Abstract
As the most serious of the many worse new pathological changes caused by diabetes, there are many risk factors for the occurrence and development of diabetic retinopathy (DR). They mainly include hyperglycemia, hypertension, hyperlipidemia and so on. Among them, hyperglycemia is the most critical cause, and plays a vital role in the pathological changes of DR. High-sucrose diets (HSDs) lead to elevated blood glucose levels in vivo, which, through oxidative stress, inflammation, the production of advanced glycation end products (AGEs) and vascular endothelial growth factor (VEGF), cause plenty of pathological damages to the retina and ultimately bring about loss of vision. The existing therapies for DR primarily target the terminal stage of the disease, when irreversible visual impairment has appeared. Therefore, early prevention is particularly critical. The early prevention of DR-related vision loss requires adjustments to dietary habits, mainly by reducing sugar intake. This article primarily discusses the risk factors, pathophysiological processes and molecular mechanisms associated with the development of DR caused by HSDs. It aims to raise awareness of the crucial role of diet in the occurrence and progression of DR, promote timely changes in dietary habits, prevent vision loss and improve the quality of life. The aim is to make people aware of the importance of diet in the occurrence and progression of DR. According to the dietary modification strategies that we give, patients can change their poor eating habits in a timely manner to avoid theoretically avoidable retinopathy and obtain an excellent prognosis.
1. Introduction
Carbohydrates are one of the three primary macronutrients essential for human energy production and are an important source of energy for maintaining normal physiological activities and functions [1]. However, long-term excessive intake of carbohydrates, which is a high-sucrose diet (HSD), especially foods containing high amounts of glucose and fructose, can lead to increased sugar intake and cause metabolic disorder, cardiovascular abnormalities [2], neurological disturbances [1,3] and inflammation [4,5,6]. This can manifest as hyperglycemia [7], obesity [8,9,10], insulin resistance [8], hypertension [11], impaired cardiac metabolic function [12], mood and behavioral disorders, impaired working memory [13], food addiction [1,14], intestinal infections and microbial dysbiosis [15,16]. In addition, HSDs can also contribute to psychopathology [17]. An HSD in women of childbearing age and during pregnancy can impact the memory processes of offspring [18] and may contribute to a predisposition to developing mental disorders in early life or adulthood.
As the most severe of the many worse new pathological microvascular changes caused by diabetes, diabetic retinopathy (DR) is the leading pathogeny of preventable vision loss in the young, especially working-age people worldwide [19]. The International Diabetes Federation (IDF) estimates that, by 2045, the global population aged 20–79 with diabetes will rise to 780 million [20]. It is estimated that 160 million adults will become DR sufferers [21]. Clinically, the confirmation of DR is primarily on the basis of the abnormal appearance of retinal vessels [22,23] (see Figure 1). There are two central disease stages. The first is non-proliferative diabetic retinopathy (NPDR), which is characterized by progressive retinal microvascular lesions. Then, it progresses to the proliferative diabetic retinopathy (PDR) stage, which is characterized by neovascularization [24,25]. NPDR involves retinal changes such as hemorrhages, microaneurysms and hard exudates, with the patients usually being asymptomatic. As NPDR progresses and retinal ischemia occurs, leading to neovascularization of the retina, it advances to PDR, and patients may experience severe visual impairment [24,25]. Additionally, diabetic macular oedema (DMO) refers to the thickening of the posterior pole of the retina and can occur at any stage [25]. Due to the late onset of clinical symptoms, early histological changes are challenging to detect during clinical examinations, and, once detected, irreversible vision loss often occurs. Beyond vision impairment, the degree of retinal damage in diabetic sufferers is significantly related to future risks of cerebrovascular accidents, myocardial infarction and mortality [26]. Therefore, for patients with DR, earlier intervention and timely diagnosis and treatments are necessary for patients with DR to reduce the potential risk of vision loss [27]. Controlling hyperglycemia is the most crucial preventive measure [28].
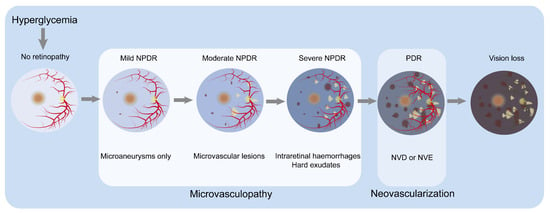
Figure 1.
Classification of DR by severity and the major clinicopathological features associated with different stages. In no retinopathy, the retina shows no microvascular abnormalities. Hyperglycemia damages the normal retina, resulting in mild NPDR, characterized by microaneurysms. In moderate NPDR, microaneurysms and other microvascular abnormalities are observed. Severe NPDR is basically characterized by one or a combination of the following: (1) more than 20 retinal hemorrhages; (2) venous beading; (3) retinal microvascular abnormalities but not meeting the criteria for PDR. The progression to the PDR stage is marked by the appearance of NVD or NVE, along with preretinal or vitreous hemorrhage. Microvasculopathy is the main characteristic of the NPDR stage, while neovascularization is the main characteristic of the PDR stage. As the condition worsens, patients experience visual loss. Abbreviations: neovascularization of optic disc, NVD; neovascularization of elsewhere, NVE; non-proliferative diabetic retinopathy, NPDR; proliferative diabetic retinopathy, PDR.
The risk factors that primarily contribute to the occurrence and progression of DR include hyperglycemia, hypertension, hyperlipidemia, diabetes duration and so on. Among these factors, hyperglycemia is the critical factor that can trigger all the related abnormalities. Although there are currently many medical treatment options targeted toward DR, they are only applicable to the terminal stage of the disease and often come with severe adverse reactions [29], posing a significant economic burden on patients and the global public healthcare system. The improper control and management of DR can lead to late-stage DR [30], which may result in blindness and increase the burden of DR disease [31]. Studies have shown that maintaining normal blood glucose levels can effectively delay the occurrence and progression of DR [32]. A high-sucrose diet and the resultant hyperglycemia exacerbate the occurrence and progression of DR, leading to vision impairment in patients.
2. Risk Factors for the Occurrence and Development of DR
The primary connected factor is blood glucose levels [33]. An HSD significantly elevates blood glucose levels [34].
2.1. Hyperglycemia
Hyperglycemia significantly affects DR [35] and is the dominating pathogeny of DR progression [36,37]. Glycated hemoglobin A1c (HbA1c) is a marker applied to measure blood glucose control [38], and its blood level represents the average blood glucose concentration over the past 120 days [39]. Strictly controlling HbA1c levels below 7% can lower the risk of DR occurrence and development [22]. Every 1% reduction in HbA1c reduces the risk of retinopathy and blindness by 40% and 15%, respectively [40]. It is a remarkable fact that earlier blood glucose control is better because long-term exposure to high blood glucose conditions can cause irreversible retinal damage even after blood glucose control is regained [41].
2.2. Hypertension
Hypertension is defined as a blood pressure reading of 130/80 mmHg or higher [42]. Hypertension stands as an independent risk factor for the onset of retinopathy in individuals diagnosed with type 2 diabetes mellitus [43]. Strictly controlling the pressure of blood can reduce the risk of DR and vision loss [44], especially when maintaining HbA1c levels around 7% simultaneously [45].
2.3. Hyperlipidemia
Hyperlipidemia refers to an elevation in circulating levels of low-density lipoprotein cholesterol (LDL-C) and very-low-density lipoprotein cholesterol (VLDL-C), accompanied by decreased levels of high-density lipoprotein cholesterol (HDL-C), which holds protective attributes [46]. Lipid-lowering agents, including statins, employed to manage hypercholesterolemia, demonstrate a reduction in the risk of DR incidence [47,48]. Intensifying blood glucose control in combination with treating hyperlipidemia can slow down the progression of DR [49].
2.4. Duration of Diabetes Mellitus
The earlier the patients have diabetes, the greater the risk of DR [50]. This phenomenon arises from the prolonged duration of diabetes, signifying a persistent impact of hyperglycemia on the body, and, consequently, an enduring assault on the retina. Research indicates that, with a diabetic duration exceeding 30 years, the prevalence of retinopathy can soar to 63% [51].
2.5. Other Risk Factors
In addition to the aforementioned primary factors, ethnic origin, pregnancy and puberty are also additional risk factors for DR. Individuals of South Asian or African descent exhibit a higher prevalence of diabetic DR compared to Caucasians [52]. Pregnancy, particularly during mid-term gestation, exerts detrimental effects on retinal vasculature due to fluctuations in estrogen levels and increased blood volume [53]. Suboptimal glycemic control during puberty correlates closely with the progression of diabetic retinopathy [54], underscoring the significance of blood glucose levels in its advancement.
3. Pathophysiology of DR
DR primarily involves retinal microangiopathy, with histological alterations occurring before the emergence of various obvious clinical symptoms. Persistent hyperglycemia assumes a pivotal role in the pathological advancement of DR by instigating and maintaining various other factors that collectively influence the development of DR.
DR encompasses a classification system comprising two fundamental clinical stages: NPDR and PDR. NPDR signifies the initial phase of DR, whereas PDR confers an escalated propensity toward vision loss relative to individuals with NPDR [55]. The primary pathology of DR involves microvascular changes in the retinal capillaries, comprising pericytes, basement membranes and endothelial cells. Pericytes possess contractile properties, maintaining capillary tone, controlling capillary diameter, regulating blood flow in the capillaries and preserving their stability [56]. Endothelial cells form tight junctions, creating an internal barrier to prevent substances from leaking out of the blood vessels [57]. During the initial phases of DR progression, elevated blood glucose levels instigate pericyte death, resulting in capillary acellular areas and the development of localized or diffuse microaneurysms due to capillary rarefaction [58]. High blood glucose damages endothelial cells, compromising the integrity of the internal barrier and causing vascular leakage [59]. Increased thickness of the basement membrane induces luminal constriction within the vasculature and vascular stiffness, thereby promoting vascular rigidity and impeding the binding efficacy of growth factors. The loss of these two cell types leads to capillary leakage and occlusion, resulting in non-perfused areas. As the disease progresses, arteriolar involvement occurs, and dilated retinal arterioles accelerate the clinical manifestation of diabetic retinopathy, including edema and hemorrhage [60]. The non-perfused areas further expand, causing capillary dilation and venous beading formation, which are known as intraretinal microvascular abnormalities [40]. NPDR is characterized by various manifestations, including small dilations of blood vessels in the microvascular network, blood leakage, irregularities in the veins of the retinal vasculature, a reduction in function capillaries and intraretinal microvascular abnormalities [61]. Notably, retinal arteriolar dilatation potentially serves as an early subclinical marker of microvascular dysfunction [22].
Extensive death of retinal microvascular cells leads to retinal hypoxia, triggering the upregulation of growth factors [62]. Various growth factors act synergistically, resulting in the formation of neovascularization [63], marking the transition to the proliferative stage of DR. Neovascularization continues to grow and forms fibrous tissue membranes. Subsequently, these fibrous tissues adhere to the vitreous. Contraction of the vitreous can cause hemorrhage, and the contracted fibrous tissue leads to retinal traction and detachment, ultimately resulting in vision loss [64].
Additionally, during the initial phases of the ailment, there is an associated impairment of both retinal ganglion and glial cells [65]. Neurologic and glial dysfunction occur concurrently with vascular abnormalities and generally precede obvious microvascular damage [66].
4. Molecular Mechanisms of HSD-Induced Development of DR
Sugar (sucrose) includes fructose and glucose [67]. An HSD can result in elevated levels of glucose in the bloodstream. Elevated blood glucose levels are pivotal in the pathogenesis of vascular complications associated with diabetes. DR represents the predominant complication of the microvasculature. Extensive research has demonstrated that hyperglycemia, especially long-term sustained high blood glucose levels, serves as a central factor in the occurrence and progression of DR, inducing a variety of biochemical abnormalities [68,69] (Figure 2).
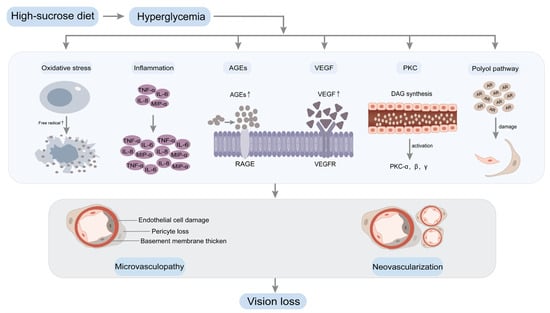
Figure 2.
Hyperglycemia induces biochemical changes in DR. A high-sucrose diet leads to increased blood glucose levels, resulting in hyperglycemia. Prolonged and sustained high blood glucose levels contribute to retinal microvasculopathy through multiple pathways, including oxidative stress, inflammation, AGEs, VEGF, PKC and the polyol pathway. Significant apoptotic events within the retinal capillary cells instigate retinal hypoxia and subsequent neovascularization. The proliferation of abundant neovascularization engenders the development of fibrous tissue covering, leading to tractional retinal detachment. Ultimately, this culminates in vision loss. Abbreviations: advanced glycation end products, AGEs; receptor for advanced glycation end products, RAGE; vascular endothelial growth factor, VEGF; vascular endothelial growth factor receptor, VEGFR; protein kinase C, PKC; diacylglycerol, DAG; aldose reductase, AR; tumor necrosis factor-α, TNF-α; macrophage inflammatory protein-1α, MIP-α; interleukin-6, IL-6; interleukin-8, IL-8.
4.1. Oxidative Stress
Under optimal physiological circumstances, the body maintains a meticulous oxidative–reductive equilibrium. However, disruption ensues when there is a disproportionate interplay between the generation and elimination of free radicals, thereby perturbing this dynamic equilibrium and instigating an upsurge in free radical production [70]. Consequently, oxidative stress manifests itself [71]. Given the retina’s unique characteristics, characterized by protracted light exposure, heightened oxygen consumption and reliance on glucose oxidation, it becomes highly susceptible to oxidative stress [72]. The confluence of hyperglycemia precipitates the accrual of reactive oxygen species (ROS) within the retina, thereby subjecting retinal and capillary cells to oxidative stress [59,73,74]. Subsequently, diverse molecular mechanisms are initiated to cause oxidative stress on retinal well-being, including the activation of the protein kinase C (PKC) pathway, formation of advanced glycation end products (AGEs), initiation of the polyol pathway and facilitation of the hexosamine pathway [75], as well as the induction of inflammatory cascades [71,76,77].
4.2. Inflammation
Hyperglycemia disrupts the balance between pro-inflammatory and anti-inflammatory responses maintained by microglia, leading to a state of inflammation [78]. This inflammatory state increases vascular permeability and can activate leukocytes, leading to capillary blockages and local retinal ischemia [79]. Elevated concentrations of pro-inflammatory cytokines and chemokines, encompassing various inflammatory mediators, have been detected in ocular samples from DR patients [80], and upregulated pro-inflammatory cytokines may directly or indirectly induce angiogenesis [81,82]. Research has shown that high-glucose-induced inflammation in DR can be alleviated by either activating [83,84,85] or inhibiting [86] specific signaling pathways.
4.3. Advanced Glycation End Products (AGEs)
Persistent hyperglycemia triggers the initiation of non-enzymatic glycation in macromolecules, encompassing proteins, consequently leading to an increase in AGEs [71]. AGEs can increase the levels of cell adhesion molecules within retinal endothelial cells, resulting in capillary occlusion [87]. They may also induce apoptosis of retinal pericytes through oxidative stress mechanisms [88]. The deposition of AGE adducts within the retinal microvascular basement membrane can cause functional impairments, such as perturbation of endothelial junctions and heightened vascular permeability [89], leading to endothelial damage and extravasation of intravascular substances. Additionally, AGEs can cause neuronal abnormalities [87].
4.4. Vascular Endothelial Growth Factor (VEGF)
In DR, progressive loss of capillaries leads to retinal hypoxia, inducing the expression of vascular endothelial growth factor (VEGF) [90]. In the context of DR, the progressive decline of capillary density culminates in retinal hypoxia, prompting the upregulation of VEGF. Subsequently, a robust neovascular response is triggered, particularly during the advanced stages of DR [91], characterized by excessive neovascularization [92]. VEGF activates PKC, orchestrating the dismantling of tight junctions, perturbation of the blood–retinal barrier (BRB) and increased permeability of capillaries [93,94].
4.5. Protein Kinase C (PKC)
Hyperglycemia causes the buildup of diacylglycerol (DAG), activating various PKC isoforms in the retina [95,96]. PKC promotes the generation of reactive oxygen species (ROS), leading to amplified vascular permeability, upregulation of VEGF expression [97], modifications in blood flow and alterations in enzyme activity [98]. These intricate processes contribute to retinal cell apoptosis, the emergence of capillaries devoid of cellular components and the disturbance of the functionality of the BRB [99].
4.6. Polyol Pathway
Hyperglycemia triggers the polyol pathway activation across diverse cellular populations, leading to excessive aldose reductase activity [75]. Consequently, retinal endothelial cells, pericyte cells and other retinal cell types endure detrimental effects mediated by oxidative stress [100], increased cellular osmotic pressure and AGEs formation [101]. It can also lead to abnormalities in neuroglia and neurons [102].
It is important to note that hyperglycemia does not singularly cause retinal damage in DR through only one of the aforementioned pathways. Instead, these pathways interact and influence each other, collectively contributing to vision loss in DR.
5. Dietary Modification Strategies
Currently, the main therapeutic approaches for DR include laser photocoagulation, intravitreal administration of pharmacological agents targeting VEGF, intravitreal injection of corticosteroids and vitrectomy [19,24]. However, these approaches primarily target the advanced phases of DR and can only partially alleviate the extent of visual decline; they cannot reverse vision damage. Additionally, these treatments are associated with high costs, multiple side effects and poor patient compliance. Therefore, early prevention is the most effective, cost-effective and beneficial primary choice for delaying vision loss in DR. Among the early prevention methods, dietary modification is one of the simplest and easiest to adhere to.
Since hyperglycemia has a significant implication in the pathogenesis and advancement of DR, dietary modification should focus on reducing sugar consumption. According to guidelines established by The World Health Organization (WHO), limiting the consumption of free sugars to less than 10% of the overall energy intake is recommended. Ideally, it can be controlled below 5% when feasible. Although slight variations exist among different countries and age groups, it is advised to minimize sugar consumption as much as possible. It is imperative to acknowledge that free sugars do not encompass naturally occurring sugars found in fresh fruits, vegetables and milk. Authentic free sugars exclusively include monosaccharides and disaccharides introduced through diverse food manufacturing procedures, commonly recognized as added sugars. Naturally existing sugars in honey and fruit juice also fall under the classification of free sugars [103]. The medical research institute advocates for a maximum threshold of 25% of daily caloric consumption as the recommended limit for added sugar intake [104].
5.1. Fruits and Vegetables
Fruits and vegetables hold significant importance as sources of a diverse array of nutrients and dietary fibers [105]. Research indicates that increasing the consumption of fruits and vegetables can effectively diminish the likelihood of developing DR [106,107] and provide protective effects against DR [108]. Fruits and leafy green vegetables contribute to delaying DR progression and mitigating visual impairment [23]. To promote better blood glucose control and mitigate inflammation in sufferers, it is recommended to consume fruits and vegetables that abound in flavonoids, such as leafy greens, fruits and berries [109,110]. These can protect against the demise of retinal ganglion cells (RGCs) triggered by oxidative stress [111].
5.2. Fish
Polyunsaturated fatty acids (PUFAs) can protect the vision of patients with DR [112], and reduce the severity of the DR condition [113]. The retina contains a significant concentration of long-chain ω-3 polyunsaturated fatty acids (LCω3PUFAs) that showcase notable anti-inflammatory and antiangiogenic properties. A daily intake of at least 500 mg of dietary LCω3PUFAs can decrease the likelihood of visual impairment in patients of DR [114,115,116,117,118]. Fish, an excellent source of omega-3 polyunsaturated fatty acids, can reduce the formation of pathological blood vessels [119,120]. Increasing fish consumption can slow down the progression of DR [121,122], thereby reducing the probability of developing severe manifestations of the condition [123].
5.3. Vitamins
5.3.1. Vitamin A
The administration of vitamin A has been observed to exhibit protective properties against retinal damage induced by hyperglycemia and contribute to the delay in retinal neovascularization formation [124]. Serum levels of vitamin A are correlated with DR [125], emphasizing its potential relevance in assessing the condition.
5.3.2. Vitamin B
Vitamin B3 is characterized by its existence in two distinct forms, namely niacin and niacinamide, each exhibiting unique molecular structures and biological functions. Niacinamide can reduce oxidative deoxyribonucleic acid (DNA) damage, promote DNA repair and alleviate retinal neurodegeneration in diabetic patients [126]. However, high-dose niacin may increase the risk of insulin resistance [127]. Substantial dietary consumption of vitamin B6 has effectively reduced the likelihood of developing DR [128]. Diminished levels of vitamin B12 in the serum have been correlated with an augmented susceptibility to DR [129], with some indications suggesting its potential role as a distinct risk factor contributing to the suffering condition [130].
Additionally, vitamins B1, B7 and B9 have also exhibited significant therapeutic potential in addressing retinal lesions associated with diabetes, underscoring their significant role in managing these pathological conditions [131,132].
5.3.3. Vitamin D
Vitamin D has properties such as lowering blood glucose, antioxidation, anti-inflammation, antiangiogenesis and neuroprotection [133,134,135,136]. There is a compelling correlation between serum vitamin D levels and the occurrence or seriousness of DR [137,138,139,140,141,142,143,144,145]. A prospective study found that maintaining adequate optimal levels of vitamin D in the bloodstream can prevent the deleterious impact of diabetes on the intricately interconnected microvasculature, including DR [146]. The association between serum levels of 25-hydroxyvitamin D and DR was supported by pertinent studies [147,148,149]. When the concentration of serum 25-hydroxyvitamin D falls below the threshold of 15.57 ng/mL, the risk of visual impairment doubles in DR patients [150]. 1,25-dihydroxyvitamin D₃ may serve as a potential protective role in retina by regulating inflammatory responses [151], and possesses inhibitory prowess against the activity of retinal VEGF and transforming growth factor [152]. Fatty fish and fish oil, notably salmon and sardines, are designated as prominent dietary reservoirs of vitamin D [133].
5.3.4. Vitamin E
Elevated blood glucose levels have been implicated as a pivotal contributor in the escalation of oxidative stress, and vitamin E can prevent lipid peroxidation, improve oxidative stress [153] and reduce the severity of diabetes-related complications [154]. Additionally, vitamin E can significantly decrease retinal capillary basement membrane thickness, protecting against retinal damage associated with DR caused by hyperglycemia-induced oxidative stress [155,156].
5.4. Non-Vitamin-A Carotenoids
Carotenoids, which are indigenous antioxidants, exhibit both anti-inflammatory and antioxidant qualities. They can reduce inflammation and oxidative stress caused by high blood glucose. Their remarkable impact involves mitigating the inflammatory response and oxidative burden arising from hyperglycemia, thereby slowing down the occurrence and development of DR [157,158]. The main carotenoids include lutein, zeaxanthin, astaxanthin and lycopene.
The macula, a region in the retina, showcases a profound abundance of lutein and zeaxanthin [159]. These two nutrients, well recognized as the macular pigment, possess antioxidant, anti-inflammatory, antiangiogenic, neuroprotective and blue-light-filtering properties in the eyes [160,161,162,163,164,165,166]. They can significantly improve retinal vascular changes caused by high blood glucose [167] and enhance macular function [168], thereby protecting and alleviating DR [169,170,171,172,173]. Carotenoid dietary intake is significantly reduced in DR patients [174]. Since the human body cannot synthesize carotenoids, it is recommended that DR patients obtain them from food sources. Lutein predominantly resides within verdant leafy vegetables such as broccoli, spinach and lettuce [175]. Zeaxanthin is mainly found in corn and corn products [175], while astaxanthin is primarily found in seafood and algae [176,177].
5.5. Flavonoids
Flavonoids are the preeminent polyphenols ubiquitously interspersed within our nutritional diet and possess anti-inflammatory and antioxidant properties. They can alleviate hyperglycemia, inhibit oxidative stress and inflammation processes [178], regulate carbohydrate and fat metabolism [179] and slow down visual loss in DR [180,181].
Anthocyanins are a class of flavonoids and essential natural bioactive pigments [182]. They are mainly found in berries and cherries [183], such as wild blueberries, cranberries, raspberry seeds and strawberries [184]. Cyanidin-3-O-glucoside (C3G) is an anthocyanin type with strong antioxidant and anti-inflammatory effects. It exhibits a therapeutic intervention for ameliorating inflammation triggered by elevated glucose levels, along with mitigating angiogenesis in DR [185]. Blueberry anthocyanins can enhance the integrity of the BRB compromised by oxidative stress [186], adverse consequences inflicted by oxidative stress and inflammation on retinal tissue homeostasis [187], and inhibit the progression of DR [188]. Blueberry anthocyanin extract protects the capillaries of the retina from elevated-glucose-level-induced damage through antioxidant and anti-inflammatory mechanisms [189].
Apart from its antioxidant properties that mitigate oxidative stress, the flavonoid naringenin and its derivatives exhibit neurotrophic effects, reducing neuronal vascular damage associated with DR [190]. Genistein, predominantly found in leguminous foods such as broccoli and cilantro, manifests anti-inflammatory properties and suppresses neovascularization in ocular tissues [191].
5.6. Dietary Fiber
Dietary fiber manifests an inhibitory impact on the kinetics of monosaccharides and fatty acid digestion and absorption, impeding their prompt assimilation, thereby reducing calorie absorption [192]. The utilization of this therapeutic approach demonstrated a reduction in the likelihood of DR [193] and has a protective effect against existing cases [108]. Investigations have elucidated that individuals with low-dietary-fiber intake have a higher risk of vision-threatening DR [194].
5.7. Other Nutrients
Whole grains are rich in soluble and insoluble fiber, which can lower blood glucose levels, ameliorate blood lipid levels and optimize gut microbiota [195]. The consistent incorporation of cheese and whole wheat bread into the diet has been found to correlate with a reduced likelihood of DR advancement [196]. Goji berries can increase the concentrations of lutein and zeaxanthin, ameliorating high-glucose-induced microstructure and physiological damage in the retina [197]. Furthermore, it confers protective effects on the retina [198]. Coconut water can lower blood sugar levels and mitigate DR damage [199].
6. Dietary Recommendations for DR Patients—The Mediterranean Diet (MedDiet)
The Mediterranean diet (MedDiet), renowned for its global recognition, originates from the Mediterranean coast regions. This dietary pattern is distinguished by its emphasis on the abundant consumption of plant-derived foods, including fruits, vegetables, legumes, grains and nuts, preferably fresh and minimally processed. It also includes a significant intake of olive oil as the foremost contributor of fat, desired consumption of dairy products like cheese and yogurt, prudent consumption of fish and poultry and moderate alcohol consumption [200,201,202] (Figure 3).
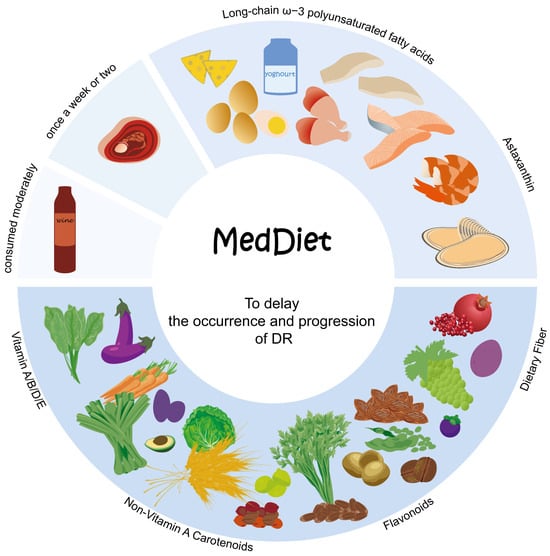
Figure 3.
The distinguishing attributes of MedDiet. First, an adequate intake of a huge variety of plant-based foods that are minimally processed, seasonally fresh and locally grown. This includes fruits, vegetables, minimally refined grains, beans and nuts, as they provide a rich array of nutrients such as vitamins, non-vitamin-A carotenoids, flavonoids and dietary fiber. Fresh fruits are incorporated as a regular dessert option. Second, there is a moderate intake of local products derived from milk, primarily yogurt, alongside fish and seafood, to obtain nutrients like LCω3PUFAs and astaxanthin. Third, the consumption of red meat, including processed variants, is limited to once a week or every two weeks, while allowing for moderate wine consumption with meals. MedDiet offers a variety of nutrients that can help to lower blood glucose levels, reduce oxidative stress and mitigate inflammation, thereby exerting a significant impact on retarding the occurrence and progression of DR.
MedDiet is rich in low glycemic foods, vitamins, minerals, antioxidants, fiber, monounsaturated fatty acids (MUFAs), PUFAs and probiotics. Research has evidenced the ability of this intervention to result in reduced levels of HbA1c, concentrations of postprandial blood glucose, oxidative stress and inflammation, and improve lipid profiles and gut immune function [201,203]. Numerous studies have demonstrated that adhering to MedDiet helps to reduce the morbidity of DR and prevents vision loss [204,205,206,207,208].
7. Other Lifestyle Recommendations
Hypertension is recognized as an additional contributing factor to the incidence of DR, and reducing elevated blood pressure levels confers valuable preventive benefits against DR [209]. The Dietary Approaches to Stop Hypertension (DASH) diet has been linked to the management of hypertension and shares similarities with MedDiet. It also possesses anti-inflammatory and antioxidant properties, which can improve blood glucose control [210]. Calorie restriction and intermittent fasting have demonstrated potency in reducing blood glucose levels in the blood circulation [211]. Maintaining a low-calorie and low-sodium intake is also beneficial for DR [212]. Hyperglycemia, hypertension and dyslipidemia represent a triad of modifiable primary risk factors for severe vision loss in sufferers [213]. Achieving optimal glycemic control, blood pressure and lipid profiles management can reduce diabetes-related vision impairment [214,215].
Furthermore, engaging in appropriate physical exercises, such as aerobic activities, strength training and yoga, can mitigate the likelihood of DR occurrence and progression [216,217].
8. Conclusions
Diabetes manifests as the foremost prevailing metabolic disorder, with diabetic retinopathy emerging as its predominant and consequential complication. Additionally, this ocular condition serves as the primary contributor to avoidable vision loss among the working-age cohort. The occurrence and advancement of DR are influenced by a variety of risk elements, including hyperglycemia, hypertension, dyslipidemia and duration of diabetes. Notably, hyperglycemia assumes a pivotal role as the primary driver behind the occurrence and progression of DR. Therefore, it is crucial to gain a comprehensive understanding of the underlying disease mechanisms through which HSDs intricately contribute to the occurrence and progression of DR. Given the inconspicuous presentation of clinical indicators amidst the nascent stages of microvascular and neuronal compromise in DR, it is challenging to raise awareness among patients. However, once clinical symptoms manifest, vision loss becomes inevitable. Existing treatment options can only partially alleviate the degree of visual impairments but cannot reverse visual damage. Therefore, early intervention is of utmost importance.
Among various early intervention methods, dietary intervention is the most cost-effective, patient-friendly, least harmful and highly beneficial approach. We primarily elucidate the complex pathogenesis of DR and the intricate biochemical mechanisms by which a high-sugar diet leads to irreversible vision loss in DR. This aims to raise awareness about the importance of daily dietary habits in the occurrence and advancement of DR. Moreover, we underscore the pronounced impact of specific nutrients in delaying the development of DR, providing dietary modification strategies for DR patients as references. It is noteworthy that the application of a balanced low-sugar diet is the primary factor in the prevention and therapy of hyperglycemia and its bad consequences, and that patients with DR must integrate the use of necessary anti-hyperglycemic and lipid-lowering medications, such as statins, alongside appropriate dietary adjustments and suitable physical exercise. A singular dietary adjustment alone is unlikely to exert a decisive impact on the progression of DR. Therefore, the adoption of a balanced low-sugar diet complements pharmacological interventions, facilitating enhanced therapeutic efficacy, alongside the indispensable inclusion of daily physical activity in routine life. We recommend that DR patients minimize their daily sugar intake while controlling the three major high-risk factors of glycemic, blood pressure and lipid levels to mitigate the potential hazards of vision loss as much as possible.
Author Contributions
Conceptualization, C.Y.; investigation, C.Y.; writing—original draft preparation, C.Y. and Y.Y.; writing—review and editing, C.Y. and J.A.; supervision, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Wenzhou Basic Scientific Research Project from Wenzhou Science and Technology Bureau (No. Y2020356).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviation
| Name | Abbreviation |
| Diabetic Retinopathy | DR |
| High-Sucrose Diets | HSDs |
| Advanced Glycation End Products | AGEs |
| International Diabetes Federation | IDF |
| Vascular Endothelial Growth Factor | VEGF |
| Vascular Endothelial Growth Factor Receptor | VEGFR |
| Proliferative Diabetic Retinopathy | PDR |
| Non-Proliferative Diabetic Retinopathy | NPDR |
| Diabetic Macular Oedema | DMO |
| Neovascularization of Optic Disc | NVD |
| Neovascularization of Elsewhere | NVE |
| Glycated Hemoglobin A1c | HbA1c |
| Low-Density Lipoprotein Cholesterol | LDL-C |
| Very-Low-Density Lipoprotein Cholesterol | VLDL-C |
| High-Density Lipoprotein Cholesterol | HDL-C |
| Protein Kinase C | PKC |
| Diacylglycerol | DAG |
| Aldose Reductase | AR |
| Tumor Necrosis Factor-α | TNF-α |
| Macrophage Inflammatory Protein-1α | MIP-1α |
| Interleukin-6 | IL-6 |
| Interleukin-8 | IL-8 |
| Receptor for Advanced Glycation End Products | RAGE |
| Reactive Oxygen Species | ROS |
| Blood–Retinal Barrier | BRB |
| World Health Organization | WHO |
| Retinal Ganglion Cells | RGCs |
| Polyunsaturated Fatty Acids | PUFAs |
| Long-chain ω-3 Polyunsaturated Fatty Acids | LCω3PUFAs |
| Deoxyribonucleic Acid | DNA |
| Cyanidin-3-O-Glucoside | C3G |
| Mediterranean Diet | MedDiet |
| Monounsaturated Fatty Acids | MUFAs |
| Dietary Approaches to Stop Hypertension | DASH |
References
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Sugars, obesity, and cardiovascular disease: Results from recent randomized control trials. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Vesela, I.; Driks, H.; Ferrario, C.R.; Mistretta, C.M.; Bradley, R.M.; Dus, M. High-sucrose diet exposure is associated with selective and reversible alterations in the rat peripheral taste system. Curr. Biol. 2022, 32, 4103–4113.e4. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, G.; Jin, L.H. A high-sugar diet affects cellular and humoral immune responses in Drosophila. Exp. Cell Res. 2018, 368, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.T.; Zhang, J.F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural. Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Palanker Musselman, L.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Sukumar Hathiramani, S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Models Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, F.; Yu, M.; Yang, X.; Yao, Z.; Li, Q.; Wei, Z.; Feng, K.; Zeng, P.; Zhao, D.; et al. Reduced Nogo expression inhibits diet-induced metabolic disorders by regulating ChREBP and insulin activity. J. Hepatol. 2020, 73, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Apaijai, N.; Arinno, A.; Palee, S.; Pratchayasakul, W.; Kerdphoo, S.; Jaiwongkam, T.; Chunchai, T.; Chattipakorn, S.C.; Chattipakorn, N. High-Saturated Fat High-Sugar Diet Accelerates Left-Ventricular Dysfunction Faster than High-Saturated Fat Diet Alone via Increasing Oxidative Stress and Apoptosis in Obese-Insulin Resistant Rats. Mol. Nutr. Food Res. 2019, 63, e1800729. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Miwa, H.; Tanaka, T.; Toriumi, K.; Kunii, Y.; Shimbo, H.; Sakamoto, T.; Hino, M.; Izumi, R.; Nagaoka, A.; et al. High-sucrose diets contribute to brain angiopathy with impaired glucose uptake and psychosis-related higher brain dysfunctions in mice. Sci. Adv. 2021, 7, eabl6077. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Araki, Y.; Hanzawa, F.; Umeki, M.; Kojima, T.; Nishimura, N.; Ikeda, S.; Mochizuki, S.; Oda, H. High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J. Nutr. Biochem. 2021, 93, 108621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Teng, Y.; Qin, N.; Ren, X.; Xia, X. A high-sucrose diet causes microbiota composition shift and promotes the susceptibility of mice to Salmonella Typhimurium infection. Food Funct. 2023, 14, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Eudave, D.M.; BeLow, M.N.; Flandreau, E.I. Effects of high fat or high sucrose diet on behavioral-response to social defeat stress in mice. Neurobiol. Stress 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mizera, J.; Kazek, G.; Niedzielska-Andres, E.; Pomierny-Chamiolo, L. Maternal high-sugar diet results in NMDA receptors abnormalities and cognitive impairment in rat offspring. FASEB J. 2021, 35, e21547. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.N.; Ulbig, M.W. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch. Arztebl. Int. 2010, 107, 75–83; quiz 84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pr. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Hong, T.; Bahrami, B.; Flood, V.; Liew, G.; Chang, A.A. Diet and risk of visual impairment: A review of dietary factors and risk of common causes of visual impairment. Nutr. Rev. 2021, 79, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Mohamed, Q.; Gillies, M.C.; Wong, T.Y. Management of diabetic retinopathy: A systematic review. JAMA 2007, 298, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, B.S.; Wu, J.; Luong, T.Q.; Gandhi, N.K.; Fong, D.S.; Chen, W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology 2021, 128, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.; Cheung, G.C.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic retinopathy for the non-ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Simó-Servat, A.; Bogdanov, P.; Simó, R. Diabetic retinopathy: New therapeutic perspectives based on pathogenic mechanisms. J. Endocrinol. Investig. 2017, 40, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Mookiah, M.R.; Acharya, U.R.; Chua, C.K.; Lim, C.M.; Ng, E.Y.; Laude, A. Computer-aided diagnosis of diabetic retinopathy: A review. Comput. Biol. Med. 2013, 43, 2136–2155. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.E.; Gardner, T.W. Functions of insulin and insulin receptor signaling in retina: Possible implications for diabetic retinopathy. Prog. Retin. Eye Res. 2003, 22, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, R.; Heath, H. Identification of fructose as the retinopathic agent associated with the ingestion of sucrose-rich diets in the rat. Metab. Clin. Exp. 1980, 29, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.T.; Li, X.F.; Sun, Y.M.; Li, Y.B.; Su, Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed. Pharmacother. 2015, 74, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. Diabetic retinopathy–biomolecules and multiple pathophysiology. Diabetes Metab. Syndr. 2015, 9, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N. Diabetic retinopathy and systemic factors. Middle East Afr. J. Ophthalmol. 2015, 22, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kohner, E.M. Diabetic retinopathy. BMJ 1993, 307, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, B.; Tang, L. Metabolic memory: Mechanisms and implications for diabetic retinopathy. Diabetes Res. Clin. Pract. 2012, 96, 286–293. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Cull, C.A.; Adler, A.I.; Matthews, D.R.; Neil, H.A.W.; Holman, R.R. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: A prospective observational study (UKPDS 75). Diabetologia 2006, 49, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998, 317, 703–713. [Google Scholar] [CrossRef]
- Preventing blindness due to diabetic retinopathy. Control glycaemia and blood pressure, and monitor the eyes. Prescrire Int. 2010, 19, 35–38.
- Su, X.; Peng, D. New insight into sortilin in controlling lipid metabolism and the risk of atherogenesis. Biol. Rev. 2020, 95, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Vania, R.; Victor, A.A. Statin reduces the incidence of diabetic retinopathy and its need for intervention: A systematic review and meta-analysis. Eur. J. Ophthalmol. 2021, 31, 1216–1224. [Google Scholar] [CrossRef]
- Chou, Y.; Ma, J.; Su, X.; Zhong, Y. Emerging insights into the relationship between hyperlipidemia and the risk of diabetic retinopathy. Lipids Health Dis. 2020, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- ACCORD Study Group; ACCORD Eye Study Group; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H. Improving the screening of risk factors in diabetic retinopathy. Expert. Rev. Endocrinol. Metab. 2022, 17, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Schmidt, S.; Lehmann, T.; Köhler, B.; Kloos, C.; Voigt, U.A.; Meller, D.; Wolf, G.; Müller, U.A.; Müller, N. Prevalence and Progression Rate of Diabetic Retinopathy in Type 2 Diabetes Patients in Correlation with the Duration of Diabetes. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2018, 126, 570–576. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Mallika, P.; Tan, A.; Aziz, S.; Asok, T.; Alwi, S.S.; Intan, G. Diabetic retinopathy and the effect of pregnancy. Malays. Fam. Physician Off. J. Acad. Fam. Physicians Malays. 2010, 5, 2–5. [Google Scholar]
- O’Hagan, M.; Harvey, J.N.; Brecon Group. Glycemic Control in Children with Type 1 Diabetes in Wales: Influence of the pediatric diabetes specialist nurse. Diabetes Care 2010, 33, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Jones, R.B.; Allison, S.P. Incidence of and risk factors for proliferative retinopathy and its association with blindness among diabetes clinic attenders. Ophthalmic. Epidemiol. 2000, 7, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the Pathogenesis of Diabetic Retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z. Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy. Front. Endocrinol. 2022, 13, 816400. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Kotwani, A. Implication of oxidative stress in progression of diabetic retinopathy. Surv. Ophthalmol. 2016, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.G. Prognosis and retinal vessel features. Ophthalmology 2007, 114, 1796–1797, author reply 1797. [Google Scholar] [CrossRef]
- Ockrim, Z.; Yorston, D. Managing diabetic retinopathy. BMJ 2010, 341, c5400. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.B.; Afzal, A.; Spoerri, P.; Pan, H.; Shaw, L.C.; Mames, R.N. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert. Opin. Investig. Drugs 2004, 13, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.I.; Khan, H.A.; Alhomida, A.S. Neurodegeneration and Neuroprotection in Diabetic Retinopathy. Int. J. Mol. Sci. 2013, 14, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Silvestri, F.; Bongiorni, S.; Taddei, A.R.; Fanelli, G.; Rinalducci, S.; De Palma, C.; Perrotta, C.; Prantera, G.; Cervia, D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol. Res. 2021, 166, 105488. [Google Scholar] [CrossRef] [PubMed]
- Kearney, F.M.; Fagan, X.J.; Al-Qureshi, S. Review of the role of refined dietary sugars (fructose and glucose) in the genesis of retinal disease. Clin. Exp. Ophthalmol. 2014, 42, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sahajpal, N.S.; Goel, R.K.; Chaubey, A.; Aurora, R.; Jain, S.K. Pathological Perturbations in Diabetic Retinopathy: Hyperglycemia, AGEs, Oxidative Stress and Inflammatory Pathways. Curr. Protein Pept. Sci. 2019, 20, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Gürler, B.; Vural, H.; Yilmaz, N.; Oguz, H.; Satici, A.; Aksoy, N. The role of oxidative stress in diabetic retinopathy. Eye 2000, 14, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Á.L. Oxidative Stress in Diabetic Retinopathy. Antioxidants 2021, 10, 50. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.G.; Estevez, J.J.; Liu, E.; Craig, J.E.; Finnie, J.W. Pericytes, inflammation, and diabetic retinopathy. Inflammopharmacology 2020, 28, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Capitao, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Song, E.; Wang, Z.; Ji, N.; Zhu, L.; Wang, K.; Sun, H.; Zhang, Y.; Zhu, Q.; Liu, X.; et al. Melatonin attenuates oxidative stress and inflammation of Muller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed. Pharmacother. 2021, 137, 111274. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Li, L.; Zhu, L.; Guo, Y.; Du, S.; Zhang, Y.; Wang, Z.; Zhang, Y.; Zhu, M. Geniposide Attenuates Hyperglycemia-Induced Oxidative Stress and Inflammation by Activating the Nrf2 Signaling Pathway in Experimental Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2021, 2021, 9247947. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Lambiase, A.; Armentano, M.; Tucciarone, G.; Sacchetti, M.; Greco, A.; Alisi, L. Diabetic retinopathy, oxidative stress, and sirtuins: An in depth look in enzymatic patterns and new therapeutic horizons. Surv. Ophthalmol. 2022, 67, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ouyang, H.; Mei, X.; Lu, B.; Yu, Z.; Chen, K.; Wang, Z.; Ji, L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-kappaB signaling pathway. FASEB J. 2019, 33, 11776–11790. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Jiang, D.Y.; Tang, L.S. Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci. 2006, 79, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Kandarakis, S.A.; Piperi, C.; Topouzis, F.; Papavassiliou, A.G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014, 42, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Frey, T.; Lin, C.; Antonetti, D.A. Protein Kinase Cβ Phosphorylates Occludin Regulating Tight Junction Trafficking in Vascular Endothelial Growth Factor–Induced Permeability In Vivo. Diabetes 2012, 61, 1573–1583. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Felinski, E.A.; Wolpert, E.B.; Sundstrom, J.M.; Gardner, T.W.; Antonetti, D.A. VEGF Activation of Protein Kinase C Stimulates Occludin Phosphorylation and Contributes to Endothelial Permeability. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5106–5115. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; Hiraoka-Yamamoto, J.; Matsumoto, M.; Clermont, A.; Leitges, M.; Marette, A.; Aiello, L.P.; Kern, T.S.; King, G.L. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009, 15, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Das Evcimen, N.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Jun, H.O.; Yu, Y.S.; Kim, K.W. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. Am. J. Pathol. 2010, 176, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Dagher, Z.; Park, Y.S.; Asnaghi, V.; Hoehn, T.; Gerhardinger, C.; Lorenzi, M. Studies of Rat and Human Retinas Predict a Role for the Polyol Pathway in Human Diabetic Retinopathy. Diabetes 2004, 53, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Guerra, M.; Singh, L.P.; Ibrahim, A.S. Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy. Antioxidants 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabetes Res. 2007, 2007, 061038. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Sugars Intake for Adults and Children [Internet]. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 4 March 2015).
- Schmidt, L.A. New unsweetened truths about sugar. JAMA Intern. Med. 2014, 174, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yoshimura, Y.; Kawasaki, R.; Kamada, C.; Tanaka, S.; Horikawa, C.; Ohashi, Y.; Araki, A.; Ito, H.; Akanuma, Y.; et al. Fruit Intake and Incident Diabetic Retinopathy with Type 2 Diabetes. Epidemiology 2013, 24, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Cheong, Z.Y.; Tan, B.; Wong, D.; Liu, X.; Chua, J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients 2022, 14, 5021. [Google Scholar] [CrossRef] [PubMed]
- Valero-Vello, M.; Peris-Martínez, C.; García-Medina, J.J.; Sanz-González, S.M.; Ramírez, A.I.; Fernández-Albarral, J.A.; Galarreta-Mira, D.; Zanón-Moreno, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the Antioxidant, Anti-Inflammatory, and Neuroprotective Potential of Natural Food and Nutritional Supplements for Ocular Health in the Mediterranean Population. Foods 2021, 10, 1231. [Google Scholar] [CrossRef]
- Mahoney, S.E.; Loprinzi, P.D. Influence of flavonoid-rich fruit and vegetable intake on diabetic retinopathy and diabetes-related biomarkers. J. Diabetes Complicat. 2014, 28, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Hanneken, A. Flavonoids Protect Retinal Ganglion Cells from Oxidative Stress–Induced Death. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4796–4803. [Google Scholar] [CrossRef] [PubMed]
- Alsbirk, K.E.; Seland, J.H.; Assmus, J. Diabetic retinopathy and visual impairment in a Norwegian diabetic coast population with a high dietary intake of fish oils. An observational study. Acta Ophthalmol. 2022, 100, e532–e538. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kawasaki, R.; Rogers, S.; Man, R.E.K.; Itakura, K.; Xie, J.; Flood, V.; Tsubota, K.; Lamoureux, E.; Wang, J.J. The Associations of Dietary Intake of Polyunsaturated Fatty Acids with Diabetic Retinopathy in Well-Controlled Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7473–7479. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y. Dietary Intake of Omega-3 Fatty Acids from Fish and Risk of Diabetic Retinopathy. JAMA 2017, 317, 2226–2227. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.-M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals with Type 2 Diabetes: Prospective Investigation from the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K. Omega-3 Fatty Acid Intake Lowers Risk of Diabetic Retinopathy. Am. J. Nurs. 2017, 117, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Gorusupudi, A.; Chang, F.Y.; Nelson, K.; Hageman, G.S.; Bernstein, P.S. n-3 PUFA Supplementation Alters Retinal Very-Long-Chain-PUFA Levels and Ratios in Diabetic Animal Models. Mol. Nutr. Food Res. 2019, 63, e1801058. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S. Oily fish intake reduces risk of diabetic retinopathy, study shows. BMJ 2016, 354, i4586. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci. Transl. Med. 2011, 3, 69ra12. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. Eating Oily Fish May Protect Against Diabetic Retinopathy. JAMA 2016, 316, 1637. [Google Scholar] [CrossRef] [PubMed]
- Kadri, R.; Vishwanath, P.; Parameshwar, D.; Hegde, S.; Kudva, A.A. Dietary associations with diabetic retinopathy—A cohort study. Indian J. Ophthalmol. 2021, 69, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Chia, A.-R.; Chee, M.L.; Man, R.E.K.; Tan, G.S.W.; Lamoureux, E.L.; Wong, T.Y.; Chong, M.F.-F.; Schmetterer, L. The relationship of dietary fish intake to diabetic retinopathy and retinal vascular caliber in patients with type 2 diabetes. Sci. Rep. 2018, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Saxena, S.; Mishra, A.; Saxena, A.; Natu, S.M. Nutrition for diabetic retinopathy: Plummeting the inevitable threat of diabetic vision loss. Eur. J. Nutr. 2017, 56, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Rostamkhani, H.; Mellati, A.A.; Tabaei, B.S.; Alavi, M.; Mousavi, S.N. Association of Serum Zinc and Vitamin A Levels with Severity of Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Study. Biol. Trace Elem. Res. 2019, 192, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Han, J.-S.; Park, C.K. Neuroprotective Effects of Nicotinamide (Vitamin B3) on Neurodegeneration in Diabetic Rat Retinas. Nutrients 2022, 14, 1162. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.T.; Ramet, M.E.; Patel, A.R.; Pandian, N.G.; Mendelsohn, M.E.; Karas, R.H. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002, 144, 165–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horikawa, C.; Aida, R.; Kamada, C.; Fujihara, K.; Tanaka, S.; Tanaka, S.; Araki, A.; Yoshimura, Y.; Moriya, T.; Akanuma, Y.; et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: Analysis of data from the Japan Diabetes Complications Study (JDCS). Eur. J. Nutr. 2020, 59, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, R.; Zhu, Y.; Wang, Z.; Hou, Y.; Su, K.; He, X.; Song, G. Meta-analysis of Serum Vitamin B12 Levels and Diabetic Retinopathy in Type 2 Diabetes. Arch. Med. Res. 2023, 54, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.V.; Prabhakar, B.; Kulkarni, Y.A. Water Soluble Vitamins and their Role in Diabetes and its Complications. Curr. Diabetes Rev. 2020, 16, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Vitamin D, the Vitamin D Receptor, Calcitriol Analogues and Their Link with Ocular Diseases. Nutrients 2022, 14, 2353. [Google Scholar] [CrossRef]
- Totolici, G.; Tiutiuca, C.; Jurja, S.; Tutunaru, D.; Pătrașcu, A.M. The role of vitamin D in the onset and progression of diabetic retinopathy. Rom. J. Ophthalmol. 2022, 66, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes/Metab. Res. Rev. 2021, 37, e3447. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef] [PubMed]
- Tecilazich, F.; Formenti, A.M.; Giustina, A. Role of vitamin D in diabetic retinopathy: Pathophysiological and clinical aspects. Rev. Endocr. Metab. Disord. 2021, 22, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D Deficiency Is Associated with Retinopathy in Children and Adolescents with Type 1 Diabetes. Diabetes Care 2011, 34, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Trott, M.; Driscoll, R.; Iraldo, E.; Pardhan, S. Associations between vitamin D status and sight threatening and non-sight threatening diabetic retinopathy: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2022, 21, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Afarid, M.; Ghattavi, N.; Johari, M. Serum Levels of Vitamin D in Diabetic Patients with and Without Retinopathy. J. Ophthalmic. Vis. Res. 2020, 15, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Atkin, S.L.; et al. Association of vitamin D2 and D3 with type 2 diabetes complications. BMC Endocr. Disord. 2020, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.-A.; Gao, F.; Qin, L.-L. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2017, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, C.; Liu, D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Eliaçık, M.; Cincik, H.; Öztürk, M.; DeFronzo, R.A.; Abdul-Ghani, M. The Impact of Vitamin D Deficiency on Retinopathy and Hearing Loss among Type 2 Diabetic Patients. Biomed. Res. Int. 2018, 2018, 2714590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Z.; Geng, T.; Zhu, K.; Li, R.; Lu, Q.; Lin, X.; Liu, S.; Chen, L.; Guo, Y.; et al. Vitamin D Status, Vitamin D Receptor Polymorphisms, and Risk of Microvascular Complications Among Individuals with Type 2 Diabetes: A Prospective Study. Diabetes Care 2023, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ashinne, B.; Rajalakshmi, R.; Anjana, R.M.; Narayan, K.M.V.; Jayashri, R.; Mohan, V.; Hendrick, A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pr. 2018, 139, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Otí, J.M.; Galván-Manso, A.I.; Callejas-Herrero, M.R.; Vara-González, L.A.; Salas-Herrera, F.; Muñoz-Cacho, P. Vitamin D Deficiency Is Significantly Associated with Retinopathy in Type 2 Diabetes Mellitus: A Case-Control Study. Nutrients 2022, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.; Ates, O.; Bilen, H.; Kocer, I. Retinal Nerve Fiber Layer Thickness in Early-Stage Diabetic Retinopathy with Vitamin D Deficiency. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Shen, J.; Liu, F.; Zeng, H.; Li, L.; Yu, H.; Lu, H.; Lu, F.; Wu, Q.; Jia, W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet. Med. 2014, 31, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Buomprisco, G.; Pascarella, A.; Pescosolido, N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 57, 559–565. [Google Scholar] [CrossRef]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxy vitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor-beta(1) in the retinas of rats with diabetes. Diabetes Res. Clin. Pr. 2012, 98, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, A.; Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; Mazzone, M.G.; Sciacca, L.; Agodi, A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients 2022, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Pazdro, R.; Burgess, J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Granado-Casas, M.; Ramírez-Morros, A.; Martín, M.; Real, J.; Alonso, N.; Valldeperas, X.; Traveset, A.; Rubinat, E.; Alcubierre, N.; Hernández, M.; et al. Type 1 Diabetic Subjects with Diabetic Retinopathy Show an Unfavorable Pattern of Fat Intake. Nutrients 2018, 10, 1184. [Google Scholar] [CrossRef] [PubMed]
- Yulek, F.; Or, M.; Ozogul, C.; Isik, A.C.; Ari, N.; Stefek, M.; Bauer, V.; Karasu, C. Effects of stobadine and vitamin E in diabetes-induced retinal abnormalities: Involvement of oxidative stress. Arch. Med. Res. 2007, 38, 503–511. [Google Scholar] [CrossRef]
- Murillo, A.G.; Fernandez, M.L. Potential of Dietary Non-Provitamin A Carotenoids in the Prevention and Treatment of Diabetic Microvascular Complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- She, C.; Shang, F.; Zhou, K.; Liu, N. Serum Carotenoids and Risks of Diabetes and Diabetic Retinopathy in a Chinese Population Sample. Curr. Mol. Med. 2017, 17, 287–297. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of beta-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Fathalipour, M.; Fathalipour, H.; Safa, O.; Nowrouzi-Sohrabi, P.; Mirkhani, H.; Hassanipour, S. The Therapeutic Role of Carotenoids in Diabetic Retinopathy: A Systematic Review. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Au Eong, K.G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Keegan, G.; Pardhan, S.; Chichger, H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Exp. Eye Res. 2020, 197, 108104. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. A Systematic Review of Carotenoids in the Management of Diabetic Retinopathy. Nutrients 2021, 13, 2441. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2008, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The colorful world of carotenoids: A profound insight on therapeutics and recent trends in nano delivery systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 3658–3697. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shalini, T.; Jose, S.S.; Prasanthi, P.S.; Balakrishna, N.; Viswanath, K.; Reddy, G.B. Carotenoid status in type 2 diabetes patients with and without retinopathy. Food Funct. 2021, 12, 4402–4410. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Glukhareva, T.V.; Danilova, I.G.; Kovaleva, E.G. Natural antioxidants in diabetes treatment and management: Prospects of astaxanthin. Crit. Rev. Food Sci. Nutr. 2022, 62, 5005–5028. [Google Scholar] [CrossRef]
- Dong, L.-Y.; Jin, J.; Lu, G.; Kang, X.-L. Astaxanthin Attenuates the Apoptosis of Retinal Ganglion Cells in db/db Mice by Inhibition of Oxidative Stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Arroo, R. The protective effects of flavonoids and carotenoids against diabetic complications—A review of in vivo evidence. Front. Nutr. 2023, 10, 1020950. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.L.; Bruno, D.F.; Ambrosio, A.F.; Santos, P.F. The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients 2020, 12, 3169. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Srirangam, R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J. Pharm. Pharmacol. 2010, 62, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Al-Dosari, D.; Alhomida, A.S. Role of Oxidative Stress in Diabetic Retinopathy and the Beneficial Effects of Flavonoids. Curr. Pharm. Des. 2018, 24, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Shafighi, N.; Barber, A.J.; Nabavi, S.M. Anthocyanins as a potential therapy for diabetic retinopathy. Curr. Med. Chem. 2015, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Yarla, N.S.; Kumar, K.E.; Lakkappa, D.B.; Kamal, M.A.; Scotti, L.; Scotti, M.T.; Ashraf, G.M.; Rao, B.S.B.; Kumari, D.S.; et al. Preventive and Therapeutic Potentials of Anthocyanins in Diabetes and Associated Complications. Curr. Med. Chem. 2018, 25, 5347–5371. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Gao, X.; Ge, X.; Cui, J.; Liu, X. Cyanidin-3-o-glucoside (C3G) inhibits vascular leakage regulated by microglial activation in early diabetic retinopathy and neovascularization in advanced diabetic retinopathy. Bioengineered 2021, 12, 9266–9278. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ye, Z.; Yang, W.; Xu, Y.-J.; Tan, C.-P.; Liu, Y. Blueberry Anthocyanins from Commercial Products: Structure Identification and Potential for Diabetic Retinopathy Amelioration. Molecules 2022, 27, 7475. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, K.; Li, P. Blueberry anthocyanins extract attenuated diabetic retinopathy by inhibiting endoplasmic reticulum stress via the miR-182/OGG1 axis. J. Pharmacol. Sci. 2022, 150, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. J. Food Biochem. 2021, 45, e13972. [Google Scholar] [CrossRef] [PubMed]
- Francisco, S.G.; Smith, K.M.; Aragones, G.; Whitcomb, E.A.; Weinberg, J.; Wang, X.; Bejarano, E.; Taylor, A.; Rowan, S. Dietary Patterns, Carbohydrates, and Age-Related Eye Diseases. Nutrients 2020, 12, 2862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, X.; Yuan, T.; Guo, C.; Zhou, Z.; Wang, L.; Dou, G. Certain Dietary Nutrients Reduce the Risk of Eye Affliction/Retinopathy in Individuals with Diabetes: National Health and Nutrition Examination Survey, 2003-2018. Int. J. Env. Res. Public Health 2022, 19, 12173. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Raman, R.; Kulothungan, V.; Sharma, T. Influence of dietary-fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (report 26). Clin. Exp. Ophthalmol. 2012, 40, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Xixi, Y.; Xiaotong, H.; Changfan, W.; Stuart, K.; Xianwen, S.; Lei, Z.; Mingguang, H. Does daily dietary intake affect diabetic retinopathy progression? 10-year results from the 45 and Up Study. Br. J. Ophthalmol. 2020, 104, 1774. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Dey, S.; Sim, R.; Lee, J.; Au Eong, K.G. Fructus lycii: A Natural Dietary Supplement for Amelioration of Retinal Diseases. Nutrients 2021, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wark, L.; Ji, H.; Willard, L.; Jaing, Y.; Han, J.; He, H.; Ortiz, E.; Zhang, Y.; Medeiros, D.M.; et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol. Nutr. Food Res. 2013, 57, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Peng, L.; Zhang, X.; Wu, Q.; Yao, J.; Xing, Q.; Zheng, Y.; Huang, X.; Chen, S.; Xie, Q. Effects of coconut water on blood sugar and retina of rats with diabetes. PeerJ 2021, 9, e10667. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, T.; Bozhinovska, N.; Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Velija Asimi, Z.; Burekovic, A. Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review. Nutrients 2021, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Mulpuri, L.; Sridhar, J.; Goyal, H.; Tonk, R. The relationship between dietary patterns and ophthalmic disease. Curr. Opin. Ophthalmol. 2023, 34, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.Z.; Man, R.E.K.; Fenwick, E.K.; Gupta, P.; Li, L.-J.; van Dam, R.M.; Chong, M.F.; Lamoureux, E.L. Dietary intake and diabetic retinopathy: A systematic review. PLoS ONE 2018, 13, e0186582. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, F.; Firouzabadi, F.D.; Moosaie, F.; Shadnoush, M.; Poopak, A.; Kermanchi, J.; Abhari, S.M.F.; Forouzanfar, R.; Mansournia, M.A.; Khosravi, A.; et al. Effects of a Mediterranean diet on the development of diabetic complications: A longitudinal study from the nationwide diabetes report of the National Program for Prevention and Control of Diabetes (NPPCD 2016-2020). Maturitas 2021, 153, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Corella, D.; Amor, A.J.; Fitó, M.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Wang, X.; Vedula, S.S.; Marrone, M.; Sleilati, G.; Hawkins, B.S.; Frank, R.N. Blood pressure control for diabetic retinopathy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Robles-Rivera, R.R.; Castellanos-Gonzalez, J.A.; Olvera-Montano, C.; Flores-Martin, R.A.; Lopez-Contreras, A.K.; Arevalo-Simental, D.E.; Cardona-Munoz, E.G.; Roman-Pintos, L.M.; Rodriguez-Carrizalez, A.D. Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2020, 2020, 3096470. [Google Scholar] [CrossRef]
- Oza, M.J.; Laddha, A.P.; Gaikwad, A.B.; Mulay, S.R.; Kulkarni, Y.A. Role of dietary modifications in the management of type 2 diabetic complications. Pharmacol. Res. 2021, 168, 105602. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.S.; Janal, M.N. High caloric and sodium intakes as risk factors for progression of retinopathy in type 1 diabetes mellitus. Arch. Ophthalmol. 2010, 128, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Wu, S.Y.; Hennis, A.; Hyman, L.; Nemesure, B.; Yang, L.; Schachat, A.P.; Barbados Eye Study, G. Hyperglycemia, blood pressure, and the 9-year incidence of diabetic retinopathy: The Barbados Eye Studies. Ophthalmology 2005, 112, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, N.; Tang, H.; Yang, X.; Yao, Q.; Zhang, L.; Zhang, H.; Zhang, Y.; Nie, X.; Liao, X.; et al. Diabetic retinopathy risk prediction in patients with type 2 diabetes mellitus using a nomogram model. Front. Endocrinol. 2022, 13, 993423. [Google Scholar] [CrossRef] [PubMed]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. The Effect of Diet and Lifestyle on the Course of Diabetic Retinopathy-A Review of the Literature. Nutrients 2022, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.P.; Lent-Schochet, D.; Lo, T.; Yiu, G. Emerging Concepts in the Treatment of Diabetic Retinopathy. Curr. Diabetes Rep. 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).