Evaluating the Therapeutic Properties of Natural Products in Orthodontic and Surgical Treatment of Dentofacial Deformities: A Systematic Review of Clinical Trials
Abstract
1. Introduction
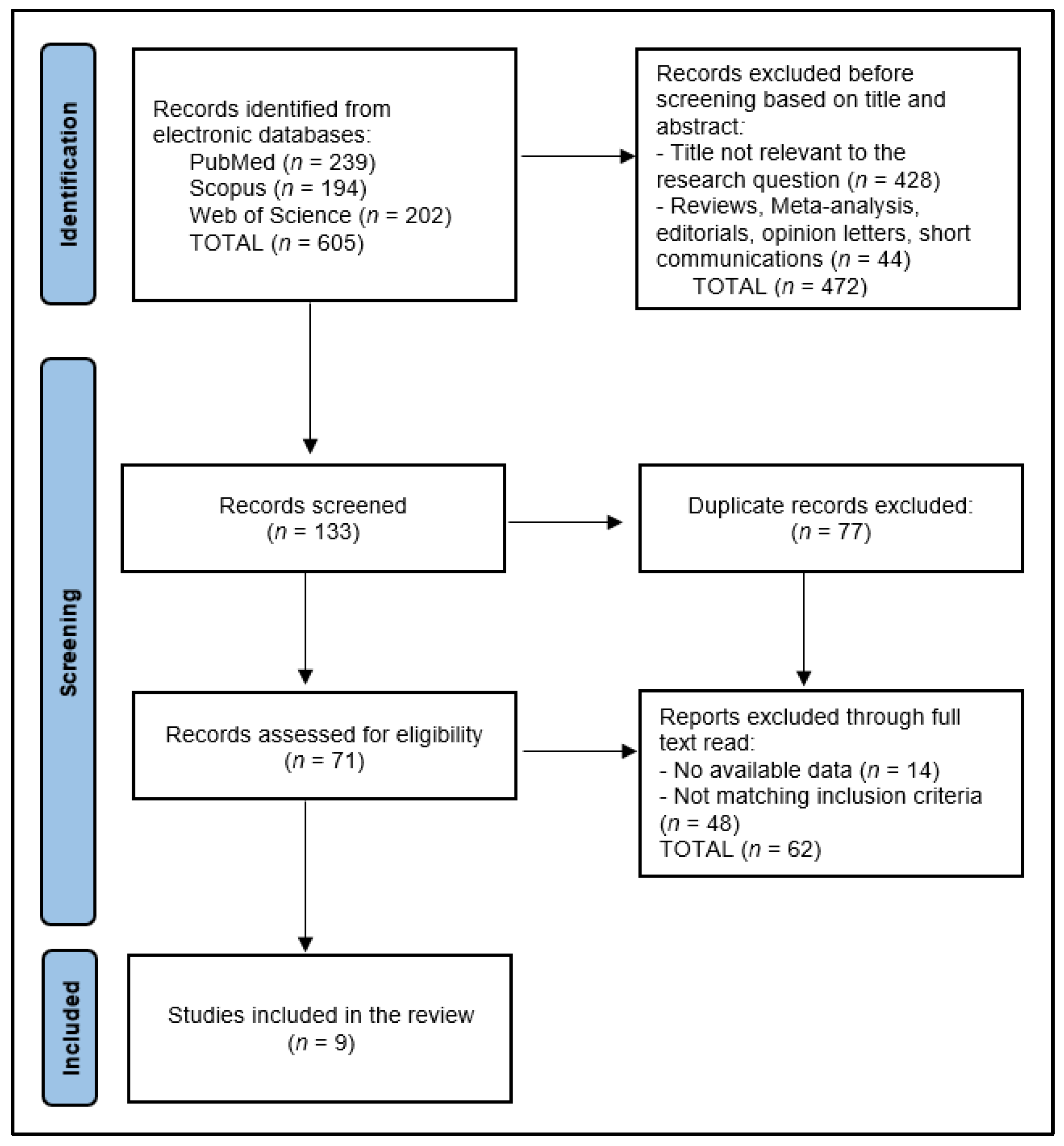
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Data Collection Process
2.4. Risk of Bias and Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Population Characteristics
3.3. Clinical Trial Assessment
3.4. Assessment of Outcomes
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AlMogbel, A. Clear Aligner Therapy: Up to date review article. J. Orthod. Sci. 2023, 12, 37. [Google Scholar] [CrossRef]
- Jaber, S.T.; Hajeer, M.Y.; Sultan, K. Treatment Effectiveness of Clear Aligners in Correcting Complicated and Severe Malocclusion Cases Compared to Fixed Orthodontic Appliances: A Systematic Review. Cureus 2023, 15, e38311. [Google Scholar] [CrossRef]
- Verrusio, C.; Iorio-Siciliano, V.; Blasi, A.; Leuci, S.; Adamo, D.; Nicolò, M. The effect of orthodontic treatment on periodontal tissue inflammation: A systematic review. Quintessence Int. 2018, 49, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Gao, Y.; Min, Q.; Li, X.; Liu, L.; Lv, Y.; Xu, W.; Liu, X.; Wang, H. Immune System Acts on Orthodontic Tooth Movement: Cellular and Molecular Mechanisms. BioMed Res. Int. 2022, 2022, 9668610. [Google Scholar] [CrossRef]
- Karthi, M.; Anbuslevan, G.J.; Senthilkumar, K.P.; Tamizharsi, S.; Raja, S.; Prabhakar, K. NSAIDs in orthodontic tooth movement. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S2), S304–S306. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Almeida, V.L.; Lopes, B.M.V.; Franco, A.; Matos, F.R.; Quintans-Júnior, L.J.; Rode, S.M.; Paranhos, L.R. The influence of non-steroidal anti-inflammatory drugs and paracetamol used for pain control of orthodontic tooth movement: A systematic review. An. Acad. Bras. Ciências 2017, 89, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Robson, J. The dangers of NSAIDs: Look both ways. Br. J. Gen. Pract. 2016, 66, 172–173. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Vonkeman, H.E.; van de Laar, M.A. Nonsteroidal anti-inflammatory drugs: Adverse effects and their prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.E.; Graatsma, H.H.; Stricker, B.H. Contraindicated NSAIDs are frequently prescribed to elderly patients with peptic ulcer disease. Br. J. Clin. Pharmacol. 2002, 53, 183–188. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Ji, H.F.; Li, X.J.; Zhang, H.Y. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucl. Int. J. Cytol. Allied Top. 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Popa, Z.; Rusu, L.; Susan, R.; Pinzaru, I.; Ardelean, E.; Borcan, F.; Voicu, M.; Sas, I.T.; Popovici, R.A.; Lazureanu, V. Obtaining and characterization of a polyurethane carrier used for eugenol as a possible remedy in oral therapies. Mater. Plast. 2018, 55, 9–13. [Google Scholar] [CrossRef]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef]
- Nunes, C.D.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Menéndez López-Mateos, C.; Menéndez López-Mateos, M.L.; Aguilar-Salvatierra, A.; Gómez-Moreno, G.; Carreño, J.C.; Khaldy, H.; Menéndez-Núñez, M. Salivary Markers of Oxidative Stress in Patients Undergoing Orthodontic Treatment with Clear Aligners versus Self-Ligating Brackets: A Non-Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3531. [Google Scholar] [CrossRef]
- Martin, B.J.; Campbell, P.M.; Rees, T.D.; Buschang, P.H. A randomized controlled trial evaluating antioxidant-essential oil gel as a treatment for gingivitis in orthodontic patients. Angle Orthod. 2016, 86, 407–412. [Google Scholar] [CrossRef]
- Leiva-Cala, C.; Lorenzo-Pouso, A.I.; Centenera-Centenera, B.; López-Palafox, J.; Gándara-Vila, P.; García-García, A.; Pérez-Sayáns, M. Clinical efficacy of an Aloe Vera gel versus a 0.12% chlorhexidine gel in preventing traumatic ulcers in patients with fixed orthodontic appliances: A double-blind randomized clinical trial. Odontology 2019, 108, 470–478. [Google Scholar] [CrossRef]
- Kamath, D.G.; Nadimpalli, H.; Nayak, S.U.; Rajendran, V.; Natarajan, S. Comparison of antiplaque and anti-gingivitis effects of aloe vera mouthwash with chlorhexidine in fixed orthodontic patients—A randomized controlled trial. Int. J. Dent. Hyg. 2023, 21, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Furtado Júnior, J.H.C.; Valadas, L.A.R.; Fonseca, S.G.D.C.; Lobo, P.L.D.; Calixto, L.H.M.; Lima, A.G.F.; de Aguiar, M.H.R.; Arruda, I.S.; Lotif, M.A.L.; Rodrigues Neto, E.M.; et al. Clinical and Microbiological Evaluation of Brazilian Red Propolis Containing-Dentifrice in Orthodontic Patients: A Randomized Clinical Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 8532701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goes, P.; Dutra, C.S.; Lisboa, M.R.; Gondim, D.V.; Leitão, R.; Brito, G.A.; Rego, R.O. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J. Oral. Sci. 2016, 58, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Yeturu, S.K.; Acharya, S.; Urala, A.S.; Pentapati, K.C. Effect of Aloe vera, chlorine dioxide, and chlorhexidine mouth rinses on plaque and gingivitis: A randomized controlled trial. J. Oral Biol. Craniofacial Res. 2016, 6, 54–58. [Google Scholar] [CrossRef]
- Atwa, A.D.; AbuShahba, R.Y.; Mostafa, M.; Hashem, M.I. Effect of honey in preventing gingivitis and dental caries in patients undergoing orthodontic treatment. Saudi Dent. J. 2014, 26, 108–114. [Google Scholar] [CrossRef]
- Golshah, A.; Mirzaeei, S.; Nikkerdar, N.; Ghorbani, F. Gingivitis Effectiveness of Emulgel Containing 2% Resveratrol in Orthodontic Patients: An 8-Week Randomized Clinical Trial. Int. J. Dent. 2021, 2021, 6615900. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar] [PubMed] [PubMed Central]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vangipuram, S.; Jha, A.; Bhashyam, M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: A randomized controlled trial. J. Clin. Exp. Dent. 2016, 8, e442–e447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papadopoulou, C.; Karamani, I.; Gkourtsogianni, S.; Seremidi, K.; Kloukos, D. A systematic review on the effectiveness of organic unprocessed products in controlling gingivitis in patients undergoing orthodontic treatment with fixed appliances. Clin. Exp. Dent. Res. 2021, 7, 664–671. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, F.; Huang, H.; Wang, M.; Rong, W.; Wang, J. Applications of Antioxidants in Dental Procedures. Antioxidants 2022, 11, 2492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vo, T.T.T.; Chu, P.M.; Tuan, V.P.; Te, J.S.; Lee, I.T. The Promising Role of Antioxidant Phytochemicals in the Prevention and Treatment of Periodontal Disease via the Inhibition of Oxidative Stress Pathways: Updated Insights. Antioxidants 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosca, O.; Bumbu, B.A.; Ancusa, O.; Talpos, S.; Urechescu, H.; Ursoniu, S.; Bloanca, V.; Pricop, M. The Role of C-Reactive Protein and Neutrophil to Lymphocyte Ratio in Predicting the Severity of Odontogenic Infections in Adult Patients. Medicina 2023, 59, 20. [Google Scholar] [CrossRef]
- Pricop, M.; Ancusa, O.; Talpos, S.; Urechescu, H.; Bumbu, B.A. The Predictive Value of Systemic Immune-Inflammation Index and Symptom Severity Score for Sepsis and Systemic Inflammatory Response Syndrome in Odontogenic Infections. J. Pers. Med. 2022, 12, 2026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Valverde, N.; López-Valverde, A.; Montero, J.; Rodríguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Antioxidant, anti-inflammatory and antimicrobial activity of natural products in periodontal disease: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1226907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovac, V.; Poljsak, B.; Perinetti, G.; Primozic, J. Systemic Level of Oxidative Stress during Orthodontic Treatment with Fixed Appliances. Biomed. Res. Int. 2019, 2019, 5063565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zieniewska, I.; Maciejczyk, M.; Zalewska, A. The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity. Int. J. Mol. Sci. 2020, 21, 9684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Čanadi Jurešić, G.; Spalj, S. Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portelli, M.; Militi, A.; Cervino, G.; Lauritano, F.; Sambataro, S.; Mainardi, A.; Nucera, R. Oxidative Stress Evaluation in Patients Treated with Orthodontic Self-ligating Multibracket Appliances: An in Vivo Case-Control Study. Open Dent. J. 2017, 11, 257–265. [Google Scholar] [CrossRef]
- Kovač, V.; Poljšak, B.; Primožič, J.; Jamnik, P. Are Metal Ions That Make up Orthodontic Alloys Cytotoxic, and Do They Induce Oxidative Stress in a Yeast Cell Model? Int. J. Mol. Sci. 2020, 21, 7993. [Google Scholar] [CrossRef]
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A. Orthodontic treatment modifies the oxidant-antioxidant balance in saliva of clinically healthy subjects. Adv. Med. Sci. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Estrada, L.; Pérez-Soto, E.; Pérez-Vielma, N.M.; Gómez-López, M.; Sánchez-Monroy, V. Oxidative stress and genotoxicity in oral epithelial cells from subjects undergoing orthodontic treatment with fixed appliances. Clin. Oral. Investig. 2023, 27, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Atuğ Özcan, S.S.; Ceylan, I.; Ozcan, E.; Kurt, N.; Dağsuyu, I.M.; Canakçi, C.F. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis. Markers 2014, 2014, 597892. [Google Scholar] [CrossRef]
- Guler, C.; Toy, E.; Ozturk, F.; Gunes, D.; Karabulut, A.B.; Otlu, O. Evaluation of salivary total oxidant-antioxidant status and DNA damage of children undergoing fixed orthodontic therapy. Angle Orthod. 2015, 85, 239–244. [Google Scholar] [CrossRef]
- Santamaria, M., Jr.; Petermann, K.D.; Vedovello, S.A.; Degan, V.; Lucato, A.; Franzini, C.M. Antimicrobial effect of Melaleuca alternifolia dental gel in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.I.; Allarakia, R.; Alamoudi, N.M.; Nalliah, R.; Allareddy, V. Long-term clinical and bacterial effects of xylitol on patients with fixed orthodontic appliances. Prog. Orthod. 2015, 16, 35. [Google Scholar] [CrossRef][Green Version]
- Ashouri Moghaddam, A.; Radafshar, G.; Jahandideh, Y.; Kakaei, N. Clinical Evaluation of Effects of Local Application of Aloe vera Gel as an Adjunct to Scaling and Root Planning in Patients with Chronic Periodontitis. J. Dent. 2017, 18, 165–172. [Google Scholar] [PubMed] [PubMed Central]
- Pressman, P.; Clemens, R.; Hayes, A.W. Aloe vera at the frontier of glycobiology and integrative medicine: Health implications of an ancient plant. SAGE Open Med. 2019, 7, 2050312119875921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tariq, R.; Khurshid, Z.; Ahmed Farooqui, W.; Adanir, N. Anti-bacterial efficacy of Aloe vera against E. Faecalis in comparison to other intracanal medicaments: A systematic review and meta-analysis. Saudi Dent. J. 2023, 35, 451–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Girão Júnior, F.J.; Valadas, L.A.R.; Bottenberg, P.; Lotif, M.A.L.; Rodrigues Neto, E.M.; Fonseca, S.G.D.C.; Bandeira, M.A.M.; Squassi, A.; Dantas, T.C.F.B.; de Sena, N.J.C.; et al. Salivary Fluoride Bioavailability after Brushing with Brazilian Red Propolis Dentifrice: A Clinical Study. Evid. Based Complement. Altern. Med. 2022, 2022, 6148137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvalho, C.; Fernandes, W.H.C.; Mouttinho, T.B.F.; Souza, D.M.; Marcucci, M.C.; D’Alpino, P.H.P. Evidence-Based Studies and Perspectives of the Use of Brazilian Green and Red Propolis in Dentistry. Eur. J. Dent. 2019, 13, 459–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rani, N.; Singla, R.K.; Narwal, S.; Tanushree Kumar, N.; Rahman, M.M. Medicinal Plants Used as an Alternative to Treat Gingivitis and Periodontitis. Evid. Based Complement. Altern. Med. 2022, 2022, 2327641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seyyedi, S.A.; Sanatkhani, M.; Pakfetrat, A.; Olyaee, P. The therapeutic effects of chamomilla tincture mouthwash on oral aphthae: A Randomized Clinical Trial. J. Clin. Exp. Dent. 2014, 6, e535–e538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eskandari, F.; Borzou, S.; Razavian, A.; Babanouri, N.; Yousefi, K. Sustained antibacterial activity of orthodontic elastomeric ligature ties coated with a novel kombucha-derived bacterial nanocellulose: An in-vitro study. PLoS ONE 2024, 19, e0292966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shinkai, R.S.A.; Azevedo, C.L.; de Campos, T.T.; Michel-Crosato, E.; Biazevic, M.G.H. Importance of phytotherapy for oral health care and quality of life in adults: A scoping review. J. Dent. Sci. 2024, 19, 751–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shinkre, R.; Rodrigues, E.; Mukherji, I.; Pandya, D.; Naik, R.; Banerjee, A. Cissus Extracts in Dentistry: A Comprehensive Review on its Untapped Potential. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S1), S60–S62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedrinha, V.F.; Santos, L.M.; Gonçalves, C.P.; Garcia, M.T.; Lameira, O.A.; Queiroga, C.L.; Marcucci, M.C.; Shahbazi, M.A.; Sharma, P.K.; Junqueira, J.C.; et al. Effects of natural antimicrobial compounds propolis and copaiba on periodontal ligament fibroblasts, molecular docking, and in vivo study in Galleria mellonella. Biomed. Pharmacother. 2024, 171, 116139. [Google Scholar] [CrossRef] [PubMed]

| Study and Author | Country | Study Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 [21] López-Mateos et al. | Spain | 2022 | Randomized clinical trial | Medium |
| 2 [22] Martin et al. | United States | 2016 | Randomized clinical trial | High |
| 3 [23] Leiva-Cala et al. | Spain | 2019 | Randomized clinical trial | High |
| 4 [24] Kamath al. | India | 2022 | Randomized clinical trial | Medium |
| 5 [25] Furtado Júnior et al. | Brazil | 2020 | Randomized clinical trial | Medium |
| 6 [26] Goes et al. | Brazil | 2016 | Randomized clinical trial | Medium |
| 7 [27] Yeturu et al. | India | 2016 | Randomized clinical trial | Medium |
| 8 [28] Atwa et al. | Egypt | 2014 | Randomized clinical trial | Medium |
| 9 [29] Golshah et al. | Iran | 2021 | Randomized clinical trial | High |
| Study Number | Sample Size (Intervention Group) * | Mean Age/Age Range | Gender Distribution (Women) | Control Group(s) |
|---|---|---|---|---|
| 1 [21] López-Mateos et al. | Total: 67 patients, of which 48 had clear aligners | Intervention group (aligners): 32.2 years Control group (brackets): 29.3 years | Intervention group (aligners): 85.4% Control group (brackets): 78.9% | 19 patients with brackets |
| 2 [22] Martin et al. | Total: 32 patients, of which 16 patients were treated with antioxidant essential oil gel | Intervention group: 15.9 years Control group: 15.1 years | Intervention group: 50% Control group: 50% | 16 patients in placebo group treated with a water-based gel |
| 3 [23] Leiva-Cala et al. | 70 patients treated with Aloe vera gel | 12 years and older, mean: 26.1 years | 63.6% | 70 patients treated with 0.12% chlorhexidine gel |
| 4 [24] Kamath al. | Total: 30 patients, of which 15 patients had fixed orthodontic appliances treated with Aloe vera mouthwash | Intervention group: 22.5 years Control group: 22.7 years | Intervention group: 60.0% Control group: 53.3% | 15 patients with fixed orthodontic appliances treated with 0.2% chlorhexidine mouthwash |
| 5 [25] Furtado Júnior et al. | Total: 52 patients, of which 46 participants were treated with fluoride dentifrice and Brazilian red propolis | 12–16 years | 50.0% | 46 participants treated with regular fluoride dentifrice |
| 6 [26] Goes et al. | Total: 30 participants with fixed orthodontic appliances, of which 10 were treated with MTC | 10–40 years, mean: 28.8 years | 86.7% | 10 patients in the placebo group, 10 patients treated with 0.12% chlorhexidine mouthwash |
| 7 [27] Yeturu et al. | Total: 90 participants undergoing fixed orthodontic treatment, of which 30 were treated with Aloe vera, 30 with chlorhexidine, and 30 with chlorine dioxide | Mean age: 21.65 years | 50.0% | NR |
| 8 [28] Atwa et al. | Total: 20 orthodontic patients treated with honey and 10% sucrose or 10% sorbitol | 12–18 years | 100% | Sucrose 10% (positive control), sorbitol 10% (negative control) |
| 9 [29] Golshah et al. | Total: 46 orthodontic patients, of which 23 were treated with emulgel | 12–25 years | Experimental: 57.9% female, Placebo: 57.9% female, Control: 57.9% female | 23 patients in the placebo group that used no product |
| Study Number | Substance | Measurement/Dose/Administration | Follow-Up | Materials Used |
|---|---|---|---|---|
| 1 [21] López-Mateos et al. | Advanced oxidative protein product of saliva; total antioxidant capacity; myeloperoxidase activity | Saliva collection: between 08:30 and 09:00 after fasting and chewing paraffin for 5 min; initial secretion discarded, followed by 5 min of collection, then frozen at −80 °C and centrifuged at 3000 rpm for 20 min | At baseline before starting treatment, and then at 30 and 90 days before placing the next apparatus in the sequence | Aligners: Invisalign® system (Align Technology, San Jose, CA, USA), worn 22 h per day Brackets: Damon System® 0.22″ self-ligating brackets Q passive self-ligating brackets (Ormco Corporation, Orange, CA, USA) combined with Damon Optimal Force Copper Ni-Ti® 0.014″ archwires |
| 2 [22] Martin et al. | Antioxidant essential oil gel (containing menthol, thymol, ferulic acid, phloretin) | Applied twice daily after brushing to the gingiva, followed by a 30-min non-rinse period | Periodic assessments at T1, T2, and T3 (approximately every 4–6 weeks) | Placebo gel or active gel (AO ProVantage Dental Gel, Periosciences, Dallas, TX, USA) applied to the gingiva of patients with fixed orthodontic appliances |
| 3 [23] Leiva-Cala et al. | Aloe vera gel vs. 0.12% chlorhexidine gel | Aloe vera or chlorhexidine gel applied twice daily after tooth brushing, massaging for 15 min | Periodic assessments; initial and 1-month follow-up | 80% of Aloe vera combined with carbopol, a cross-linked acrylic acid hydrophilic polymer, and ascorbic acid. The CHX formulation used is commercially available as 0.12% Lacer Bioadhesive Gel (Lacer S.A., Barcelona, Spain) |
| 4 [24] Kamath al. | Aloe vera mouthwash vs. 0.2% chlorhexidine mouthwash | Aloe vera or chlorhexidine mouthwash used twice daily, 10 mL for 1 min | Assessments at baseline, 21 days, and 35 days | Aloe vera mouthwash consisted of pure Aloe vera juice (Aloe vera Juice, Patanjali Ayurveda Ltd., Kochi, India), derived from the pulp of the leaf. Composition of each 10 mL: 99.6% (w/v) Aloe vera juice; 0.02% (w/v) citric acid crystal; 0.02% (w/v) sodium benzoate crystal (preservative); and 0.2% Chlohex plus mouthwash |
| 5 [25] Furtado Júnior et al. | Fluoride dentifrice with and without Brazilian red propolis | Dentifrice used twice daily with standard brushing | Baseline and after 28 days | First, 150 g of the red propolis extract was taken and extracted with 1 L of cereal alcohol of 96° graduation and then diluted to a concentration of 1%. BRP extract at 1% concentration (previously studied antimicrobial concentration) was incorporated into the fluoridated dentifrice (1500 ppm). |
| 6 [26] Goes et al. | 1% Matricaria chamomilla L. mouthwash | Used twice daily, 15 mL for 1 min | Baseline and after 15 days | The mouthwash was prepared from Matricaria chamomilla L. extract; placebo and 0.12% chlorhexidine used as controls |
| 7 [27] Yeturu et al. | Aloe vera, chlorine dioxide, chlorhexidine | Mouth rinse used twice daily; 10 mL for 1 min | Baseline and after 15 days | NR |
| 8 [28] Atwa et al. | Honey | Patients were then asked to chew and ingest 10 g of pure undiluted honey in 2 min or rinse with 15 mL of 10% sucrose or sorbitol solutions (positive and negative controls, respectively) for 1 min | pH measured prior to baseline and at 2, 5, 10, 20, and 30 min after treatment. | Honey, sucrose, and sorbitol |
| 9 [29] Golshah et al. | emulgel containing 2% resveratrol | 5 mL applied and massaged onto the gums nightly for 30 s | Baseline, 4 weeks, and 8 weeks | Emulgel containing 2% resveratrol; identical placebo emulgel without resveratrol; no product for control |
| Study Number | Therapeutic Effects | Other Outcomes | Interpretation |
|---|---|---|---|
| 1 [21] López-Mateos et al. | AOPP (μM)–Aligners: T0 (47.1)–T90 (79.5) *. Self-ligating brackets: T0 (57.8)–T90 (80.2). MPO (mUl/mL)–Aligners: T0 (8.4) to T30 (13.9) and T90 (12.7). Self-ligating brackets: T0 (14.3)–T30 (9), and T90 (12). TAC (μM)–Aligners: T0 (50)–T90 (51.6). Self-ligating brackets: T0 (49.1)–T30 (53.5), and T90 (49.8). | NR | Overall, aligners showed a significant increase in AOPP over the 90 days, particularly in the later stages, whereas changes in MPO and TAC were not significant for either orthodontic technique. |
| 2 [22] Martin et al. | BOP: T1 − T2 = −13.6 in treatment group vs. −3.0 in placebo group *; GI: T1 − T3 = −0.02 in treatment group vs. +0.06 in placebo group *; PD: T1 − T2 = −0.03 in treatment group vs. +0.05 in placebo group * | The plaque index showed no significant differences between the treatment and control groups at any point during the study period. | Topical gel effectively reduced inflammation markers initially; however, the effects were not sustained post-discontinuation of the gel application. |
| 3 [23] Leiva-Cala et al. | Inflammation: 10.0% with Aloe vera vs. 51.4% with chlorhexidine * | Ulceration: 5.7% with Aloe vera vs. 81.4% with chlorhexidine * | Aloe vera gel demonstrated effective prevention of oral inflammation in orthodontic patients, significantly outperforming chlorhexidine gel without adverse effects |
| 4 [24] Kamath al. | GI: T1 − T2 = −0.64 in the Aloe vera group vs. −0.54 in the chlorhexidine group *; BOP: T1 − T2 = −23.7 in the Aloe vera group vs. −29.2 in the chlorhexidine group * | PI (Plaque Index): T1 − T2 = −0.56 in the Aloe vera group vs. −1.07 in the chlorhexidine group * | Both mouthwashes effectively managed plaque and gingivitis in orthodontic patients, suggesting Aloe vera as a viable alternative to chlorhexidine with fewer side effects. |
| 5 [25] Furtado Júnior et al. | GBI: T1 − T2 = −11.11 in the control group vs. −17.35 in the BRP group; * Gram-negative bacteria: T1 − T2 = +0.45 in the control group vs. −0.61 in the BRP group; * S. mutans: T1 − T2 = −0.06 in the control group vs. −0.33 in the BRP group.* | CFU counts for S. mutans and Gram-negative bacteria were significantly lower in the BRP group compared to the control group after 28 days | BRP dentifrice demonstrated better clinical and microbiological activity after 28 days, significantly reducing oral bacteria levels and improving gingival health compared to regular fluoride dentifrice |
| 6 [26] Goes et al. | VPI: Significant decrease in the MTC group (−25.6%) and the chlorhexidine group (−39.9%) compared with placebo; GBI: Significant decrease in the MTC group (−29.9%) and the chlorhexidine group (−32.0%) compared with placebo | Improvement in oral hygiene and reduction of gingival inflammation | MTC mouthwash significantly reduced plaque accumulation and gingival bleeding, offering an effective herbal alternative to chlorhexidine with fewer side effects |
| 7 [27] Yeturu et al. | Plaque score reduction: Aloe vera (−20.38%), chlorhexidine (−31.59%), chlorine dioxide (−30.29%); Gingival score reduction: Aloe vera (−9.88%), chlorhexidine (−16.30%), and chlorine dioxide (−12.22%) | NR | Chlorhexidine had the highest effectiveness in reducing plaque and gingival scores, with chlorine dioxide also being a cost-effective alternative |
| 8 [28] Atwa et al. | pH: Significant pH changes observed. Honey (6.85 to 5.86, then recovery to 6.84); sucrose (6.82 to 5.28); sorbitol stable around 6.86. Bacterial counts: Streptococcus mutans reduced from 255.6 to 104.4 CFU, Lactobacilli from 100.2 to 42.2 CFU, and P. gingivalis from 56.4 to 42 CFU with honey. | NR | Honey demonstrated a rapid recovery in plaque pH and significantly reduced bacterial counts compared to sucrose and sorbitol, suggesting strong antibacterial effects beneficial for orthodontic patients. |
| 9 [29] Golshah et al. | GI: Significant reduction from T0 to T2 in the experimental group (1.00 to 0.77); BOP: No significant change; PPD: decrease from T0 to T2 in the experimental group (2.23 to 1.29); HI: significant reduction from T0 to T2 in the experimental group (1.52 to 0.68) * | NR | Emulgel containing 2% resveratrol significantly improved clinical parameters of gingival health over 8 weeks, reducing GBI, HI, and PD effectively compared to placebo and control groups. Demonstrates potential for use in managing gingivitis in orthodontic patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talpos Niculescu, S.; Avramut, R.; Hajaj, T.; Nikolajevic-Stoican, N.; Maracineanu, R.; Perdiou, A.; Talpos Niculescu, R.; Pricop, M.; Ghircau-Radu, R.; Luca, M.M.; et al. Evaluating the Therapeutic Properties of Natural Products in Orthodontic and Surgical Treatment of Dentofacial Deformities: A Systematic Review of Clinical Trials. Nutrients 2024, 16, 1941. https://doi.org/10.3390/nu16121941
Talpos Niculescu S, Avramut R, Hajaj T, Nikolajevic-Stoican N, Maracineanu R, Perdiou A, Talpos Niculescu R, Pricop M, Ghircau-Radu R, Luca MM, et al. Evaluating the Therapeutic Properties of Natural Products in Orthodontic and Surgical Treatment of Dentofacial Deformities: A Systematic Review of Clinical Trials. Nutrients. 2024; 16(12):1941. https://doi.org/10.3390/nu16121941
Chicago/Turabian StyleTalpos Niculescu, Serban, Robert Avramut, Tareq Hajaj, Nicoleta Nikolajevic-Stoican, Raluca Maracineanu, Antonis Perdiou, Roxana Talpos Niculescu, Marius Pricop, Roxana Ghircau-Radu, Magda Mihaela Luca, and et al. 2024. "Evaluating the Therapeutic Properties of Natural Products in Orthodontic and Surgical Treatment of Dentofacial Deformities: A Systematic Review of Clinical Trials" Nutrients 16, no. 12: 1941. https://doi.org/10.3390/nu16121941
APA StyleTalpos Niculescu, S., Avramut, R., Hajaj, T., Nikolajevic-Stoican, N., Maracineanu, R., Perdiou, A., Talpos Niculescu, R., Pricop, M., Ghircau-Radu, R., Luca, M. M., & Popa, M. (2024). Evaluating the Therapeutic Properties of Natural Products in Orthodontic and Surgical Treatment of Dentofacial Deformities: A Systematic Review of Clinical Trials. Nutrients, 16(12), 1941. https://doi.org/10.3390/nu16121941








