Association of Pro-Inflammatory Diet, Smoking, and Alcohol Consumption with Bladder Cancer: Evidence from Case–Control and NHANES Studies from 1999 to 2020
Abstract
1. Introduction
2. Methods
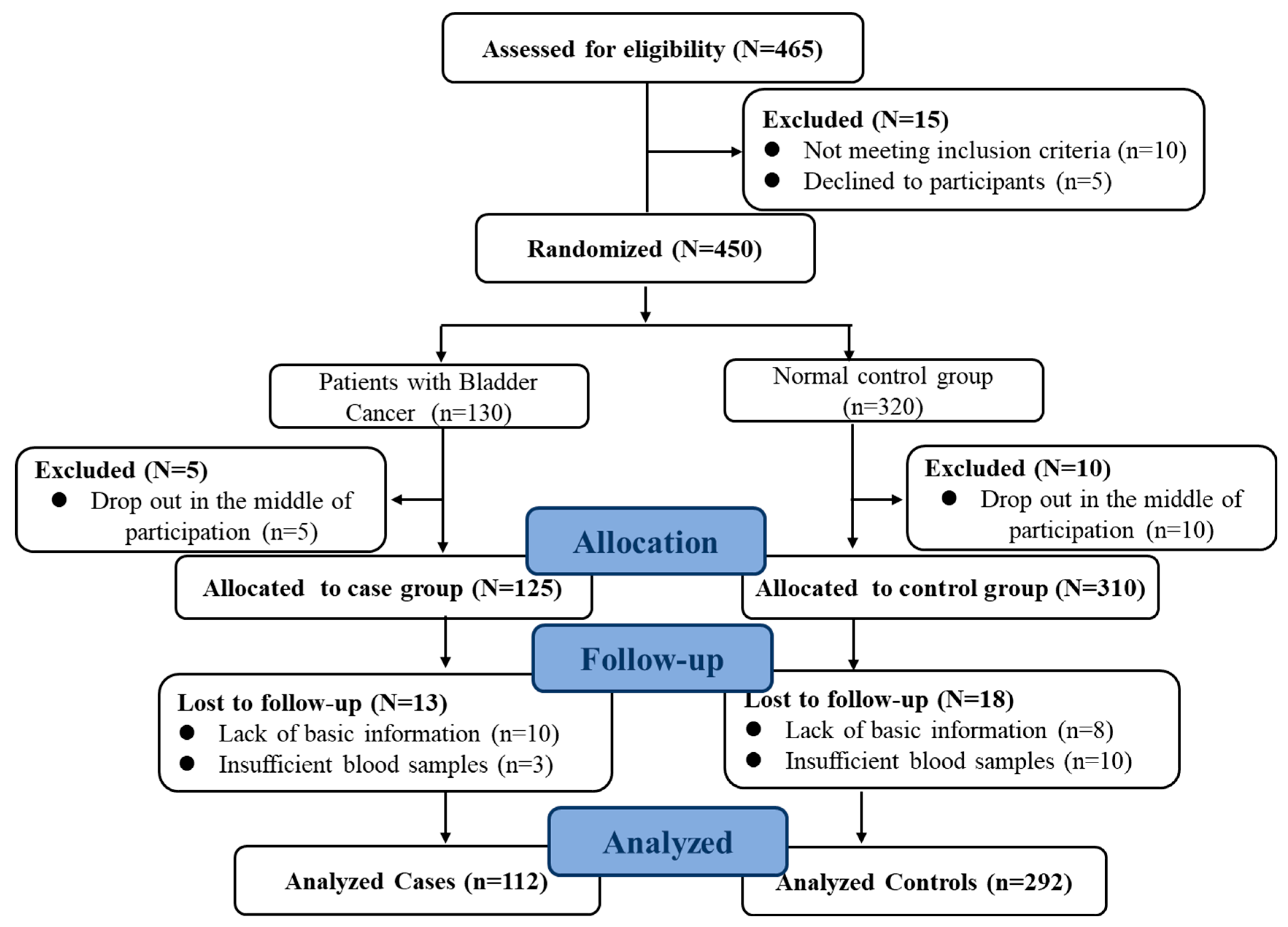
2.1. Participants
2.2. Dietary Data Collection and Assessment
2.3. Diet Inflammatory Index Calculation
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. More Grains, Red Meat, Soybean Oil and Energy in the Cancer Group
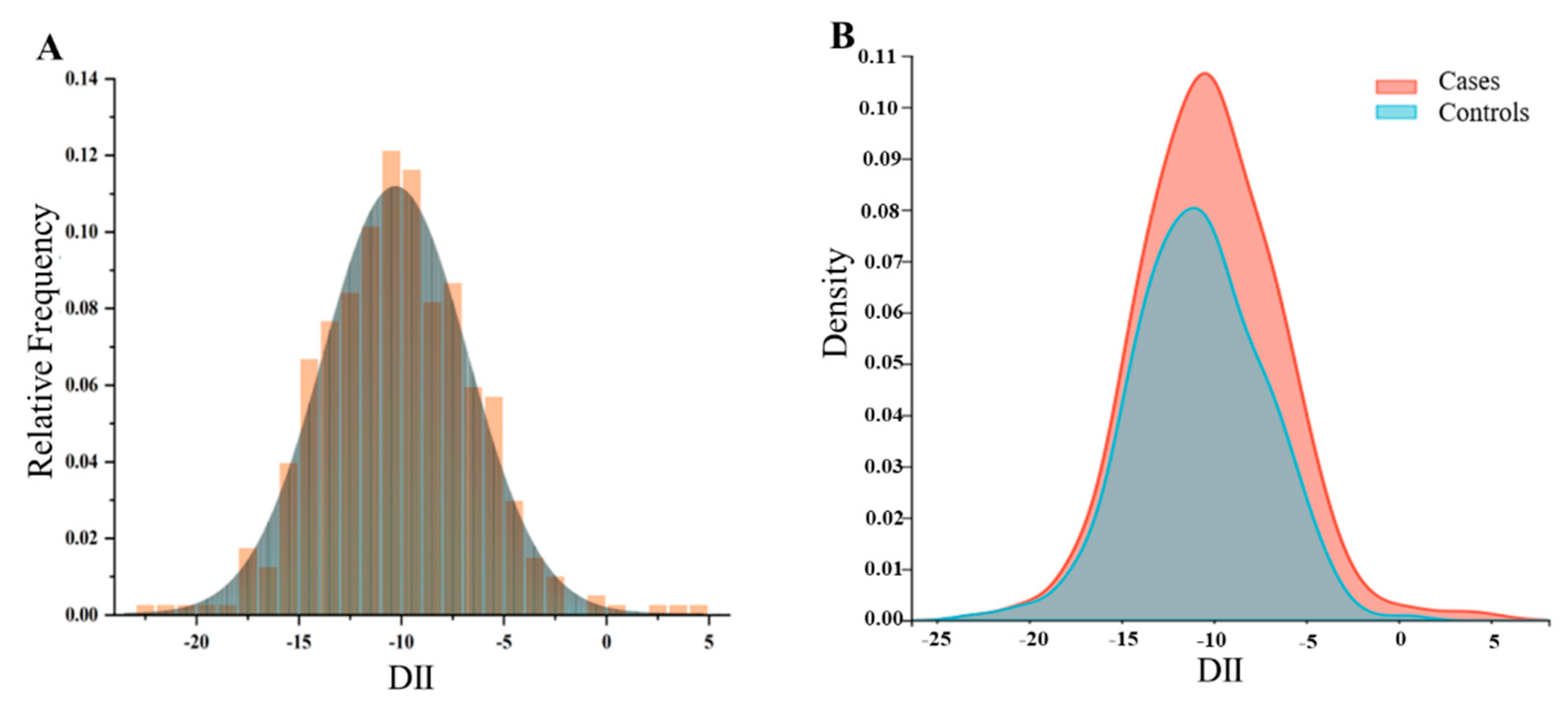
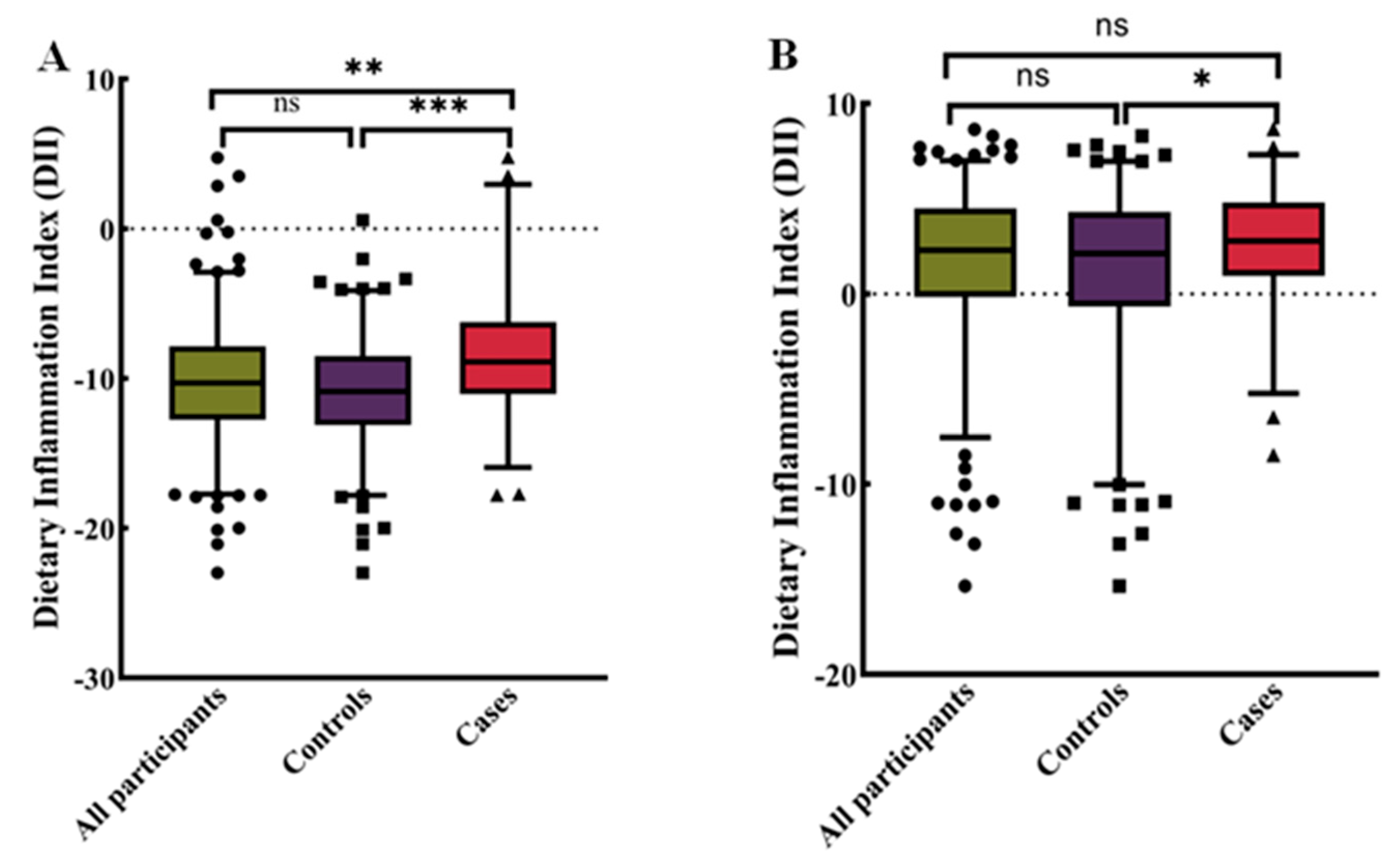
3.3. Higher Dietary Inflammation Index in the Cancer Group
3.4. The Higher Diet Inflammation Index Associated with Higher Risk of Bladder Cancer
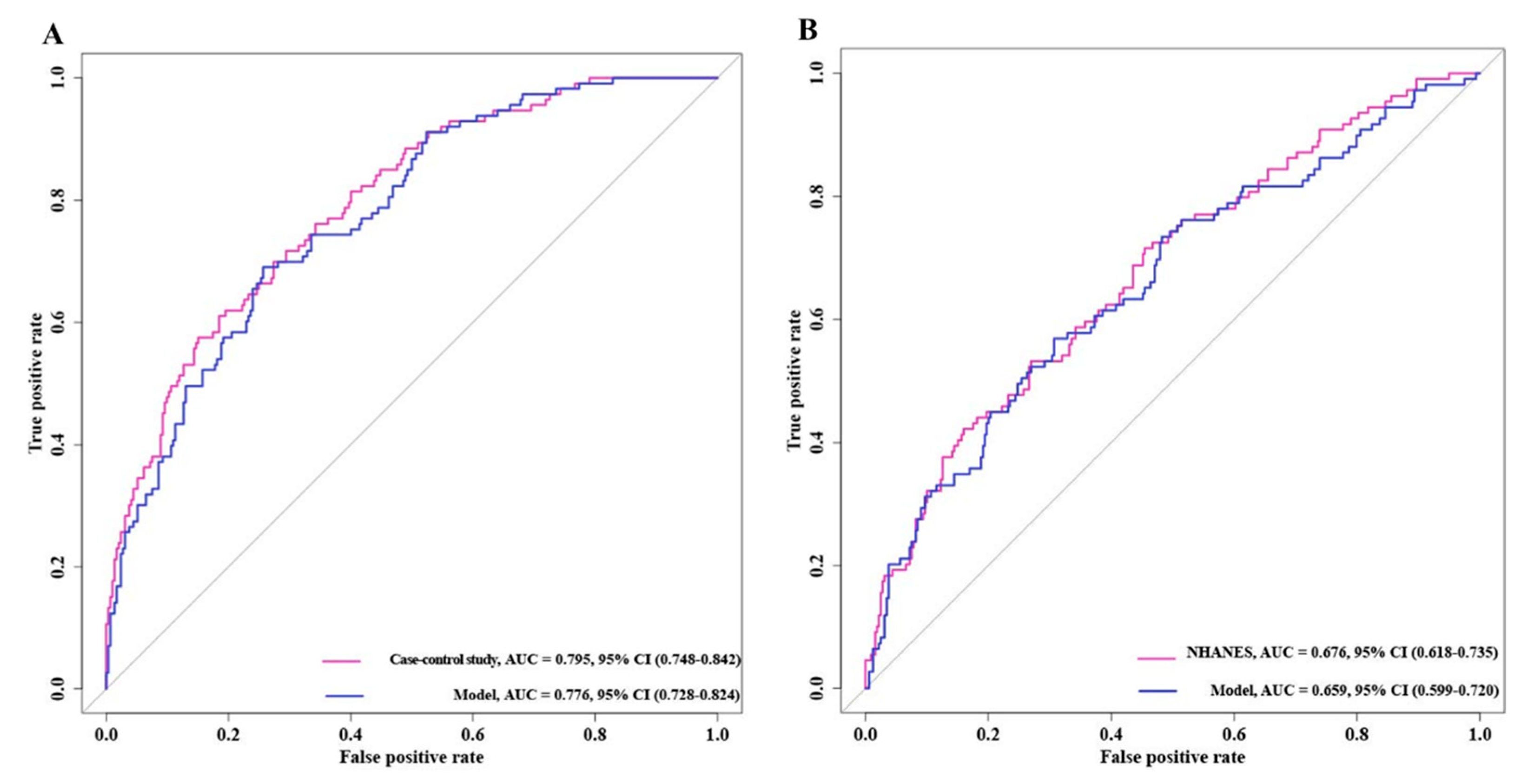
3.5. Receiver Operating Characteristic Curves of Bladder Cancer Risk Model
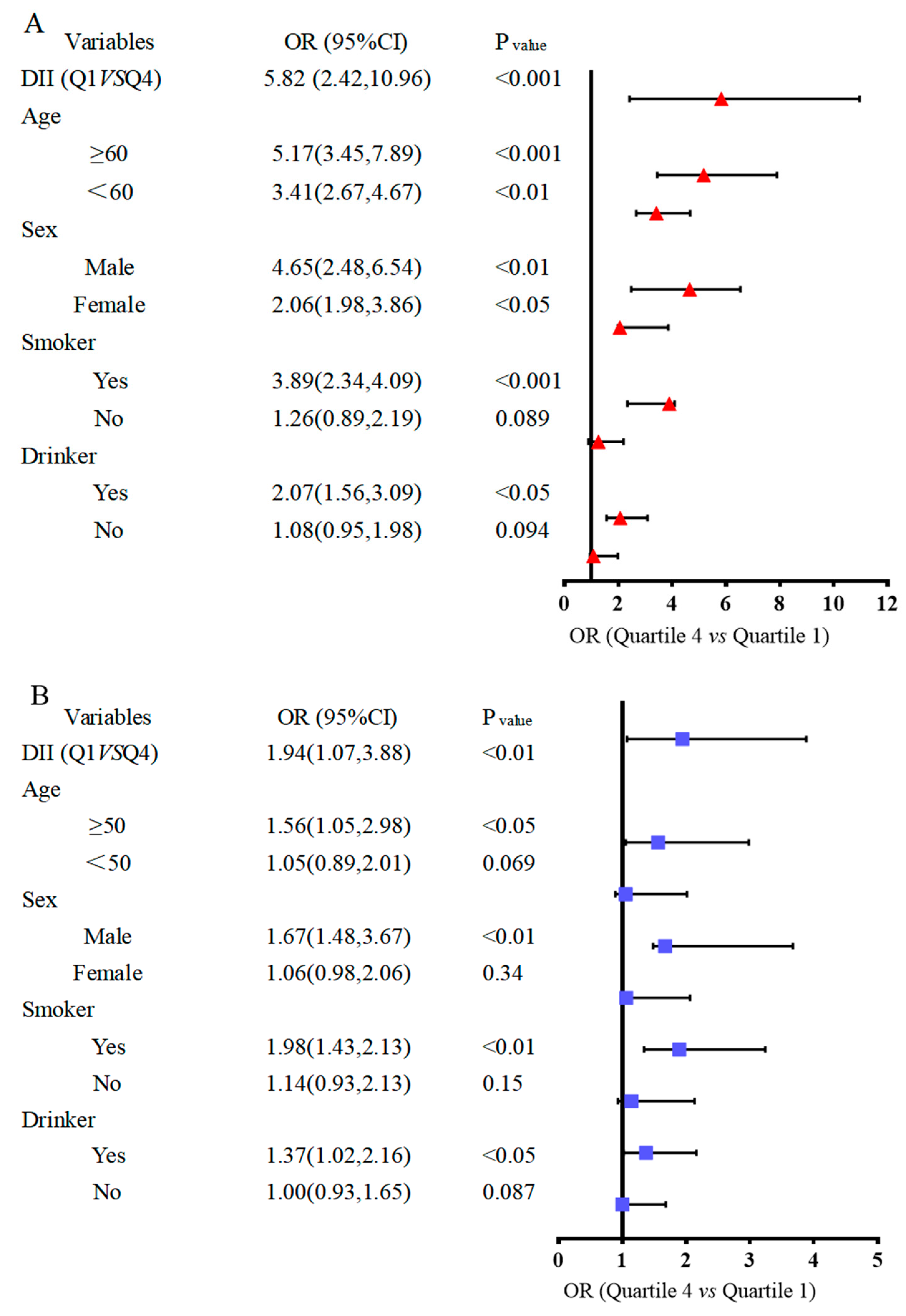
3.6. Subgroup Analysis between Dietary Inflammation Index and the Risk of Bladder Cancer
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- McIsaac, D.I.; Gill, M.; Boland, L.; Hutton, B.; Branje, K.; Shaw, J.; Grudzinski, A.L.; Barone, N.; Gillis, C.; Akhtar, S.; et al. Prehabilitation in adult patients undergoing surgery: An umbrella review of systematic reviews. Br. J. Anaesth. 2022, 128, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.L.; Witjes, J.A.; Agarwal, P.K.; Anderson, C.B.; Bivalacqua, T.J.; Bochner, B.H.; Boormans, J.L.; Chang, S.S.; Domínguez-Escrig, J.L.; McKiernan, J.M.; et al. ICUD-SIU International Consultation on Bladder Cancer 2017: Management of non-muscle invasive bladder cancer. World J. Urol. 2019, 37, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.I.; Brausi, M.; Clark, P.E.; Cookson, M.S.; Grossman, H.B.; Khochikar, M.; Kiemeney, L.A.; Malavaud, B.; Sanchez-Salas, R.; Soloway, M.S.; et al. Epidemiology, prevention, screening, diagnosis, and evaluation: Update of the ICUD-SIU joint consultation on bladder cancer. World J. Urol. 2019, 37, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Erlich, A.; Zlotta, A.R. Molecular Characterization of Bladder Cancer. Curr. Urol. Rep. 2018, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hu, F.B. Dietary patterns and long-term health: Implications for chronic disease risk. Lancet Diabetes Endocrinol. 2023, 11, 187–196. [Google Scholar]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? The Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V. Nutrient profiling of foods: Creating a nutrient-rich food index. Nutr. Rev. 2008, 66, 23–39. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Rossi, M.; Libra, M.; Montella, M.; Serraino, D.; La Vecchia, C. Dietary inflammatory index and risk of bladder cancer in a large Italian case-control study. Urology 2017, 100, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Mostafid, H. Prevention of bladder cancer incidence and recurrence: Nutrition and lifestyle. Curr. Opin. Urol. 2018, 28, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, I.; Jakimovski, D.; Shivappa, N.; Hébert, J.R.; Vahid, F.; Nedjat, S.; Mansournia, M.A.; Weinstock-Guttman, B. Dietary inflammatory index and risk of multiple sclerosis: Findings from a large population-based incident case-control study. Clin. Nutr. 2020, 39, 3402–3407. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Staritsky, L.E.; van Zutphen, M.; Geijsen, A.J.; Kok, D.E.; Kruyt, F.; Veenstra, R.P.; Bilgen, E.J.S.; Kouwenhoven, E.A.; de Wilt, J.H.; et al. The association between the adapted dietary inflammatory index and colorectal cancer recurrence and all-cause mortality. Clin. Nutr. 2021, 40, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Shivappa, N.; Petimar, J.; Hodgson, M.E.; Nichols, H.B.; Steck, S.E.; Hébert, J.R.; Sandler, D.P. Dietary inflammatory potential, oxidative balance score, and risk of breast cancer: Findings from the Sister Study. Int. J. Cancer 2021, 149, 615–626. [Google Scholar] [CrossRef]
- Lozano-Lorca, M.; Salcedo-Bellido, I.; Olmedo-Requena, R.; Castaño-Vinyals, G.; Amiano, P.; Shivappa, N.; Hébert, J.R.; Pérez-Gómez, B.; Gracia-Lavedan, E.; Gómez-Acebo, I.; et al. Dietary inflammatory index and prostate cancer risk: MCC-Spain study. Prostate Cancer Prostatic Dis. 2022, 25, 568–575. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Mirsafa, F.; Rashidkhani, B. Increased inflammatory potential of diet is associated with increased risk of bladder cancer in an Iranian case-control study. Nutr. Cancer 2019, 71, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-L.; Ren, Z.-J.; Zhang, Q.; Ren, P.-W.; Yang, B.; Liu, L.-R.; Dong, Q. Meta-analysis of the association between the inflammatory potential of diet and urologic cancer risk. PLoS ONE 2018, 13, e0204845. [Google Scholar] [CrossRef]
- Luo, J.; Shivappa, N.; Hebert, J.R.; Xu, X. Dietary inflammatory index and bladder cancer risk: A prospective study. Eur. J. Clin. Nutr. 2020, 74, 1428–1433. [Google Scholar] [CrossRef]
- Teng, C.; Zheng, S.; Wan, W.; Liu, L.; Yu, S.; Cao, M.; Lu, W.; Shan, Y. Fatty foods and the risk of bladder cancer: A case-control study. Nutrition 2023, 106, 111868. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES dietary data: Focus on collection, release, analytical considerations, and uses to inform public policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Paulose-Ram, R.; Graber, J.E.; Woodwell, D.; Ahluwalia, N. The National Health and Nutrition Examination Survey (NHANES), 2021–2022: Adapting data collection in a COVID-19 environment. Am. J. Public Health 2021, 111, 2149–2156. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010; Vital and Health Statistics. Series 10, Data from the National Health Survey; National Center for Health Statistics: Hyattsville, MD, USA, 2013; Volume 8, pp. 1–37. [Google Scholar]
- Xu, K.Y.; Borodovsky, J.T.; Presnall, N.; Mintz, C.M.; Hartz, S.M.; Bierut, L.J.; Grucza, R.A. Association between benzodiazepine or Z-drug prescriptions and drug-related poisonings among patients receiving buprenorphine maintenance: A case-crossover analysis. Am. J. Psychiatry 2021, 178, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Eijkemans, M.J.; Harrell, F.E., Jr.; Habbema, J.D.F. Prognostic modeling with logistic regression analysis: In search of a sensible strategy in small data sets. Med. Decis. Mak. 2001, 21, 45–56. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Richman, E.L.; Chan, J.M. The role of diet in influencing prostate cancer risk. Nat. Rev. Urol. 2023, 20, 237–253. [Google Scholar]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, S.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Sung, K.J.; Sung, Y.H.; Choi, C.M.; Yun, M.; Yi, Y.S.; et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1. 4. Cancer Lett. 2020, 475, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2023, 59, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an insitu DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, H. Pro-inflammatory cytokines as therapeutic targets in cancer. Nat. Rev. Cancer 2023, 23, 123–139. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Garavello, W.; Serraino, D.; La Vecchia, C. Inflammatory potential of diet and risk of oral and pharyngeal cancer in a large case-control study from Italy. Int. J. Cancer 2017, 141, 471–479. [Google Scholar] [CrossRef]
- Sharma, I.; Zhu, Y.; Woodrow, J.R.; Mulay, S.; Parfrey, P.S.; Mclaughlin, J.R.; Hebert, J.R.; Shivappa, N.; Li, Y.; Zhou, X.; et al. Inflammatory diet and risk for colorectal cancer: A population-based case-control study in Newfoundland, Canada. Nutrition 2017, 42, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.E.; Akinyemiju, T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Tabung, F.K.; Shariat, S.F.; Moschini, M.; Devore, E.; Papantoniou, K.; Yang, L.; Strohmaier, S.; Rohrer, F.; Markt, S.C.; et al. Association between inflammatory potential of diet and bladder cancer risk: Results of 3 united states prospective cohort studies. J. Urol. 2019, 202, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Lin, J.F.; Tian, T.; Xie, D.; Xu, R.H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Bosetti, C.; Zucchetto, A.; Montella, M.; Serraino, D.; La Vecchia, C.; Hébert, J.R. Association between dietary inflammatory index and prostate cancer among Italian men. Br. J. Nutr. 2015, 113, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M. Bladder cancer. Nat. Rev. Dis. Primers 2023, 9, 1–23. [Google Scholar]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Gakis, G. The role of inflammation in bladder cancer. In Inflammation and Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 183–196. [Google Scholar]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Dai, Y.N.; Yi, W.Y.; Zeegers, M.P.; Wesselius, A.; Wesselius, A. The Association between Dietary Inflammatory Potential and Urologic Cancers: A Meta-analysis. Adv. Nutr. 2024, 15, 100124. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Metab. 2023, 35, 86–110. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, L.; Ghotbabadi, Z.R.; Gholipour, A.; Ehymayed, H.M.; Najafiyan, B.; Amirlou, P.; Yasamineh, S.; Gholizadeh, O.; Emtiazi, N. A state-of-the-art review on the NRF2 in Hepatitis virus-associated liver cancer. Cell Commun. Signal. 2023, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.S.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Pharoah, P.D.; Michailidou, K.; Tyrer, J.; Brook, M.N.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl. Cancer Inst. 2015, 107, djv036. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Landry, D.; Little, J.; Minelli, C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med. Res. Methodol. 2017, 17, 146. [Google Scholar] [CrossRef]
- Paeratakul, S.; Popkin, B.M.; Kohlmeier, L.; Hertz-Picciotto, I.; Guo, X.; Edwards, L.J. Measurement error in dietary data: Implications for the epidemiologic study of the diet–disease relationship. Eur. J. Clin. Nutr. 1998, 52, 722–727. [Google Scholar] [CrossRef]
| Variable | All Participants | Controls | Cases | p-Values |
|---|---|---|---|---|
| (n = 405) | (n = 292) | (n = 113) | ||
| Age, year | 62.7 ± 11.8 (25~80) | 62.6 ± 12.3 (26~80) | 63.1 ± 10.5 (25~80) | 0.71 |
| Sex, n (%) | 0.49 | |||
| Male | 233 (57.5) | 155 (53.1) | 78 (69.0) | - |
| Female | 172 (42.5) | 137 (46.9) | 35 (31.0) | - |
| Degree of education, n (%) | <0.001 | |||
| High school and above | 111 (27.4) | 100 (34.2) | 11 (9.7) | - |
| Senior high school | 89 (22.0) | 64 (21.9) | 25 (22.1) | - |
| Primary education and below | 205 (50.6) | 128 (43.8) | 77 (68.1) | - |
| Occupation, n (%) | <0.001 | |||
| Unemployed | 41 (10.1) | 23 (7.9) | 18 (15.9) | - |
| Peasant | 49 (12.1) | 13 (4.5) | 36 (31.9) | - |
| Worker | 139 (34.3) | 111 (38.0) | 28 (24.8) | - |
| Officer | 176 (43.5) | 145 (49.7) | 31 (27.4) | - |
| Smoking status, n (%) | 0.001 | |||
| Current smoker | 108 (26.7) | 53 (18.2) | 55 (48.7) | 71 (16.6) |
| Never or former smoker | 297 (73.3) | 239 (81.8) | 58 (51.3) | 357 (83.4) |
| Alcohol consumption, n (%) | <0.01 | |||
| Current drinker | 121 (29.9) | 76 (26.0) | 45 (39,8) | 31 (7.2) |
| Never or former drinker | 284 (70.1) | 216 (74.0) | 68 (60.2) | 397 (92.8) |
| Physical activity, n (%) | <0.01 | |||
| Inactive | 130 (32.1) | 93 (31.8) | 37 (32.7) | 262 (61.2) |
| Moderately inactive | 160 (39.5) | 126 (43.2) | 34 (30.1) | 73 (17.1) |
| Moderately active | 68 (16.8) | 49 (16.8) | 19 (16.8) | 78 (18.2) |
| Active | 47 (11.6) | 24 (8.2) | 23 (20.4) | 15 (3.5) |
| Variable | All Participants | Controls | Cases | p-Values |
|---|---|---|---|---|
| (n = 405) | (n = 292) | (n = 113) | ||
| Food intake (g/d) | ||||
| Vegetables | 337.7 ± 218.9 | 381.1 ± 227.8 | 225.7 ± 143.1 | <0.001 |
| Fruits | 203.7 ± 175.6 | 228.1 ± 183.1 | 140.7 ± 140.0 | <0.001 |
| Grains | 340.4 ± 147.3 | 307.9 ± 135.0 | 424.4 ± 144.8 | <0.001 |
| Whole grain | 24.1 ± 34.6 | 26.8 ± 38.7 | 17.1 ± 18.9 | <0.01 |
| Tubers | 35.8 ± 31.9 | 34.8 ± 29.8 | 38.3 ± 36.8 | 0.32 |
| Eggs | 33.9 ± 24.5 | 35.3 ± 23.6 | 30.6 ± 26.7 | 0.083 |
| Milk and dairy products | 100.7 ± 111.9 | 114.3 ± 115.6 | 65.6 ± 93.6 | <0.001 |
| Beans | 29.2 ± 29.4 | 30.6 ± 31.1 | 25.7 ± 24.2 | 0.13 |
| Nuts | 14.8 ± 22.8 | 17.0 ± 24.0 | 9.3 ± 18.0 | <0.01 |
| Red meats | 45.6 ± 36.9 | 42.6 ± 34.1 | 53.2 ± 42.4 | <0.01 |
| Poultry | 9.5 ± 11.0 | 8.4 ± 8.9 | 12.2 ± 15.0 | 0.13 |
| Fish and shrimp | 8.8 ± 15.1 | 10.3 ± 15.4 | 5.2 ± 13.7 | <0.01 |
| Soybean oil | 39.8 ± 17.8 | 44.2 ± 12.2 | 49.8 ± 10.3 | <0.001 |
| Nutrients intake | ||||
| Energy (Kcal) | 2048.2 ± 686.5 | 1940.2 ± 634.1 | 2327.3 ± 739.1 | <0.001 |
| Protein (g) | 56.0 ± 12.0 | 58.9 ± 11.1 | 48.4 ± 11.1 | 0.72 |
| Fats (g) | 83.9 ± 18.1 | 82.4 ± 15.4 | 87.7 ± 23.4 | <0.05 |
| Carbohydrate (g) | 261.4 ± 94.4 | 247.1 ± 90.4 | 298.2 ± 95.0 | <0.001 |
| Dietary fiber (g) | 14.3 ± 7.8 | 15.4 ± 8.3 | 11.3 ± 5.5 | <0.001 |
| Cholesterol (mg) | 295.9 ± 161.6 | 311.3 ± 154.8 | 255.8 ± 172.4 | <0.05 |
| Vitamin A (mg) | 0.036 ± 0.025 | 0.038 ± 0.024 | 0.032 ± 0.021 | <0.05 |
| Vitamin B1 (mg) | 0.86 ± 0.55 | 0.84 ± 0.55 | 0.92 ± 0.56 | 0.18 |
| Vitamin B6 (mg) | 0.26 ± 0.15 | 0.28 ± 0.16 | 0.20 ± 0.13 | <0.001 |
| Vitamin C (mg) | 109.8 ± 73.6 | 124.2 ± 78.2 | 72.8 ± 41.4 | <0.001 |
| Vitamin D (μg) | 1.8 ± 1.7 | 2.1 ± 1.8 | 0.99 ± 1.2 | <0.001 |
| Vitamin E (mg) | 54.3 ± 18.3 | 50.4 ± 14.7 | 64.1 ± 22.6 | <0.001 |
| Folic acid (μg) | 114.6 ± 93.0 | 137.2 ± 96.2 | 56.1 ± 48.1 | <0.001 |
| Nicotinic acid (mg) | 15.2 ± 11.1 | 15.6 ± 12.3 | 14.2 ± 7.2 | 0.26 |
| Magnesium (mg) | 345.1 ± 172.6 | 356.6 ± 190.4 | 315.4 ± 109.9 | <0.05 |
| Iron (mg) | 18.6 ± 7.6 | 19.0 ± 8.1 | 17.5 ± 6.0 | <0.05 |
| Zinc (mg) | 9.6 ± 3.8 | 9.6 ± 3.9 | 9.9 ± 3.5 | 0.47 |
| Selenium (μg) | 35.0 ± 16.1 | 34.9 ± 16.1 | 35.5 ± 16.2 | 0.74 |
| Variable | All Participants | Controls | Cases | p-Values |
|---|---|---|---|---|
| (n = 428) | (n= 319) | (n = 109) | ||
| Nutrients intake | ||||
| Energy (Kcal) | 1924.4 ± 719.9 | 1902.4 ± 702.8 | 1988.8 ± 767.3 | 0.28 |
| Protein (g) | 71.3 ± 31.7 | 71.5 ± 32.2 | 70.8 ± 30.1 | 0.85 |
| Fats (g) | 73.0 ± 32.4 | 72.1 ± 31.8 | 75.5 ± 34.3 | 0.35 |
| Carbohydrate (g) | 244.7 ± 100.6 | 242.4 ± 96.7 | 251.3 ± 98.6 | 0.46 |
| Dietary fiber (g) | 15.0 ± 8.9 | 14.9 ± 8.8 | 15.2 ± 9.4 | 0.69 |
| Cholesterol (mg) | 250.9 ± 185.2 | 254.7 ± 190.1 | 239.8 ± 170.4 | 0.47 |
| Vitamin A (mg) | 0.061 ± 0.056 | 0.061 ± 0.060 | 0.059 ± 0.036 | 0.79 |
| Vitamin B1 (mg) | 1.5 ± 0.70 | 1.5 ± 0.70 | 1.51 ± 0.71 | 0.85 |
| Vitamin B6 (mg) | 1.9 ± 1.6 | 1.81 ± 1.20 | 1.97 ± 2.52 | 0.39 |
| Vitamin C (mg) | 85.5 ± 82.6 | 86.7 ± 84.4 | 82.2 ± 77.2 | 0.63 |
| Vitamin D (μg) | 3.7 ± 4.1 | 3.8 ± 4.2 | 3.3. ± 3.7 | 0.30 |
| Vitamin E (mg) | 6.7 ± 4.4 | 6.7 ± 4.5 | 6.6 ± 3.9 | 0.73 |
| Folic acid (μg) | 193.9 ± 156.7 | 191.4 ± 142.5 | 201.1 ± 193.0 | 0.58 |
| Nicotinic acid (mg) | 21.6 ± 12.5 | 21.5 ± 11.2 | 22.1 ± 15.7 | 0.66 |
| Magnesium (mg) | 258.7 ± 125.6 | 259.4 ± 130.7 | 256.5 ± 110.0 | 0.83 |
| Iron (mg) | 13.8 ± 6.2 | 13.7 ± 6.1 | 14.2 ± 6.6 | 0.49 |
| Zinc (mg) | 10.5 ± 7.2 | 10.6 ± 7.9 | 10.2 ± 4.3 | 0.47 |
| Selenium (μg) | 97.7 ± 45.7 | 97.8 ± 45.8 | 97.4 ± 45.5 | 0.94 |
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| ORs (95%CIs) | p Value | ORs (95%CIs) | p Value | |
| In case–control study | ||||
| Age (year) | 1.00 (0.99–1.02) | 0.73 | 0.98 (0.95–1.00) | 0.18 |
| Sex a | 1.32 (1.05–1.84) | 0.056 | 1.56 (1.25–1.98) | <0.05 |
| Degree of education b | ||||
| Reference | 1 | |||
| 2 | 3.55 (1.64–7.71) | 0.001 | 5.12 (1.95–10.43) | 0.001 |
| 3 | 5.47 (2.76–10.84) | <0001 | 7.22 (2.84–11.36) | <0001 |
| Occupation c | ||||
| Reference | 1 | |||
| 2 | 3.54 (1.46, 8.57) | <0.01 | 3.49 (1.31, 9.34) | <0.05 |
| 3 | 0.32 (0.15, 0.68) | <0.01 | 0.25 (0.11, 0.59) | <0.05 |
| 4 | 0.27 (0.13, 0.57) | <0.001 | 0.47 (0.20, 1.11) | 0.086 |
| Smoking status d | 4.27 (2.66, 6.87) | <0.001 | 2.58 (1.39, 4.78) | <0.001 |
| Alcohol consumption e | 1.88 (1.19, 2.98) | <0.05 | 0.82 (0.44, 1.52) | 0.52 |
| Physical activity f | ||||
| Reference | 1 | |||
| 2 | 0.41 (0.19, 0.88) | <0.05 | 0.79 (0.29, 2.10) | 0.63 |
| 3 | 0.28 (0.14, 0.56) | <0.001 | 0.53 (0.22, 1.27) | 0.15 |
| 4 | 0.42 (0.21, 0.83) | <0.05 | 0.69 (0.28, 1.70) | 0.42 |
| Dietary Inflammation Index (DII) | ||||
| Quartile 1 (−22.98~−13.12) | 1 | |||
| Quartile 2 (−13.11~−10.86) | 1.48 (0.67, 3.29) | 0.34 | 2.00 (0.76, 5.26) | 0.16 |
| Quartile 3 (−10.85~−8.50) | 2.62 (1.25, 5.50) | <0.05 | 2.91 (1.19, 7.14) | <0.05 |
| Quartile 4 (−8.49~0.57) | 4.33 (2.14, 8.78) | <0.0001 | 5.82 (2.42, 10.96) | <0.001 |
| In NHANES trial | ||||
| Age (year) | 1.00 (0.99, 1.02) | 0.39 | 1.00 (0.99, 1.02) | 0.77 |
| Sex a | 1.03 (0.67, 1.60) | 0.89 | 1.28 (0.74, 2.21) | 0.38 |
| Degree of education b | ||||
| Reference | 1 | |||
| 2 | 2.45 (1.45, 4.11) | 0.001 | 3.49 (1.92, 6.36) | <0001 |
| 3 | 1.18 (0.56, 2.45) | 0.67 | 1.25 (0.54, 2.89) | 0.61 |
| Occupation c | ||||
| Reference | 1 | |||
| 2 | 1.22(0.66, 1.57) | 0.17 | 0.97 (0.44, 2.12) | 0.34 |
| 3 | 0.87 (0.49, 1.55) | 0.65 | 2.49 (1.02, 6.09) | <0.05 |
| 4 | 1.45(0.75, 2.81) | 0.27 | 3.47 (1.20, 7.11) | <0.05 |
| Smoking status d | 2.22 (1.30, 3.79) | <0.05 | 2.11 (1.15, 3.88) | <0.001 |
| Alcohol consumption e | 2.62 (1.24, 5.51) | <0.05 | 2.41 (1.04, 5.59) | <0.05 |
| Physical activity f | ||||
| Reference | 1 | |||
| 2 | 2.40 (0.50, 5.52) | 0.28 | 1.60 (0.28, 9.18) | 0.60 |
| 3 | 2.99(0.62, 7.35) | 0.17 | 1.66 (0.28, 9.85) | 0.58 |
| 4 | 2.06 (0.45, 6.37) | 0.35 | 1.26 (0.25, 6.53) | 0.78 |
| Dietary Inflammation Index (DII) | ||||
| Quartile 1 (−15.36~−0.66) | 1 | |||
| Quartile 2 (−0.65~2.13) | 1.74 (0.89, 3.41) | 0.11 | 1.56 (0.77, 3.15) | 0.22 |
| Quartile 3 (2.14~4.30) | 1.61 (0.82, 3.16) | 0.17 | 1.30 (0.63, 2.66) | 0.48 |
| Quartile 4 (4.31~8.30) | 2.00 (1.03, 3.87) | <0.05 | 1.94 (1.07, 3.88) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.; Lu, W.; Che, J.; Wu, Y.; Meng, D.; Shan, Y. Association of Pro-Inflammatory Diet, Smoking, and Alcohol Consumption with Bladder Cancer: Evidence from Case–Control and NHANES Studies from 1999 to 2020. Nutrients 2024, 16, 1793. https://doi.org/10.3390/nu16111793
Teng C, Lu W, Che J, Wu Y, Meng D, Shan Y. Association of Pro-Inflammatory Diet, Smoking, and Alcohol Consumption with Bladder Cancer: Evidence from Case–Control and NHANES Studies from 1999 to 2020. Nutrients. 2024; 16(11):1793. https://doi.org/10.3390/nu16111793
Chicago/Turabian StyleTeng, Chunying, Weihong Lu, Jiawen Che, Yanhong Wu, Danqun Meng, and Yujuan Shan. 2024. "Association of Pro-Inflammatory Diet, Smoking, and Alcohol Consumption with Bladder Cancer: Evidence from Case–Control and NHANES Studies from 1999 to 2020" Nutrients 16, no. 11: 1793. https://doi.org/10.3390/nu16111793
APA StyleTeng, C., Lu, W., Che, J., Wu, Y., Meng, D., & Shan, Y. (2024). Association of Pro-Inflammatory Diet, Smoking, and Alcohol Consumption with Bladder Cancer: Evidence from Case–Control and NHANES Studies from 1999 to 2020. Nutrients, 16(11), 1793. https://doi.org/10.3390/nu16111793






