Nutritional Strategies against Diabetic Nephropathy: Insights from Animal Studies and Human Trials
Abstract
1. Introduction
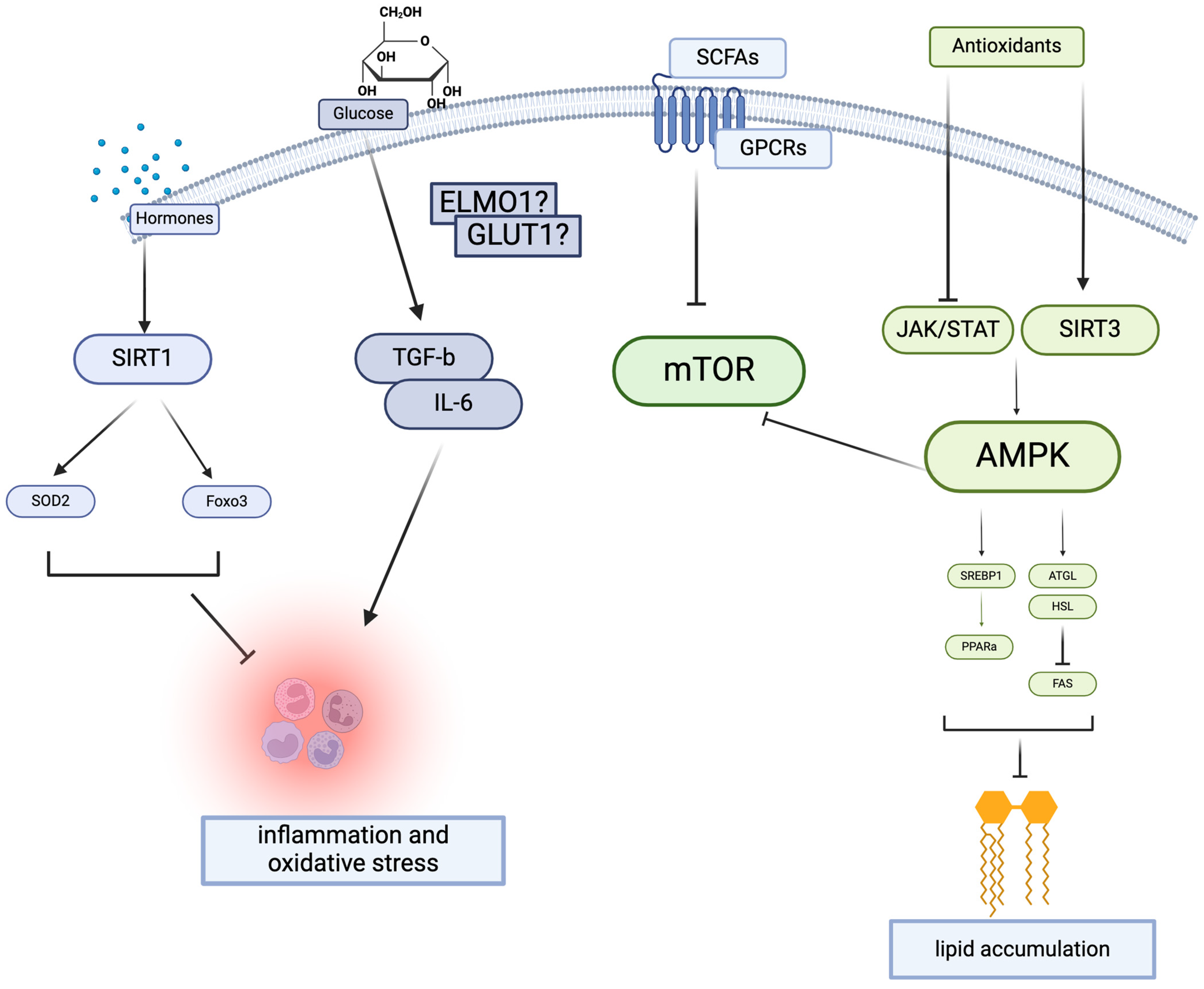
2. Mechanistic Studies
2.1. Nutritional Compound: Autophagy, Nutrients Metabolism, and Oxidative Stress
2.1.1. Lipid Metabolism and AMPK Signaling
2.1.2. Nutritional Compounds
2.2. Gut–Kidney Axis
2.2.1. Fiber Intake and Gut Microbiota
2.2.2. SCFA Cellular Receptors
2.3. Vitamin D: Autophagy and Metabolism
3. Human Level Trials
3.1. Protein Restriction on DN Progression
3.2. Antioxidants: Polyphenols and Flavonoids
3.3. Salt Intake
3.4. Omega-3 and Omega-6 Fatty Acids
4. Conclusions
Funding
Conflicts of Interest
References
- Lim, A. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renovasc Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Cabala, S.; Ozgo, M.; Herosimczyk, A. The Kidney-Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression. Metabolites 2024, 14, 78. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Goldstein-Fuchs, J.; Kalantar-Zadeh, K. Nutrition Intervention for Advanced Stages of Diabetic Kidney Disease. Diabetes Spectr. 2015, 28, 181–186. [Google Scholar] [CrossRef]
- Tamadon, M.R.; Zahmatkesh, M.; Beladi Mousavi, S.S. Administration of antioxidants in chronic kidney disease. J. Nephropharmacol. 2015, 4, 9–11. [Google Scholar]
- Dennis, J.M.; Witting, P.K. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef]
- Chow, F.Y.; Nikolic-Paterson, D.J.; Ozols, E.; Atkins, R.C.; Tesch, G.H. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J. Am. Soc. Nephrol. 2005, 16, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Grobe, A.C.; Wells, S.M.; Benavidez, E.; Oishi, P.; Azakie, A.; Fineman, J.R.; Black, S.M. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: Role of NADPH oxidase and endothelial NO synthase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1069–L1077. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xing, X.; Li, M.; Liu, Y.; Xu, A.; Zhang, J. Podocyte injury of diabetic nephropathy: Novel mechanism discovery and therapeutic prospects. Biomed. Pharmacother. 2023, 168, 115670. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Quianzon, C.C.; Cheikh, I. History of insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 21, 18701. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Shin, Y.K.; Jeong, J.H. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARdelta-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Byun, W.S.; Kang, M.J.; Han, J.A.; Moon, J.; Shin, M.J.; Lee, H.J.; Chung, J.H.; Lee, J.S.; Son, C.G.; et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKalpha2. FEBS J. 2020, 287, 2087–2104. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, C.; Zhang, Y.; Xu, D.; Gui, L.; Lu, Y.; Zhang, Q. Liraglutide regulates lipid metabolism via FGF21-LKB1-AMPK-ACC1 pathway in white adipose tissues and macrophage of type 2 diabetic mice. Biochem. Biophys. Res. Commun. 2021, 548, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Wang, R.; Hu, D.; Zhao, X.; Hu, W. Correlation of serum meteorin-like concentrations with diabetic nephropathy. Diabetes Res. Clin. Pract. 2020, 169, 108443. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, Y.; Wang, M.; Hou, Y.; Huang, W.; Zhou, D.; Wang, Z.; Yang, S.; Tang, W.; Zhen, J.; et al. Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab. 2020, 32, 1052–1062.e8. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Yi, B.; Yao, B.Q.; Xia, T.; Yang, Y.F.; Zhang, Z.H.; Chen, C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol. Res. 2020, 156, 104778. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Garcia-Mayorga, E.A.; Diaz-Avila, D.L.; Garza-Veloz, I.; Martinez-Fierro, M.L.; Gonzalez-Mateo, G.T. Potential Therapeutic Effects of Natural Plant Compounds in Kidney Disease. Molecules 2021, 26, 6096. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing nutritional component chrysin as a therapeutic agent: Bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef] [PubMed]
- Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, H.; Xu, N.; Zhou, S.; Peng, Y.; Zhu, J.; Liu, S.; Chang, Y. Chrysin improves diabetic nephropathy by regulating the AMPK-mediated lipid metabolism in HFD/STZ-induced DN mice. J. Food Biochem. 2022, 46, e14379. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Fan, Y.J. Resveratrol improves lipid metabolism in diabetic nephropathy rats. Front. Biosci. 2020, 25, 1913–1924. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K.S. SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxid. Med. Cell. Longev. 2016, 2016, 7296092. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, H.; Li, J.; Ma, T.; Zhou, S.; Zhang, Z.; Miao, L.; Cai, L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin. Sci. 2020, 134, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Ma, Y.; Cai, F.; Huang, X.; Xiao, L.; Zhong, C.; Ren, P.; Luo, Q.; Chen, J.; Han, F. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 2022, 76, 294–303. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, J.; Cai, Y.; Fu, M.; Li, W.; Shi, L.; Liu, J.; Dong, R.; Xu, X.; Tu, L.; et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol. Res. 2022, 183, 106367. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Tougaard, N.H.; Frimodt-Moller, M.; Salmenkari, H.; Stougaard, E.B.; Zawadzki, A.D.; Mattila, I.M.; Hansen, T.W.; Legido-Quigley, C.; Horkko, S.; Forsblom, C.; et al. Effects of Butyrate Supplementation on Inflammation and Kidney Parameters in Type 1 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 3573. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Xia, W.J.; Cui, N.; Bai, J.; Dai, Z.M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; De Paepe, K.; Vanholder, R.; Kerckhof, F.M.; Van Biesen, W.; Van de Wiele, T.; Verbeke, F.; Speeckaert, M.; Joossens, M.; Couttenye, M.M.; et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020, 97, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Atoh, K.; Itoh, H.; Haneda, M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: Relation to renal function. Diabetes Res. Clin. Pract. 2009, 83, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Menez, S.; Hanouneh, M.; Shafi, T.; Jaar, B.G. Indoxyl sulfate is associated with mortality after AKI—More evidence needed! BMC Nephrol. 2019, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Oladi-Ghadikolaei, R.; Aliasgharzadeh, A.; Shayanfar, A.; Soleymani, J.; Moradi, M.; Jouyban, A.; Tayebi Khosroshahi, H. Serum Levels of Indoxyl Sulfate and P-cresol in Type II Diabetic Patients with and without Nephropathy. Iran. J. Kidney Dis. 2023, 17, 126–134. [Google Scholar] [PubMed]
- Florens, N.; Yi, D.; Juillard, L.; Soulage, C.O. Using binding competitors of albumin to promote the removal of protein-bound uremic toxins in hemodialysis: Hope or pipe dream? Biochimie 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Jukema, J.W.; Timal, R.J.; Rotmans, J.I.; Hensen, L.C.R.; Buiten, M.S.; de Bie, M.K.; Putter, H.; Zwinderman, A.H.; van Erven, L.; Krol-van Straaten, M.J.; et al. Prophylactic Use of Implantable Cardioverter-Defibrillators in the Prevention of Sudden Cardiac Death in Dialysis Patients. Circulation 2019, 139, 2628–2638. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.B.; Lu, J.; Chen, P.P.; Lu, C.C.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; Ruan, X.Z.; Liu, B.C.; et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics 2020, 10, 2803–2816. [Google Scholar] [CrossRef] [PubMed]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Bae, E.H.; Kim, I.J.; Ma, S.K.; Choi, C.; Lee, J.; Kim, S.W. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am. J. Physiol. Renal Physiol. 2010, 298, F301–F313. [Google Scholar] [CrossRef] [PubMed]
- Hamzawy, M.; Gouda, S.A.A.; Rashid, L.; Attia Morcos, M.; Shoukry, H.; Sharawy, N. The cellular selection between apoptosis and autophagy: Roles of vitamin D, glucose and immune response in diabetic nephropathy. Endocrine 2017, 58, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, Y.; Liang, Y.; Fu, S.; Yang, C.; Liu, K.; Chen, Y. Vitamin D protects glomerular mesangial cells from high glucose-induced injury by repressing JAK/STAT signaling. Int. Urol. Nephrol. 2021, 53, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Guo, Y.; Zhou, M.; Zhang, X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism 2014, 63, 1324–1333. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Li, L.; Wu, B.; Liu, J.Y.; Yang, L.B. Vitamin D receptor gene polymorphisms and type 2 diabetes: A meta-analysis. Arch. Med. Res. 2013, 44, 235–241. [Google Scholar] [CrossRef]
- Khodir, S.A.; Samaka, R.M.; Ameen, O. Autophagy and mTOR Pathways Mediate the Potential Renoprotective Effects of Vitamin D on Diabetic Nephropathy. Int. J. Nephrol. 2020, 2020, 7941861. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Gruden, G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin. Sci. 2022, 136, 493–520. [Google Scholar] [CrossRef]
- Li, A.; Yi, B.; Han, H.; Yang, S.; Hu, Z.; Zheng, L.; Wang, J.; Liao, Q.; Zhang, H. Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway. Autophagy 2022, 18, 877–890. [Google Scholar] [CrossRef]
- Atia, T.; Iqbal, M.Z.; Fathy Ahmed, H.; Sakr, H.I.; Abdelzaher, M.H.; Morsi, D.F.; Metawee, M.E. Vitamin D Supplementation Could Enhance the Effectiveness of Glibenclamide in Treating Diabetes and Preventing Diabetic Nephropathy: A Biochemical, Histological and Immunohistochemical Study. J. Evid. Based Integr. Med. 2022, 27, 2515690X221116403. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Komine, M.; Watabe, Y.; Shimaoka, S.; Sato, F.; Kake, K.; Nishina, H.; Ohtsuki, M.; Nakagawa, H.; Tamaki, K. The action of a novel vitamin D3 analogue, OCT, on immunomodulatory function of keratinocytes and lymphocytes. Arch. Dermatol. Res. 1999, 291, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Gong, J.; Liu, Y.; Xiang, R.; Tan, X. Loss of vitamin D receptor in chronic kidney disease: A potential mechanism linking inflammation to epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2012, 303, F1107–F1115. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Hami, M.; Hatefi, A. The effects of calcitriol on albuminuria in patients with type-2 diabetes mellitus. Saudi J. Kidney Dis. Transpl. 2012, 23, 1215–1220. [Google Scholar] [CrossRef]
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. 2019, 13, 542–547. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mortazavi, M.; Iraj, B.; Askari, G. Whether vitamin D3 is effective in reducing proteinuria in type 2 diabetic patients? J. Res. Med. Sci. 2013, 18, 374–377. [Google Scholar] [PubMed]
- Mustafar, R.; Mohd, R.; Ahmad Miswan, N.; Cader, R.; Gafor, H.A.; Mohamad, M.; Shah, S.A.; Kamaruddin, N.A.; Chiew Tong, N.K. The effect of calcium with or without calcitriol supplementation on renal function in patients with hypovitaminosis d and chronic kidney disease. Nephrourol. Mon. 2014, 6, e13381. [Google Scholar] [CrossRef] [PubMed]
- Senyigit, A. The association between 25-hydroxy vitamin D deficiency and diabetic complications in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Fawaz, L.A.; Sedik, E.E.; Nour, Z.A.; Elsayed, R.M. Vitamin D status in diabetic patients (type 2) and its relation to glycemic control & diabetic nephropathy. Diabetes Metab. Syndr. 2019, 13, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Mirhosseini, M.; Kabiri, M.; Kheiri, S. Effect of vitamin D on proteinuria in type 2 diabetic patients. J. Nephropathol. 2017, 6, 10–14. [Google Scholar] [CrossRef]
- Kim, M.J.; Frankel, A.H.; Donaldson, M.; Darch, S.J.; Pusey, C.D.; Hill, P.D.; Mayr, M.; Tam, F.W. Oral cholecalciferol decreases albuminuria and urinary TGF-beta1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 2011, 80, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Takashi, Y.; Tanabe, M. Significance of Metformin Use in Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 4239. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes, C.K.D.W.G. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105 (Suppl. 4), S117–S314. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Tanaka, S.; Wakui, H.; Azushima, K.; Tsukamoto, S.; Yamaji, T.; Urate, S.; Suzuki, T.; Abe, E.; Taguchi, S.; Yamada, T.; et al. Effects of a High-Protein Diet on Kidney Injury under Conditions of Non-CKD or CKD in Mice. Int. J. Mol. Sci. 2023, 24, 7778. [Google Scholar] [CrossRef]
- Fotheringham, A.K.; Solon-Biet, S.M.; Bielefeldt-Ohmann, H.; McCarthy, D.A.; McMahon, A.C.; Ruohonen, K.; Li, I.; Sullivan, M.A.; Whiddett, R.O.; Borg, D.J.; et al. Kidney disease risk factors do not explain impacts of low dietary protein on kidney function and structure. iScience 2021, 24, 103308. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Obeid, W.; Hiremath, S.; Topf, J.M. Protein Restriction for CKD: Time to Move On. Kidney360 2022, 3, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A. Dietary protein restriction in CKD: The debate continues. Am. J. Kidney Dis. 2009, 53, 189–191. [Google Scholar] [CrossRef]
- Jiang, S.; Fang, J.; Li, W. Protein restriction for diabetic kidney disease. Cochrane Database Syst. Rev. 2023, 1, CD014906. [Google Scholar] [CrossRef]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Protein intake is not associated with progression of diabetic kidney disease in patients without macroalbuminuria. Diabetes Metab. Res. Rev. 2019, 35, e3150. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
- Meng, Y.; Bai, H.; Yu, Q.; Yan, J.; Zhao, L.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. High-Resistant Starch, Low-Protein Flour Intervention on Patients with Early Type 2 Diabetic Nephropathy: A Randomized Trial. J. Ren. Nutr. 2019, 29, 386–393. [Google Scholar] [CrossRef]
- Kamper, A.L.; Strandgaard, S. Long-Term Effects of High-Protein Diets on Renal Function. Annu. Rev. Nutr. 2017, 37, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Jalilpiran, Y.; Nekouimehr, M.; Fattahi, S.; Mokhtari, P.; Jayedi, A.; Yekaninejad, M.S.; Mirzaei, K. Dietary protein sources and risk of diabetic nephropathy in women: A case-control study. BMC Endocr. Disord. 2021, 21, 174. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Zou, L.; Jin, L.; Yang, B.; Shu, Y.; Gong, R. Dose-response relationship between dietary antioxidant intake and diabetic kidney disease in the US adults with diabetes. Acta Diabetol. 2023, 60, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Gowd, V.; Kang, Q.; Wang, Q.; Wang, Q.; Chen, F.; Cheng, K.W. Resveratrol: Evidence for Its Nephroprotective Effect in Diabetic Nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, S.; Zhang, L.; Li, Y. Resveratrol prevents renal lipotoxicity in high-fat diet-treated mouse model through regulating PPAR-alpha pathway. Mol. Cell Biochem. 2016, 411, 143–150. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xiong, J.; Nie, L.; Yu, Y.; Guan, X.; Xu, X.; Xiao, T.; Yang, K.; Liu, L.; Zhang, D.; et al. Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J. Mol. Med. 2016, 94, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Lin, J.H.; Hammes, H.P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef]
- Liu, F.; Nie, J.; Deng, M.G.; Yang, H.; Feng, Q.; Yang, Y.; Li, X.; Li, X.; Yang, X.; Li, W.; et al. Dietary flavonoid intake is associated with a lower risk of diabetic nephropathy in US adults: Data from NHANES 2007–2008, 2009–2010, and 2017–2018. Food Funct. 2023, 14, 4183–4190. [Google Scholar] [CrossRef]
- Hodson, E.M.; Cooper, T.E. Altered dietary salt intake for preventing diabetic kidney disease and its progression. Cochrane Database Syst. Rev. 2023, 1, CD006763. [Google Scholar] [CrossRef]
- Kotake, Y.; Karashima, S.; Kawakami, M.; Hara, S.; Aono, D.; Konishi, S.; Kometani, M.; Mori, H.; Takeda, Y.; Yoneda, T.; et al. Impact of salt intake on urinary albumin excretion in patients with type 2 diabetic nephropathy: A retrospective cohort study based on a generalized additive model. Endocr. J. 2022, 69, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Kinguchi, S.; Wakui, H.; Ito, Y.; Kondo, Y.; Azushima, K.; Osada, U.; Yamakawa, T.; Iwamoto, T.; Yutoh, J.; Misumi, T.; et al. Relationship between basal sodium intake and the effects of dapagliflozin in albuminuric diabetic kidney disease. Sci. Rep. 2021, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Chewcharat, A.; Chewcharat, P.; Rutirapong, A.; Papatheodorou, S. The effects of omega-3 fatty acids on diabetic nephropathy: A meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0228315. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Nagawa, D.; Nakata, M.; Narita-Kinjo, I.; Fujita, T.; Murakami, R.; Shimada, M.; Okita, A.; Sekino, K.; Tazawa, A.; et al. Dietary Intake of Polyunsaturated Fatty Acids and Diabetic Nephropathy: Cohort Analysis of the Tsugaru Study. In Vivo 2023, 37, 1890–1893. [Google Scholar] [CrossRef]
- Zong, G.; Liu, G.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Associations Between Linoleic Acid Intake and Incident Type 2 Diabetes among U.S. Men and Women. Diabetes Care 2019, 42, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Kakoki, M.; Bahnson, E.M.; Hagaman, J.R.; Siletzky, R.M.; Grant, R.; Kayashima, Y.; Li, F.; Lee, E.Y.; Sun, M.T.; Taylor, J.M.; et al. Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. JCI Insight 2019, 4, e127660. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Zhang, Y.; Zhang, X.; Huang, Q.; Guo, X. Association of Apolipoprotein E Gene Polymorphism with Type 2 Diabetic Nephropathy in the Southern Chinese Population. Int. J. Gen. Med. 2023, 16, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, I.; Kytoudis, K.; Papathanasiou, A.A.; Zaragotas, D.; Melistas, L.; Kitsios, G.D.; Yiannakouris, N.; Zintzaras, E. XbaI GLUT1 gene polymorphism and the risk of type 2 diabetes with nephropathy. Dis. Markers 2009, 27, 29–35. [Google Scholar] [CrossRef]
- Reynolds, K.M.; Horimoto, A.; Lin, B.M.; Zhang, Y.; Kurniansyah, N.; Yu, B.; Boerwinkle, E.; Qi, Q.; Kaplan, R.; Daviglus, M.; et al. Ancestry-driven metabolite variation provides insights into disease states in admixed populations. Genome Med. 2023, 15, 52. [Google Scholar] [CrossRef]
- Chen, Y.; Bi, S.; Gu, J.; Che, Q.; Liu, R.; Li, W.; Dai, T.; Wang, D.; Zhang, X.; Zhang, Y. Achieving personalized nutrition for patients with diabetic complications via 3D food printing. IJB 2024, 10, 1862. [Google Scholar] [CrossRef]
- Schechter, M.; Leibowitz, G.; Mosenzon, O. Paving the way to precision medicine for diabetic kidney disease: The PRIORITY trial. Ann. Transl. Med. 2020, 8, 1698. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, L.; Fan, Y.; Ma, J. Vitamin D receptor gene polymorphisms in association with diabetic nephropathy: A systematic review and meta-analysis. BMC Med. Genet. 2017, 18, 95. [Google Scholar] [CrossRef]
- Woods, A.; Azzout-Marniche, D.; Foretz, M.; Stein, S.C.; Lemarchand, P.; Ferre, P.; Foufelle, F.; Carling, D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell Biol. 2000, 20, 6704–6711. [Google Scholar] [CrossRef]
| Metabolic Change | Pathway/Target | Model | Effects on DN |
|---|---|---|---|
| Lipid Handling | Sirt3–AMPK (Metrnl) [21] Sirt1–AMPK-SREBP1 (JAM) [22] | STZ + HFD mice, JAML KO mice | Attenuated renal injury, altered lipid synthesis |
| AMPK-SREBP1c-PPARa (chrysin) [28] AMPKa/mTOR (resveratrol) [30] AKT/glycogen-Nrf2 (SFN) [34] | Various STZ mice/rats | Improved lipid metabolism, decreased lipotoxicity | |
| Inflammation and oxidative stress | Butyrate-GPR43 (F. prausnitzii) acetate/GPR43 [35] Sirt1-FOXO3a/SOD2 [32,33] | GPCR43 KO mice, DM rats | Modulated inflammation, ameliorated oxidative stress, regulated cytokine expression and renal function |
| Gut Microbiota | Inulin-fermented SCFAs (ITFs) [37] GPR43/109A (dietary fiber) [53] | db/db mice, Gpr KO mice | Improved renal function and glucose and lipid metabolism, reduced fibrosis |
| Vitamin D Signaling | VDR Signaling (calcitriol, paricalcitol) [62] JAK/STAT (vitamin D) [57] TGF-β and inflammation response [44,58] | STZ-treated rats, VDR KO mice | Ameliorated proteinuria, enhanced autophagy, reduced inflammation |
| Observational Studies: | ||||
|---|---|---|---|---|
| Nutritional Exposures | Disease Stage | Study Design | Participants | Outcomes |
| Dietary antioxidant intake | DN | Meta-analysis [94] | 5676 | Antioxidants lower kidney disease risk and mortality |
| Dietary flavonoid intake | DN and non-DN | Meta-analysis [100] | 1949 | Fewer flavonoids associated with DN progression |
| Salt intake | DN | Retrospective observational [102] | 269 | Higher salt intake increases SBP, HbA1c, and UAE |
| Omega-6 fatty acid intake | Stage 2 DN | Prospective cohort [105] | 123 | n-6 fatty acids with greater association with UAE |
| Basal sodium intake and dapagliflozin treatment | Type 2 diabetes | Secondary analysis of cohort [103] | 86 | Dapagliflozin decreases BP and eGFR in high-salt-intake group |
| Protein intake | Diabetes with diminished renal function | Retrospective cohort [88] | 144 | No change in UAE and eGFR with restricted protein diet |
| Clinical Trials: | ||||
| Nutritional Intervention | Disease Stage | Study Design | Participants | Outcomes |
| Altered salt intake | Type 1 or 2 diabetes | Meta-analysis of RCT [101] | 313 | No significant change in eGFR, reduced body weight, BP |
| Omega-3 fatty acids | Type 1 or 2 diabetes | Meta-analysis of RCTs [104] | 344 | No significant changes in BP, lower proteinuria |
| Low-protein diet | DN | Meta-analysis of RCTs [87] | 486 | No significant effect of protein restriction on DN |
| High-resistant starch and low-protein diet | Early DN | RCT [90] | 75 | Improved renal panel, blood glucose level |
| Mediterranean vs. Western diet | DN | Case-control study [92] | 105 | Improved in renal panel with Mediterranean diet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Franceschini, N.; Townley-Tilson, W.H.D.; Maeda-Smithies, N. Nutritional Strategies against Diabetic Nephropathy: Insights from Animal Studies and Human Trials. Nutrients 2024, 16, 1918. https://doi.org/10.3390/nu16121918
Zhou J, Franceschini N, Townley-Tilson WHD, Maeda-Smithies N. Nutritional Strategies against Diabetic Nephropathy: Insights from Animal Studies and Human Trials. Nutrients. 2024; 16(12):1918. https://doi.org/10.3390/nu16121918
Chicago/Turabian StyleZhou, Jiayi, Nora Franceschini, W. H. Davin Townley-Tilson, and Nobuyo Maeda-Smithies. 2024. "Nutritional Strategies against Diabetic Nephropathy: Insights from Animal Studies and Human Trials" Nutrients 16, no. 12: 1918. https://doi.org/10.3390/nu16121918
APA StyleZhou, J., Franceschini, N., Townley-Tilson, W. H. D., & Maeda-Smithies, N. (2024). Nutritional Strategies against Diabetic Nephropathy: Insights from Animal Studies and Human Trials. Nutrients, 16(12), 1918. https://doi.org/10.3390/nu16121918







