Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substance
2.2. Main Reagents and Instruments
2.2.1. Main Reagents
2.2.2. Main Instruments
2.3. Experimental Cells and Treatmentss
2.3.1. Preparation of PA Solution
2.3.2. Establishment of the HepG2-IR Cell Model
2.3.3. Cell Groups
2.4. The Experimental Method
2.4.1. Cell Viability Assay
2.4.2. Glucose Consumption Measurement
2.4.3. Glycogen Content Determination
2.4.4. Liver Enzyme Assay
2.4.5. Oxidative Stress Biomarker Detection
2.4.6. Western Blot
- (1)
- Tissue protein extraction was performed by employing pre-chilled RIPA protein extraction reagent, augmented with a protease inhibitor cocktail and a phosphatase inhibitor specifically targeting phosphorylated proteins. Prior to extraction, 0.1M PMSF stock solution was added to achieve a final concentration of 1 mM PMSF. Tissue samples were homogenized in lysis buffer at a 1:9 (weight/volume) ratio using a Fluka electric tissue homogenizer at 15,000 rpm for three cycles of 10 s with 10 s intervals, while maintaining low temperature in an ice/water mixture. Following homogenization, the samples underwent a 20 min incubation on ice, followed by centrifugation at 13,000 rpm for 20 min at 4 °C. Subsequently, the resultant supernatant was gathered, divided into aliquots, and stored appropriately. Then, protein concentration was quantified using the BCA method, and adjustments were made by diluting with RIPA buffer and incorporating 5× reducing sample buffer to achieve a final concentration of 2 mg/mL, followed by protein denaturation through boiling for 5 min.
- (2)
- Western blot: Either 12% or 8% separation gels were prepared based on the target protein’s molecular weight, incorporating a 5% stacking gel. A total of 20 μg of protein was loaded per well. Electrophoresis conditions entailed applying a constant 90 V for approximately 20 min to the stacking gel and 160 V to the separation gel, with endpoint determination using pre-stained protein markers. Wet transfer was performed at a constant current of 300 mA using a 0.45 μm pore size NC membrane, transferring for 1 h for 12% separation gels or 2 h for 8% separation gels. Post-transfer, the membrane was stained with Ponceau S solution to evaluate transfer efficiency and mark the lanes. The membrane was blocked in 3% BSA-TBST at room temperature for 30 min with gentle shaking. The primary antibody was diluted in 3% BSA-TBST, incubating initially at room temperature for 10 min, followed by overnight incubation at 4 °C. The next day, the membrane was equilibrated to room temperature and incubated for an additional 30 min. The membrane was washed with TBST five times for 3 min each. The secondary antibody, goat anti-rabbit IgG (H+L) HRP, was diluted in 5% skim milk-TBST at a ratio of 1:10,000 and shaken gently at room temperature for 40 min. Subsequently, the membrane was washed with TBST six times for 3 min each. Finally, visualization was carried out using ECL chemiluminescence detection reagent, and grayscale intensity was analyzed using Image-J software (Total Lab Quant V11.5, Newcastle upon, Tyne, UK).
2.5. Statistical Analysis
3. Results
3.1. IR-HepG2 Cell Model Construction
3.2. The Effect of Exogenous NTs on the Viability of IR-HepG2 Cells
3.3. The Effect of Exogenous NTs on Glucose Consumption in IR-HepG2 Cells
3.4. The Effect of Exogenous NTs on Glycogen Synthesis in IR-HepG2 Cells
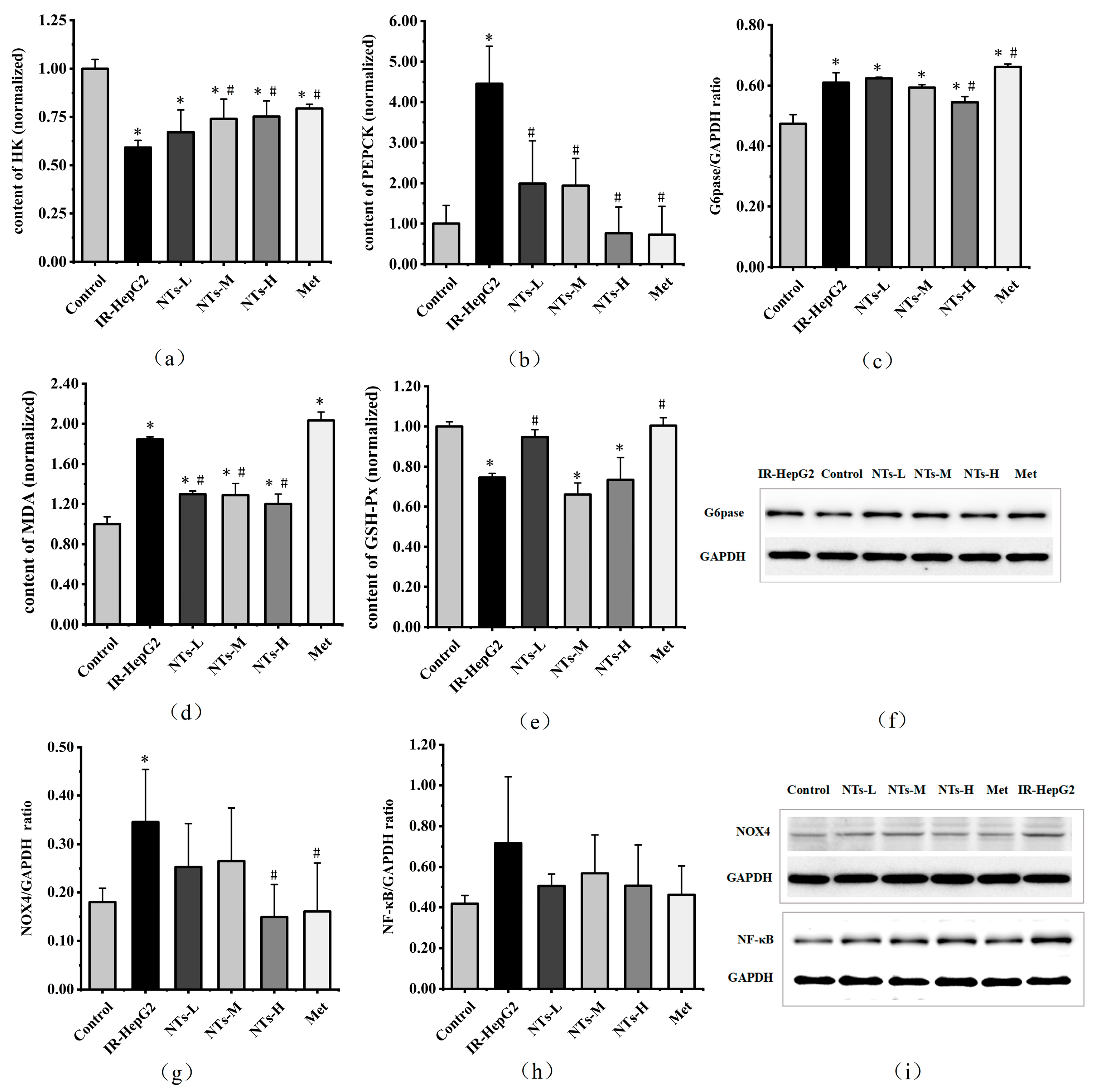
3.5. The Effect of Exogenous NTs on Glycolysis/Gluconeogenesis in IR-HepG2 Cells
3.6. The Effect of Exogenous NTs on Oxidative Stress in IR-HepG2 Cells
3.7. The Effect of Exogenous NTs on NF-κB in IR-HepG2 Cells
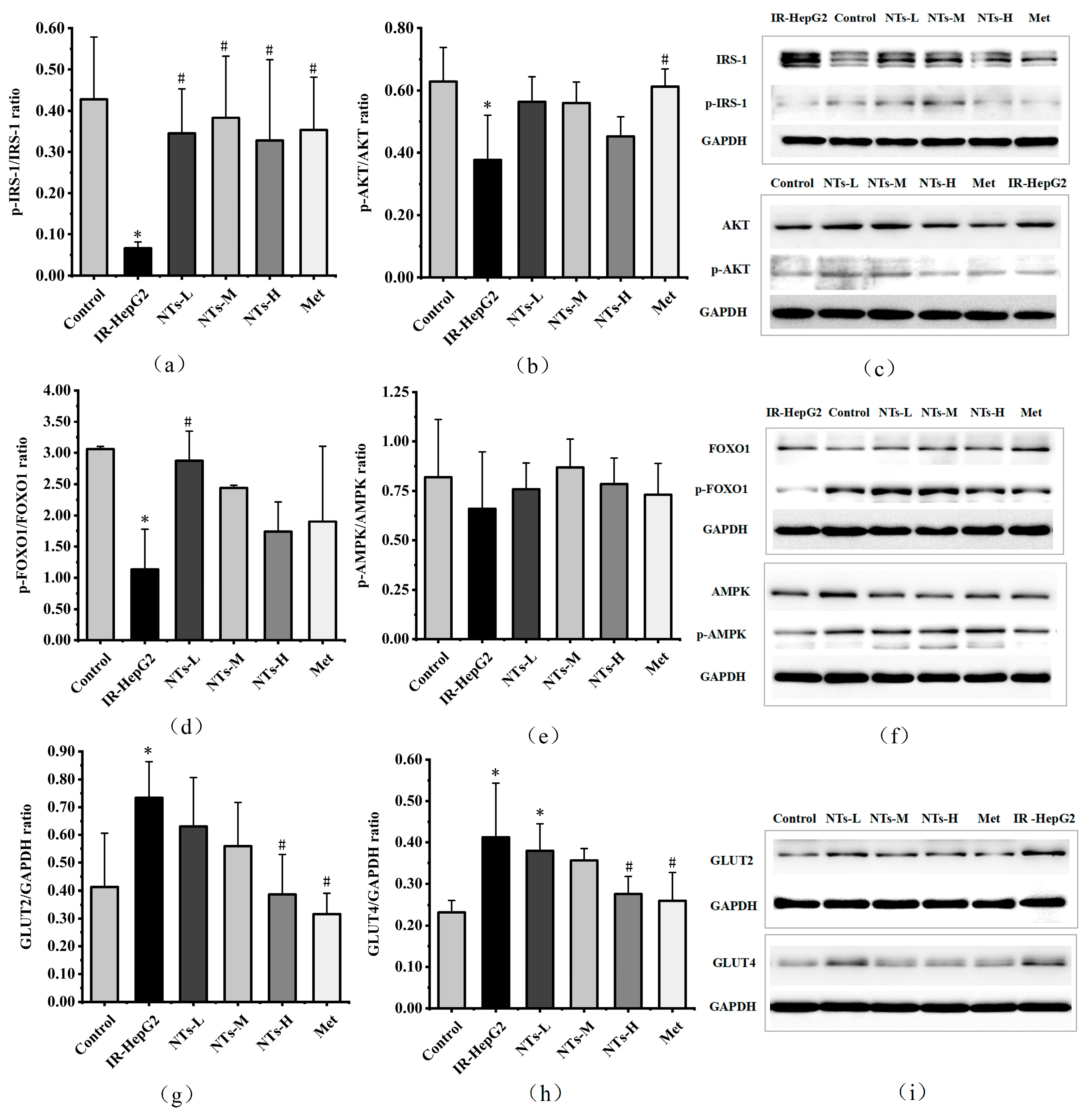
3.8. The Effect of Exogenous NTs on Insulin Signaling Pathway Proteins in IR-HepG2 Cells
3.9. The Effect of Exogenous NTs on AMPK Activity in IR-HepG2 Cells
3.10. The Effect of Exogenous NTs on Glucose Transporter Proteins in IR-HepG2 Cells
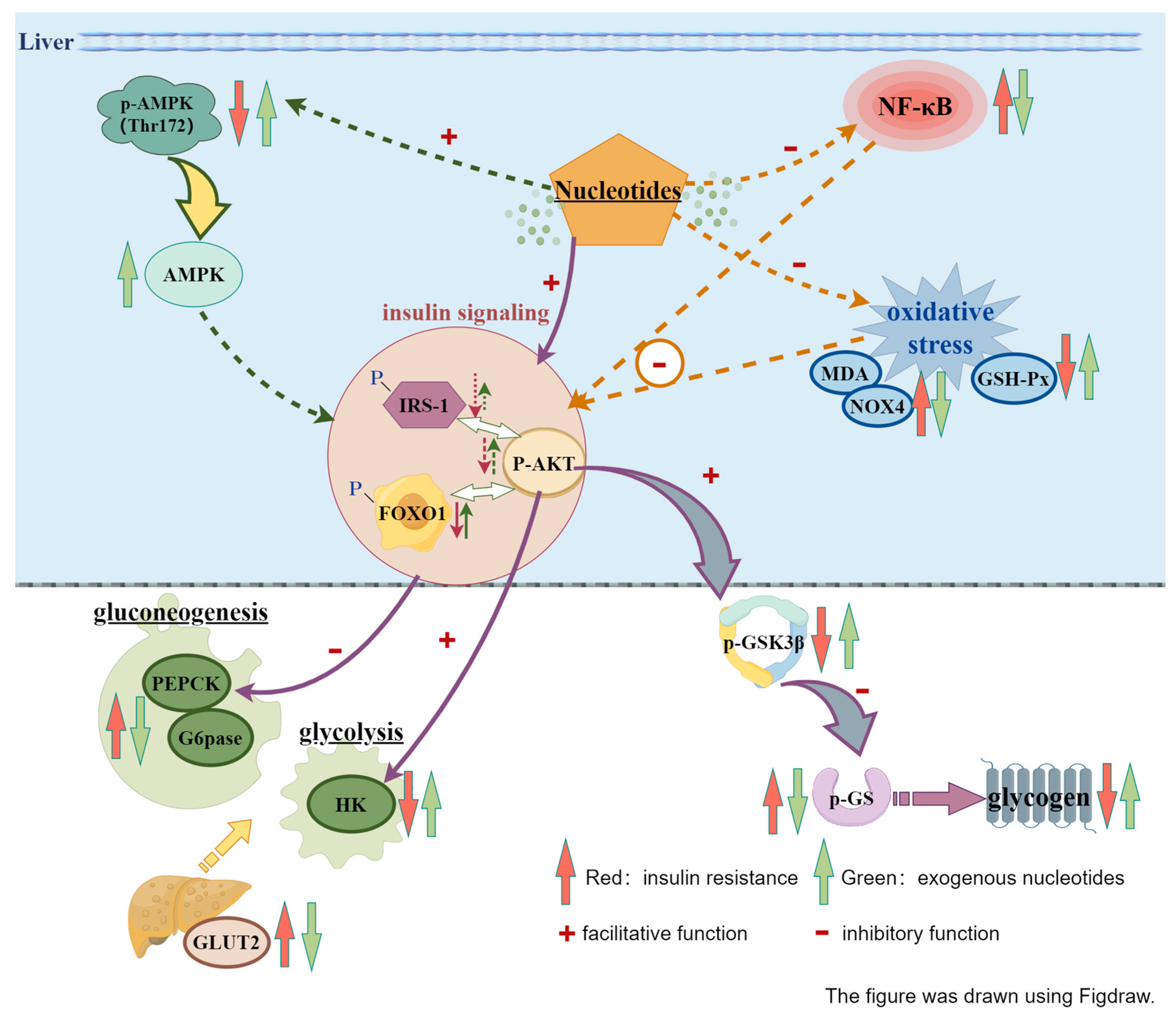
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| FOXO1 | Forkhead box O1 |
| G6pase | Glucose-6-phosphatase |
| GLUT2 | Glucose transporter 2 |
| GLUT4 | Glucose transporter 4 |
| GS | Glycogen synthase |
| GSK3β | Glycogen synthase kinase |
| GSH-Px | Glutathione peroxidase |
| HK | Hexokinase |
| IR | Insulin resistance |
| IRS-1 | Insulin receptor substrate-1 |
| MDA | Malondialdehyde |
| Met | Metformin |
| NF-κB | Nuclear factor-kappa B |
| NOX4 | NADPH oxidase 4 |
| NTs | Nucleotides |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PI3K | Phosphatidylinositol 3-hydroxy kinase |
| SOD | Superoxide dismutase |
References
- Ogawa, W.; Araki, E.; Ishigaki, Y.; Hirota, Y.; Maegawa, H.; Yamauchi, T.; Yorifuji, T.; Katagiri, H. New classification and diagnostic criteria for insulin resistance syndrome. Endocr. J. 2022, 69, 107–113. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Gijbels, A.; Jardon, K.M.; Siebelink, E.; Hul, G.B.; Wanders, L.; Erdos, B.; Péter, S.; Singh-Povel, C.M.; Bosch, J.d.V.-V.D.; et al. Cardiometabolic health improvements upon dietary intervention are driven by tissue-specific insulin resistance phenotype: A precision nutrition trial. Cell Metab. 2023, 35, 71–83.e5. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Haus, J.M.; Filion, J.; Pagadala, M.R.; Godin, J.-P.; Kochhar, S.; Ross, A.B.; Kirwan, J.P. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: A randomized-controlled trial. Metab. Clin. Exp. 2018, 82, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effects of Low-Carbohydrate versus Mediterranean Diets on Weight Loss, Glucose Metabolism, Insulin Kinetics and β-Cell Function in Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Mangogna, A.; Di Girolamo, F.G.; Fiotti, N.; Vinci, P.; Landolfo, M.; Mearelli, F.; Biolo, G. High-protein diet with excess leucine prevents inactivity-induced insulin resistance in women. Clin. Nutr. 2023, 42, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Javid, A.Z.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The Impact of Resveratrol Supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phytother. Res. PTR 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Pingali, U.; Vuppalanchi, B.; Nutalapati, C.; Gundagani, S. Aqueous Azadirachta indica (Neem) Extract Attenuates Insulin Resistance to Improve Glycemic Control and Endothelial Function in Subjects with Metabolic Syndrome. J. Med. Food 2021, 24, 1135–1144. [Google Scholar] [CrossRef]
- Mohammad, A.; Shahnaz, T.; Sorayya, K. Effect of 8 weeks’ supplementation grape seed extract on insulin resistance in iranian adolescents with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. 2021, 15, 197–203. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Alizadeh, M.; Jamalzehi, A.; Parastouei, K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: A randomized clinical trial. Phytother. Res. PTR 2021, 35, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2016, 15, 43. [Google Scholar] [CrossRef]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. 2018, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: A randomised, double-blind, placebo-controlled pilot study. Br. J. Nutr. 2014, 112, 438–445. [Google Scholar] [CrossRef]
- Rudolph, F.B. The biochemistry and physiology of nucleotides. J. Nutr. 1994, 124, 124s–127s. [Google Scholar] [CrossRef]
- Carver, J.D. Dietary nucleotides: Cellular immune, intestinal and hepatic system effects. J. Nutr. 1994, 124, 144s–148s. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yi, L.; Xu, W.; Zhou, H.; Zhang, Y.; Zhang, W.; Mai, K. Effects of dietary nucleotides on growth, non-specific immune response and disease resistance of sea cucumber Apostichopus japonicas. Fish Shellfish Immunol. 2015, 47, 1–6. [Google Scholar] [CrossRef]
- Xie, C.Y.; Wang, Q.; Li, G.; Fan, Z.; Wang, H.; Wu, X. Dietary supplement with nucleotides in the form of uridine monophosphate or uridine stimulate intestinal development and promote nucleotide transport in weaned piglets. J. Sci. Food Agric. 2019, 99, 6108–6113. [Google Scholar] [CrossRef]
- Ding, T.; Xu, M.; Li, Y. An Overlooked Prebiotic: Beneficial Effect of Dietary Nucleotide Supplementation on Gut Microbiota and Metabolites in Senescence-Accelerated Mouse Prone-8 Mice. Front. Nutr. 2022, 9, 820799. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Wang, N.; Xu, M.; Mao, R.; Li, Y. Dietary Nucleotides Supplementation and Liver Injury in Alcohol-Treated Rats: A Metabolomics Investigation. Molecules 2016, 21, 435. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wu, Z.; Xu, L.; Fang, Y.; Xu, Y. Maternal supplementation of nucleotides improves the behavioral development of prenatal ethanol-exposed mice. Cogn. Affect. Behav. Neurosci. 2014, 14, 879–890. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Liu, R.; Wei, C.; Wang, X.; Yu, X.; Li, Z.; Mao, R.; Hu, J.; Zhu, N.; Liu, X.; et al. Exogenous Nucleotides Ameliorate Ageing-Related Intestinal Inflammation in Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2023, 15, 2533. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, R.; Guo, Q.; Wang, S.; Zhao, M.; Zhang, Z.; Wang, J.; Li, Y. Dietary nucleotides extend the life span in Sprague-Dawley rats. J. Nutr. Health Aging 2013, 17, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Wei, C.; Xu, M.; Li, Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2022, 14, 2796. [Google Scholar] [CrossRef] [PubMed]
- Siahanidou, T.; Mandyla, H.; Papassotiriou, I.; Anagnostakis, D. Serum lipids in preterm infants fed a formula supplemented with nucleotides. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 56–60. [Google Scholar] [PubMed]
- Wu, X.; Zhu, N.; He, L.; Xu, M.; Li, Y. 5’-Cytimidine Monophosphate Ameliorates H2O2-Induced Muscular Atrophy in C2C12 Myotubes by Activating IRS-1/Akt/S6K Pathway. Antioxidants 2024, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liang, Y.; Su, Y.; Wang, L. DhHP-6 ameliorates hepatic oxidative stress and insulin resistance in type 2 diabetes mellitus through the PI3K/AKT and AMPK pathway. Biochem. J. 2020, 477, 2363–2381. [Google Scholar] [CrossRef]
- Karki, S.; Farb, M.G.; Ngo, D.T.; Myers, S.; Puri, V.; Hamburg, N.M.; Carmine, B.; Hess, D.T.; Gokce, N. Forkhead box O-1 modulation improves endothelial insulin resistance in human obesity. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1498–1506. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, X.; Wu, D.; Xing, S.; Bian, F.; Li, W.; Chi, J.; Bai, X.; Wu, G.; Chen, X.; et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br. J. Pharmacol. 2015, 172, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.N.; Hardie, D.G.; Morrice, N.; Tornqvist, H.E. 5’-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 2001, 276, 46912–46916. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guan, H.; Tan, X.; Jiang, Y.; Li, F.; Sun-Waterhouse, D.; Li, D. Enhanced alleviation of insulin resistance via the IRS-1/Akt/FOXO1 pathway by combining quercetin and EGCG and involving miR-27a-3p and miR-96-5p. Free. Radic. Biol. Med. 2022, 181, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Tzatsos, A.; Kandror, K.V. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol. 2006, 26, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, A.J.; Birk, J.B.; Eilertsen, E.; Stuenæs, J.T.; Wojtaszewski, J.F.; Jensen, J. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E231–E240. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, M.H.; El-Awady, M.S.; Megahed, N.; Gameil, N.M. The ergogenic supplement β-hydroxy-β-methylbutyrate (HMB) attenuates insulin resistance through suppressing GLUT-2 in rat liver. Can. J. Physiol. Pharmacol. 2016, 94, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Dutta, S.; Velpandian, T.; Mathur, S.R. Psidium guajava Linn. leaf extract affects hepatic glucose transporter-2 to attenuate early onset of insulin resistance consequent to high fructose intake: An experimental study. Pharmacogn. Res. 2015, 7, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef]
- Aravinthan, A.; Challis, B.; Shannon, N.; Hoare, M.; Heaney, J.; Alexander, G.J.M. Selective insulin resistance in hepatocyte senescence. Exp. Cell Res. 2015, 331, 38–45. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Y.; Yang, C.; Lu, S.; Zhao, L.; Liu, X.; Zhou, D.; Luo, L.; Yin, Z. γ-glutamylcysteine alleviates insulin resistance and hepatic steatosis by regulating adenylate cyclase and IGF-1R/IRS1/PI3K/Akt signaling pathways. J. Nutr. Biochem. 2023, 119, 109404. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Role of hyperketonemia in inducing oxidative stress and cellular damage in cultured hepatocytes and type 1 diabetic rat liver. Cell. Physiol. Biochem. 2015, 37, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zou, M.-H. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 2012, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Lee, D.Y.; Roman, L.J.; Khazim, K.; Gorin, Y. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol. Cell. Biol. 2013, 33, 3439–3460. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Li, Y.; Xu, M. Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways. Nutrients 2024, 16, 1801. https://doi.org/10.3390/nu16121801
Song L, Li Y, Xu M. Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways. Nutrients. 2024; 16(12):1801. https://doi.org/10.3390/nu16121801
Chicago/Turabian StyleSong, Lixia, Yong Li, and Meihong Xu. 2024. "Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways" Nutrients 16, no. 12: 1801. https://doi.org/10.3390/nu16121801
APA StyleSong, L., Li, Y., & Xu, M. (2024). Exogenous Nucleotides Ameliorate Insulin Resistance Induced by Palmitic Acid in HepG2 Cells through the IRS-1/AKT/FOXO1 Pathways. Nutrients, 16(12), 1801. https://doi.org/10.3390/nu16121801






