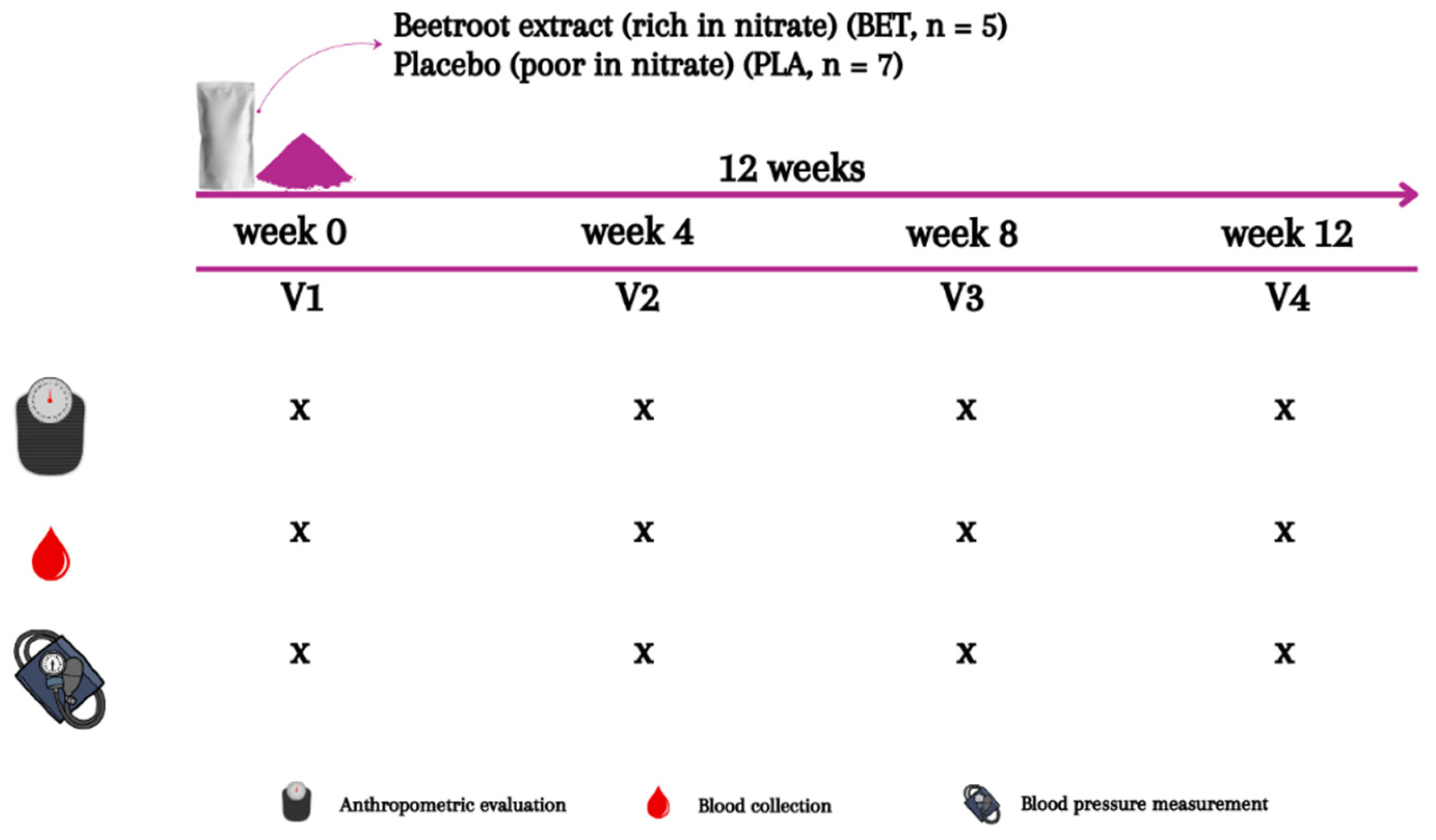
2.2. Experimental Design
2.3. Beetroot Extract
2.4. Anthropometric Measurements
2.5. Biochemical Analysis
2.6. Hemodynamic Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, M.D.; Mingorance, C.; Rodríguez-Rodríguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2010, 9, 142–152. [Google Scholar] [CrossRef]
- Šilhavý, J.; Mlejnek, P.; Šimáková, M.; Malínská, H.; Marková, I.; Hüttl, M.; Miklánková, D.; Kazdová, L.; Vrbacký, M.; Pecinová, A.; et al. Hypolipidemic Effects of Beetroot Juice in SHR-CRP and HHTg Rat Models of Metabolic Syndrome: Analysis of Hepatic Proteome. Metabolites 2023, 13, 192. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell. Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63, E239–E245. [Google Scholar] [CrossRef]
- Van der Avoort, C.M.T.; Van Loon, L.J.C.; Hopman, M.T.E.; Verdijk, L.B. Increasing vegetable intake to obtain the health promoting and ergogenic effects of dietary nitrate. Eur. J. Clin. Nutr. 2018, 72, 1485–1489. [Google Scholar] [CrossRef]
- Brzezińska-Rojek, J.; Rutkowska, M.; Ośko, J.; Konieczka, P.; Prokopowicz, M.; Grembecka, M. Evaluation of the Safety and Potential Benefits of Beetroot-Based Dietary Supplements According to Their Elemental Composition. Biol. Trace Elem. Res. 2023, 202, 3318–3332. [Google Scholar] [CrossRef]
- Mitrevski, J.; Pantelić, N.; Dodevska, M.S.; Kojić, J.S.; Vulić, J.J.; Zlatanović, S.; Gorjanović, S.; Laličić-Petronijević, J.; Marjanović, S.; Antić, V.V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. [Google Scholar] [CrossRef]
- Hopper, I.; Connell, C.; Briffa, T.; De Pasquale, C.G.; Driscoll, A.; Kistler, P.M.; Macdonald, P.S.; Sindone, A.; Thomas, L.; Atherton, J.J. Nutraceuticals in Patients with Heart Failure: A Systematic Review. J. Card. Fail. 2020, 26, 166–179. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Kiran Kumar Vuppala, P.S.L. Standardized Beetroot Extract in Cardiovascular and Exercise Performance: A Randomized, Double Blind, Placebo Controlled, Crossover, Two Group, Two Periods, Clinical Study to Evaluate the Efficacy and Safety. Int. J. Innov. Res. Med. Sci. 2017, 1, 376–383. [Google Scholar] [CrossRef]
- Strilchuk, L.; Cincione, R.I.; Fogacci, F.; Cicero, A.F.G. Dietary interventions in blood pressure lowering: Current evidence in 2020. Kardiol. Pol. 2020, 78, 659–666. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef]
- Schneider, A.C.; Hughes, W.E.; Ueda, K.; Bock, J.M.; Casey, D.P. Reduced blood pressure responsiveness to skeletal muscle metaboreflex activation in older adults following inorganic nitrate supplementation. Nitric Oxide 2018, 78, 81–88. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Bešter, P.K.; Lobnik, F.; Eržen, I.; Kastelec, D.; Zupan, M. Prediction of cadmium concentration in selected home-produced vegetables. Ecotoxicol. Env. Environ. Saf. 2013, 96, 182–190. [Google Scholar] [CrossRef]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Puchalski, C. Accumulation of cadmium, lead and mercury in seedlings of selected sugar beet varieties as a result of simulated soil contamination. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 351–354. [Google Scholar] [CrossRef][Green Version]
- Ćwieląg-Drabek, M.; Piekut, A.; Gut, K.; Grabowski, M. Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Sci. Rep. 2020, 10, 3363. [Google Scholar] [CrossRef]
- Jean, J.; Sirot, V.; Hulin, M.; Le Calvez, E.; Zinck, J.; Noël, L.; Vasseur, P.; Nesslany, F.; Gorecki, S.; Guérin, T.; et al. Dietary exposure to cadmium and health risk assessment in children—Results of the French infant total diet study. Food Chem. Toxicol. 2018, 115, 358–364. [Google Scholar] [CrossRef]
- Manwani, S.; Devi, P.; Singh, T.; Yadav, C.S.; Awasthi, K.K.; Bhoot, N.; Awasthi, G. Heavy metals in vegetables: A review of status, human health concerns, and management options. Environ. Environ. Sci. Pollut. Res. Int. 2023, 30, 71940–71956. [Google Scholar] [CrossRef]
- WHO. Preventing Disease through Healthy Environments; WHO: Geneva, Switzerland, 2021; Volume 5. [Google Scholar]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Cobas, R.; Rodacki, M.; Giacaglia, L.; Calliari, L.E.P.; Noronha, R.M.; Valerio, C.; Custódio, J.; Scharf, M.; Barcellos, C.R.G.; Tomarchio, M.P.; et al. Diagnóstico do diabetes e rastreamento do diabetes tipo 2. In Diretriz da Sociedade Brasileira de Diabetes; Sociedade Brasileira de Diabetes: São Paulo, Brazil, 2023. [Google Scholar] [CrossRef]
- Diniz, M.F.H.S.; Beleigoli, A.M.R.; Schmidt, M.I.; Duncan, B.B.; Ribeiro, A.L.P.; Vidigal, P.G.; Benseñor, I.M.; Lotufo, P.A.; Santos, I.S.; Griep, R.H.; et al. Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study. Cad. Saude Publica 2020, 36, e00072120. [Google Scholar] [CrossRef]
- Endukuru, C.K.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B. Cut-off Values and Clinical Utility of Surrogate Markers for Insulin Resistance and Beta-Cell Function to Identify Metabolic Syndrome and Its Components among Southern Indian Adults. J. Obes. Metab. Syndr. 2020, 29, 281–291. [Google Scholar] [CrossRef]
- Faludi, A.A.; Izar, M.C.O.; Saraiva, J.F.K.; Chacra, A.P.M.; Bianco, H.T.; Afiune, A.; Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [CrossRef]
- Molla, N.H.; Kathak, R.R.; Sumon, A.H.; Barman, Z.; Mou, A.D.; Hasan, A.; Mahmud, F.; Fariha, K.A.; Ali, N. Assessment of the relationship between serum uric acid levels and liver enzymes activity in Bangladeshi adults. Sci. Rep. 2021, 11, 20114. [Google Scholar] [CrossRef]
- Szwarcwald, C.L.; Malta, D.C.; Pereira, C.A.; Figueiredo, A.W.; Almeida, W.D.S.; Machado, I.E.; Bacal, N.S.; Silva, A.G.D.; Silva Júnior, J.B.D.; Rosenfeld, L.G. Reference values for laboratory tests of cholesterol, glycosylated hemoglobin and creatinine of the Brazilian adult population. Rev. Bras. Epidemiol. 2019, 22 (Suppl. 2), e190002.supl.190002. [Google Scholar] [CrossRef]
- Keller, R.M.; Beaver, L.P.; Prater, M.C.; Hord, N.G. Dietary Nitrate and Nitrite Concentrations in Food Patterns and Dietary Supplements. Nutr. Today 2020, 55, 218–226. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines of Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Volino-Souza, M.; Oliveira, G.V.; Pinheiro, V.D.S.; Conte-Junior, C.A.; Alvares, T.D.S. The effect of dietary nitrate on macro- and microvascular function: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 1225–1236. [Google Scholar] [CrossRef]
- Croitoru, M.D. Nitrite and nitrate can be accurately measured in samples of vegetal and animal origin using an HPLC-UV/VIS technique. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 911, 154–161. [Google Scholar] [CrossRef]
- Townsend, J.R.; Hart, T.L.; Haynes, J.T.; Woods, C.A.; Toy, A.M.; Pihera, B.C.; Aziz, M.A.; Zimmerman, G.A.; Jones, M.D.; Vantrease, W.C.; et al. Influence of Dietary Nitrate Supplementation on Physical Performance and Body Composition Following Offseason Training in Division I Athletes. J. Diet. Suppl. 2022, 19, 534–549. [Google Scholar] [CrossRef]
- Quinn, F.; Armbruster, D. The Immunoassay Handbook, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Taher, J.; Cosme, J.; Renley, B.A.; Daghfal, D.J.; Yip, P.M. A novel Sigma metric encompasses global multi-site performance of 18 assays on the Abbott Alinity system. Clin. Biochem. 2019, 63, 106–112. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef]
- Zhan, S.-Y.; Shao, Q.; Liu, L.; Fan, X.-H. A simple and accurate method to determine nitrite and nitrate in serum based on high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 2013, 27, 1547–1553. [Google Scholar] [CrossRef]
- Barros-Santos, E.; de Oliveira, G.V.; Volino-Souza, M.; Alvares, T.S. Dietary nitrate improves skeletal muscle microvascular oxygenation in HIV-infected patients receiving highly active antiretroviral therapy: A randomised, double-blind, cross-over, placebo-controlled study. Br. J. Nutr. 2020, 124, 1277–1284. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- de Oliveira, G.V.; Nascimento, L.A.D.D.; Volino-Souza, M.; Mesquita, J.S.; Alvares, T.S. Beetroot-based gel supplementation improves handgrip strength and forearm muscle O. Appl. Physiol. Nutr. Metab. 2018, 43, 920–927. [Google Scholar] [CrossRef]
- Narayanan, M.V.; Rasane, P.; Singh, J.; Kaur, S.; Avinashe, H.; Gunjal, M.; Kaur, J.; Bhadariya, V. Beetroot Bioactive and its Associated Health Benefits: Considerations for Utilization of Beetroot in Value-added Products. Recent. Adv. Food Nutr. Agric. 2023, 14, 155–166. [Google Scholar] [CrossRef]
- James, P.E.; Willis, G.R.; Allen, J.D.; Winyard, P.G.; Jones, A.M. Nitrate pharmacokinetics: Taking note of the difference. Nitric Oxide 2015, 48, 44–50. [Google Scholar] [CrossRef]
- Miller, G.D.; Collins, S.; Ives, J.; Williams, A.; Basu, S.; Kim-Shapiro, D.B.; Berry, M.J. Efficacy and Variability in Plasma Nitrite Levels during Long-Term Supplementation with Nitrate Containing Beetroot Juice. J. Diet. Suppl. 2023, 20, 885–910. [Google Scholar] [CrossRef]
- Zafeiridis, A.; Triantafyllou, A.; Papadopoulos, S.; Koletsos, N.; Touplikioti, P.; Zafeiridis, A.S.; Gkaliagkousi, E.; Dipla, K.; Douma, S. Dietary nitrate improves muscle microvascular reactivity and lowers blood pressure at rest and during isometric exercise in untreated hypertensives. Microcirculation 2019, 26, e12525. [Google Scholar] [CrossRef]
- van der Avoort, C.M.T.; Ten Haaf, D.S.M.; Bongers, C.C.W.G.; van Oorschot, F.; Verdijk, L.B.; van Loon, L.J.C.; Hopman, M.T.E. Increasing Nitrate-Rich Vegetable Intake Lowers Ambulatory Blood Pressure in (pre)Hypertensive Middle-Aged and Older Adults: A 12-Wk Randomized Controlled Trial. J. Nutr. 2021, 151, 2667–2679. [Google Scholar] [CrossRef]
- de Oliveira, G.; Morgado, M.; Pierucci, A.; Alvares, T. A single dose of a beetroot-based nutritional gel improves endothelial function in the elderly with cardiovascular risk factors. J. Funct. Foods 2016, 26, 301–308. [Google Scholar] [CrossRef]
- Volino-Souza, M.; de Oliveira, G.V.; Alvares, T.S. A single dose of beetroot juice improves endothelial function but not tissue oxygenation in pregnant women: A randomised clinical trial. Br. J. Nutr. 2018, 120, 1006–1013. [Google Scholar] [CrossRef]
- Somani, Y.B.; Soares, R.N.; Gosalia, J.; Delgado, J.M.; Flanagan, M.; Basu, S.; Kim-Shapiro, D.B.; Murias, J.M.; Proctor, D.N. A single dose of dietary nitrate supplementation protects against endothelial ischemia-reperfusion injury in early postmenopausal women. Appl. Physiol. Nutr. Metab. 2022, 47, 749–761. [Google Scholar] [CrossRef]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Ali, A. Performance and Health Benefits of Dietary Nitrate Supplementation in Older Adults: A Systematic Review. Nutrients 2017, 9, 1171. [Google Scholar] [CrossRef]
- Carter, S.J.; Gruber, A.H.; Raglin, J.S.; Baranauskas, M.N.; Coggan, A.R. Potential health effects of dietary nitrate supplementation in aging and chronic degenerative disease. Med. Hypotheses 2020, 141, 109732. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 12. [Google Scholar] [CrossRef]
- Pinheiro, V.; Volino-Souza, M.; de Oliveira, G.; Conte-Junior, C.; Alvares, T. Effect of high-nitrate beetroot juice consumption on thyroid gland hormones and iodine levels in adults. Food Biosci. 2021, 40, 2212–4292. [Google Scholar] [CrossRef]
- McMahon, N.F.; Brooker, P.G.; Pavey, T.; Leveritt, M.D. Assessment of dietary nitrate supplementation: Prevalence of use, knowledge, attitudes and beliefs among active Australians. Front. Nutr. 2023, 10, 1291431. [Google Scholar] [CrossRef]
- Kemmner, S.; Lorenz, G.; Wobst, J.; Kessler, T.; Wen, M.; Günthner, R.; Stock, K.; Heemann, U.; Burkhardt, K.; Baumann, M.; et al. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: A pilot study. Nitric Oxide 2017, 64, 7–15. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Norouzirad, R.; Mirmiran, P.; Gaeini, Z.; Jeddi, S.; Shokri, M.; Azizi, F.; Ghasemi, A. Effect of inorganic nitrate on metabolic parameters in patients with type 2 diabetes: A 24-week randomized double-blind placebo-controlled clinical trial. Nitric Oxide 2021, 107, 58–65. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Brandt, K.; Fell, D.; Warner, S.; Ryan, L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J. Nutr. Sci. 2014, 3, e9. [Google Scholar] [CrossRef]
- Lotfi, M.; Azizi, M.; Tahmasebi, W.; Bashiri, P. Efficacy of Beetroot Juice Consumption on the Lipid Profile of Females Soccer Players. Med. Lab. J. 2020, 14, 26–30. [Google Scholar] [CrossRef]
- Holy, B.; Isaac, N.; Ngove, B. Post-prandial effect of beetroot (beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects. Eur. J. Pharm. Med. Res. 2017, 4, 60–62. [Google Scholar]
- Amirpoor, A.; Zavar, R.; Amerizadeh, A.; Asgary, S.; Moradi, S.; Farzaei, M.H.; Masoumi, G.; Sadeghi, M. Effect of Beetroot Consumption on Serum Lipid Profile: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 100887. [Google Scholar] [CrossRef]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 2017, 9, 1270. [Google Scholar] [CrossRef]
- Siervo, M.; Shannon, O.; Kandhari, N.; Prabhakar, M.; Fostier, W.; Köchl, C.; Rogathi, J.; Temu, G.; Stephan, B.C.M.; Gray, W.K.; et al. Nitrate-Rich Beetroot Juice Reduces Blood Pressure in Tanzanian Adults with Elevated Blood Pressure: A Double-Blind Randomized Controlled Feasibility Trial. J. Nutr. 2020, 150, 2460–2468. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Martínez, J.A.; Portillo, M.P. Current Knowledge on Beetroot Bioactive Compounds: Role of Nitrate and Betalains in Health and Disease. Foods 2021, 10, 1314. [Google Scholar] [CrossRef]
- Broxterman, R.M.; La Salle, D.T.; Zhao, J.; Reese, V.R.; Richardson, R.S.; Trinity, J.D. Influence of dietary inorganic nitrate on blood pressure and vascular function in hypertension: Prospective implications for adjunctive treatment. J. Appl. Physiol. 2019, 127, 1085–1094. [Google Scholar] [CrossRef]
- Fan, X. Statistical Significance and Effect Size in Education Research: Two Sides of a Coin. J. Educ. Res. 2001, 94, 275–282. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]

| BET | PLA | Reference Value [21,22,23,24,25,26,27,28,29] | p | |
|---|---|---|---|---|
| Demographics | ||||
| N (male) | 5 (1) | 7 (2) | - | - |
| Age (years) | 64 ± 5 | 69 ± 5 | - | 0.111 |
| Anthropometric measurements | ||||
| Weight (kg) | 68.7 ± 15.8 | 64.8 ± 9.8 | - | 0.535 |
| Height (m) | 1.64 ± 0.13 | 1.61 ± 0.07 | - | 0.611 |
| BMI (kg/m2) | 25.4 ± 4.4 | 24.5 ± 2.2 | 22.00–27.00 | 0.701 |
| Biochemical analysis | ||||
| Glucose (mg/dL) | 93.40 ± 17.00 | 103.28 ± 21.16 | <100.00 | 0.409 |
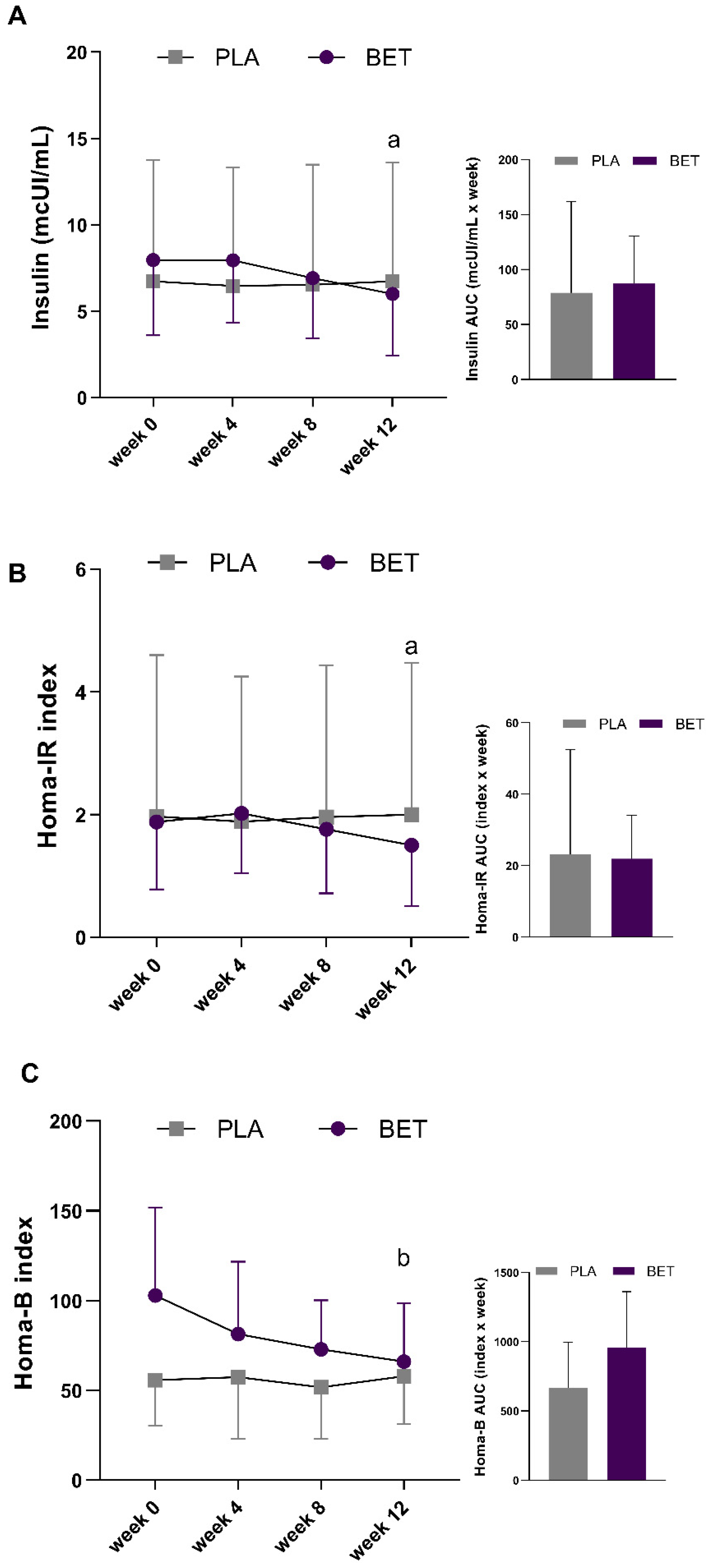
| Insulin (mcIU/mL) | 7.96 ± 4.36 | 6.73 ± 7.03 | <25.00 | 0.738 |
| HOMA-IR index | 1.88 ± 1.10 | 1.97 ± 2.62 | <2.35 | 0.944 |
| HOMA-B index | 102.96 ± 48.75 | 55.78 ± 25.45 | >94.74 | 0.052 |
| HbA1C | 5.54 ± 0.42 | 5.80 ± 0.42 | <5.70 | 0.505 |
| TG (mg/dL) | 102.80 ± 62.43 | 102.43 ± 50.37 | <150.00 | 0.545 |
| TC (mg/dL) | 198.00 ± 21.76 | 171.14 ± 32.32 | <190.00 | 0.139 |
| HDL-c (mg/dL) | 60.20 ± 10.73 | 62.14 ± 15.50 | >40.00 | 0.815 |
| LDL-c (mg/dL) | 116.00 ± 24.30 | 90.43 ± 32.04 | <130.00 | 0.116 |
| VLDL-c (mg/dL) | 22.00 ± 9.80 | 18.57 ± 9.25 | - | 0.550 |
| Creatinine (mg/dL) | 0.78 ± 0.13 | 0.81 ± 0.13 | 0.50–1.30 | 0.669 |
| Uric acid (mg/dL) | 4.38 ± 0.88 | 4.80 ± 0.53 | <7.00 | 0.324 |
| GGT (IU/L) | 28.80 ± 28.08 | 28.28 ± 11.82 | <55.00 | 0.966 |
| ALP (IU/L) | 66.60 ± 15.66 | 66.28 ± 16.40 | <128.00 | 0.974 |
| AST (IU/L) | 30.20 ± 17.02 | 23.00 ± 6.30 | <35.00 | 0.323 |
| ALT (IU/L) | 24.00 ± 16.47 | 17.71 ± 5.31 | <45.00 | 0.187 |
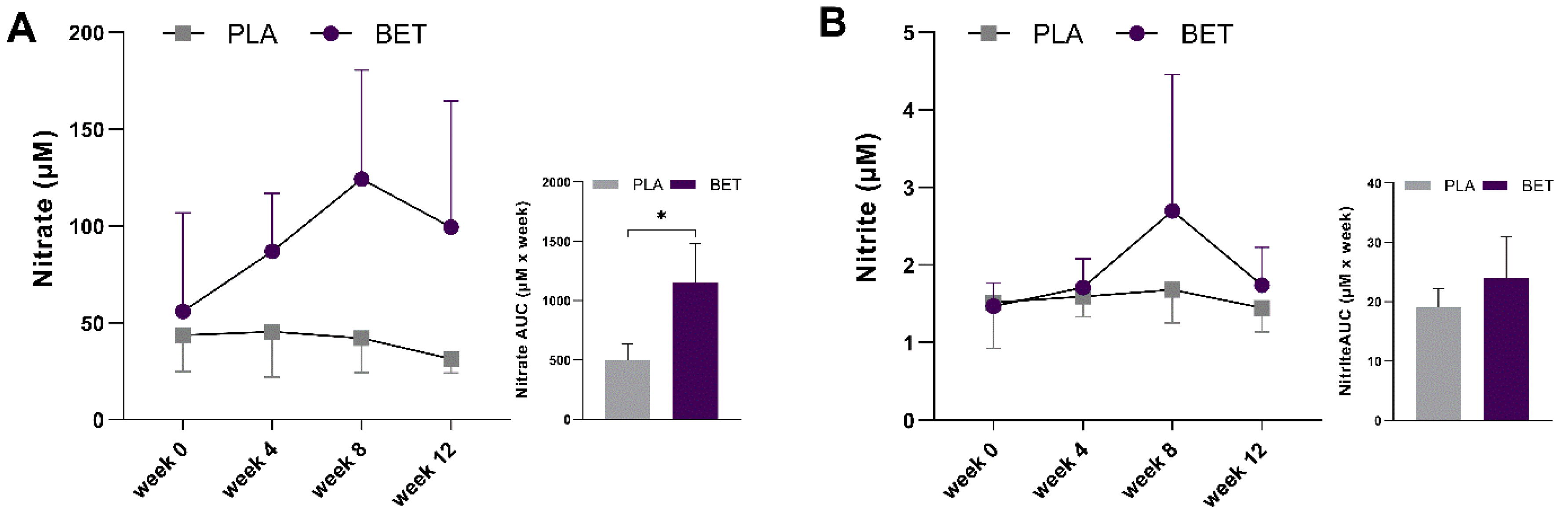
| Nitrate (µM) | 55.90 ± 50.79 | 43.60 ± 18.79 | <59.60 | 0.565 |
| Nitrite (µM) | 1.47 ± 0.30 | 1.51 ± 0.59 | <1.50 | 0.628 |
| Hemodynamic analysis | ||||
| SBP (mmHg) | 126.80 ± 6.26 | 119.85 ± 7.82 | <140.00 | 0.132 |
| DBP (mmHg) | 86.00 ± 11.13 | 74.42 ± 7.52 | <90.00 | 0.056 |
| HR (bpm) | 73.80 ± 10.44 | 64.71 ± 10.62 | <80.00 | 0.172 |
| Proximate Composition | |
| Carbohydrates (%) w/w | 80 |
| Sugars (%) w/w | 61 |
| Fat (%) w/w | 0.5 |
| Protein (%) w/w | 6.3 |
| Ash (%) w/w | 5.2 |
| Moisture (%) w/w | 3.5 |
| Mineral | |
| Calcium (%) w/w | 0.1 |
| Potassium (%) w/w | 1.0 |
| Sodium (%) w/w | 0.7 |
| Iron (%) w/w | n.d. |
| Heavy metal | |
| Lead (µg/g) ppm | <0.2 |
| Arsenic (µg/g) ppm | <0.2 |
| Cadmium (µg/g) ppm | <0.2 |
| Mercury (µg/g) ppm | <0.1 |
| AUC | Mean Difference | 95% Confidence Interval | |||
|---|---|---|---|---|---|
| BET | PLA | Lower Limit | Upper Limit | ||
| Anthropometric measurements | |||||
| Body mass (kg) | 829.2 ± 205.2 | 771.4 ± 110.2 | −57.79 ± 90.97 | −260.48 | 144.90 |
| Height (m) | 19.33 ± 0.89 | 19.70 ± 1.53 | −0.37 ± 0.70 | −1.92 | 1.19 |
| BMI (kg/m2) | 305.9 ± 53.3 | 295.8 ± 24.3 | −10.12 ± 22.61 | −60.50 | 40.27 |
| Biochemical analysis | |||||
| Glucose (mg/dL) | 1196.4 ± 230.3 | 1271.0 ± 308.3 | 74.60 ± 163.76 | −290.28 | 439.48 |
| Insulin (mcIU/mL) | 87.3 ± 43.1 | 78.9 ± 82.8 | −8.38 ± 40.78 | −99.25 | 82.48 |
| HOMA-IR index | 21.9 ± 12.1 | 23.1 ± 29.4 | 1.18 ± 14.07 | −30.16 | 32.53 |
| HOMA-B index | 955.6 ± 405.7 | 664.0 ± 332.0 | −291.55 ± 212.71 | −765.50 | 182.40 |
| TG (mg/dL) | 1247.6 ± 386.0 | 1253.9 ± 637.1 | 6.26 ± 322.39 | −712.07 | 724.58 |
| TC (mg/dL) | 2331.8 ± 252.0 | 2070.7 ± 423.7 | −261.08 ± 213.63 | 213.63 | −737.08 |
| HDL-c (mg/dL) | 701.8 ± 95.4 | 709.7 ± 123.1 | 7.91 ± 66.08 | −139.32 | 155.15 |
| LDL-c (mg/dL) | 1405.2 ± 281.1 | 1136.0 ± 373.5 | −269.20 ± 198.84 | −712.24 | 173.84 |
| VLDL-c (mg/dL) | 225.2 ± 66.7 | 225.0 ± 110.8 | −0.02 ± 55.98 | −124.93 | 124.53 |
| Creatinine (mg/dL) | 9.2 ± 1.0 | 9.8 ± 1.6 | 0.57 ± 0.80 | −1.20 | 2.36 |
| Uric acid (mg/dL) | 55.7 ± 12.7 | 56.2 ± 6.1 | 0.52 ± 5.47 | −11.68 | 12.72 |
| GGT (IU/L) | 365.4 ± 400.4 | 344.4 ± 124.7 | −24.04 ± 158.70 | −374.64 | 332.55 |
| ALP (IU/L) | 785.6 ± 201.3 | 859.1 ± 193.9 | 73.54 ± 115.30 | −183.36 | 330.44 |
| AST (IU/L) | 342.8 ± 146.5 | 266.4 ± 61.7 | −76.37 ± 61.02 | −212.35 | 59.61 |
| ALT (IU/L) | 263.8 ± 145.9 | 181.4 ± 50.9 | −82.37 ± 58.76 | −213.30 | 48.55 |
| Nitrate (µM) | 1155.5 ± 325.7 * | 499.9 ± 134.0 | −655.66 ± 154.22 | −1052.65 | −258.67 |
| Nitrite (µM) | 24.1 ± 6.9 | 19.0 ± 3.2 | −5.00 ± 3.30 | −13.38 | 3.37 |
| Hemodynamic analysis | |||||
| SBP (mmHg) | 1535.4 ± 84.3 | 1443.7 ± 92.9 | − 91.68 ± 52.42 | −208.49 | 25.12 |
| DBP (mmHg) | 986.0 ± 75.5 | 896.3 ± 86.1 | −89.71 ± 48.03 | −196.74 | 17.31 |
| HR (bpm) | 815.8 ± 78.3 | 740.9 ± 70.5 | −74.94 ± 43.16 | −171.10 | 21.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, V.d.S.; Junior, O.J.F.R.; Ortmann, C.F.; Pande, A.; Conte-Junior, C.A.; Alvares, T.S. Evaluation of 12-Week Standardized Beetroot Extract Supplementation in Older Participants: A Preliminary Study of Human Health Safety. Nutrients 2024, 16, 1942. https://doi.org/10.3390/nu16121942
Pinheiro VdS, Junior OJFR, Ortmann CF, Pande A, Conte-Junior CA, Alvares TS. Evaluation of 12-Week Standardized Beetroot Extract Supplementation in Older Participants: A Preliminary Study of Human Health Safety. Nutrients. 2024; 16(12):1942. https://doi.org/10.3390/nu16121942
Chicago/Turabian StylePinheiro, Vivian dos Santos, Olavo João Frederico Ramos Junior, Caroline Flach Ortmann, Anurag Pande, Carlos Adam Conte-Junior, and Thiago Silveira Alvares. 2024. "Evaluation of 12-Week Standardized Beetroot Extract Supplementation in Older Participants: A Preliminary Study of Human Health Safety" Nutrients 16, no. 12: 1942. https://doi.org/10.3390/nu16121942
APA StylePinheiro, V. d. S., Junior, O. J. F. R., Ortmann, C. F., Pande, A., Conte-Junior, C. A., & Alvares, T. S. (2024). Evaluation of 12-Week Standardized Beetroot Extract Supplementation in Older Participants: A Preliminary Study of Human Health Safety. Nutrients, 16(12), 1942. https://doi.org/10.3390/nu16121942








