Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. RNA Isolation and cDNA Preparation
2.4. Quantitative Real-Time RT-PCR Analysis
2.5. Plasma Membrane Isolation Procedure
2.6. Western Blot Assay
2.7. Adenylate Cyclase Activity Assay
2.8. 5′-Nucleotidase Activity Assay
2.9. Statistical and Data Analysis
3. Results
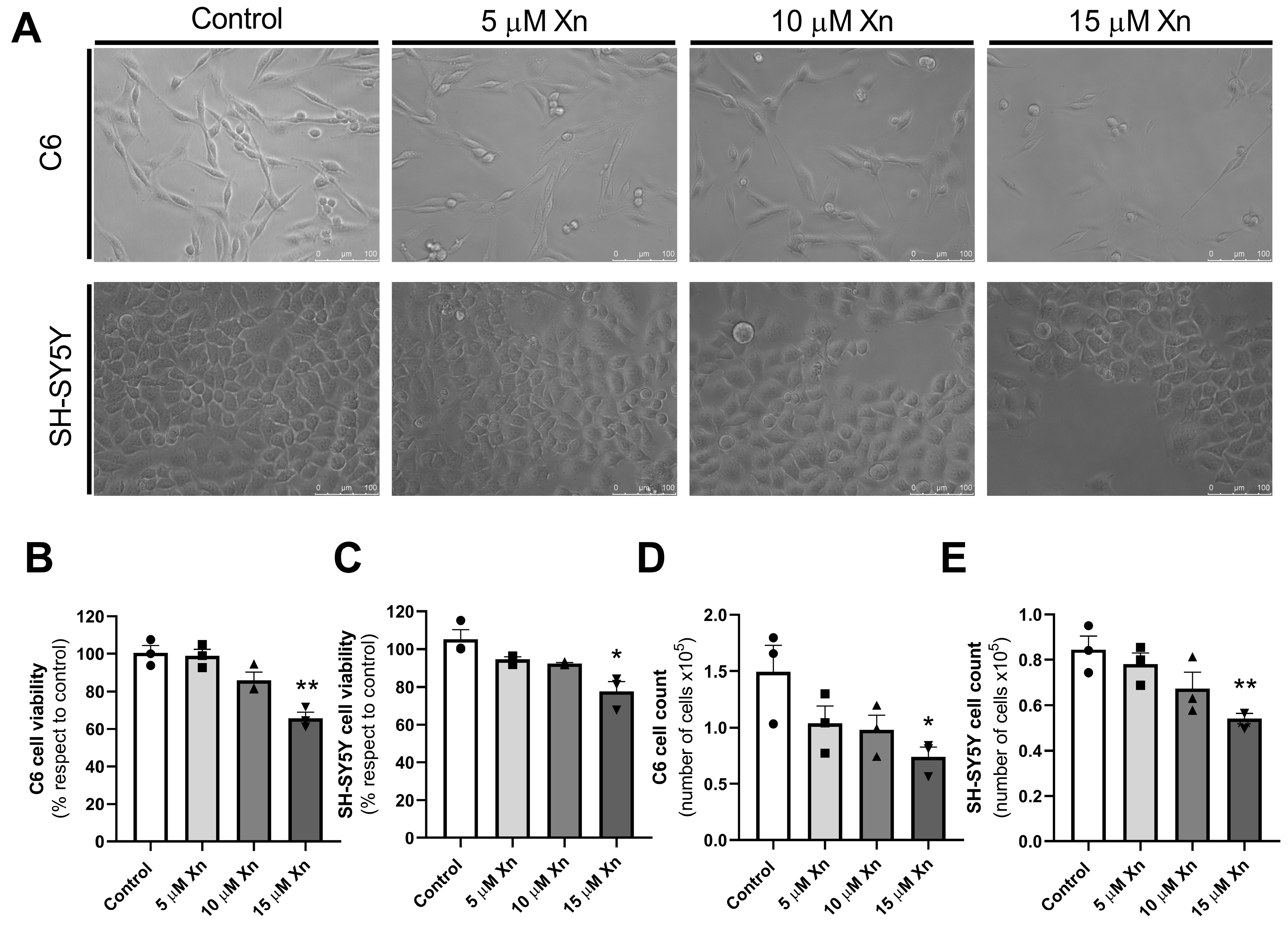
3.1. Effect of Xn on C6 and SH-SY5Y Cell Lines Viability
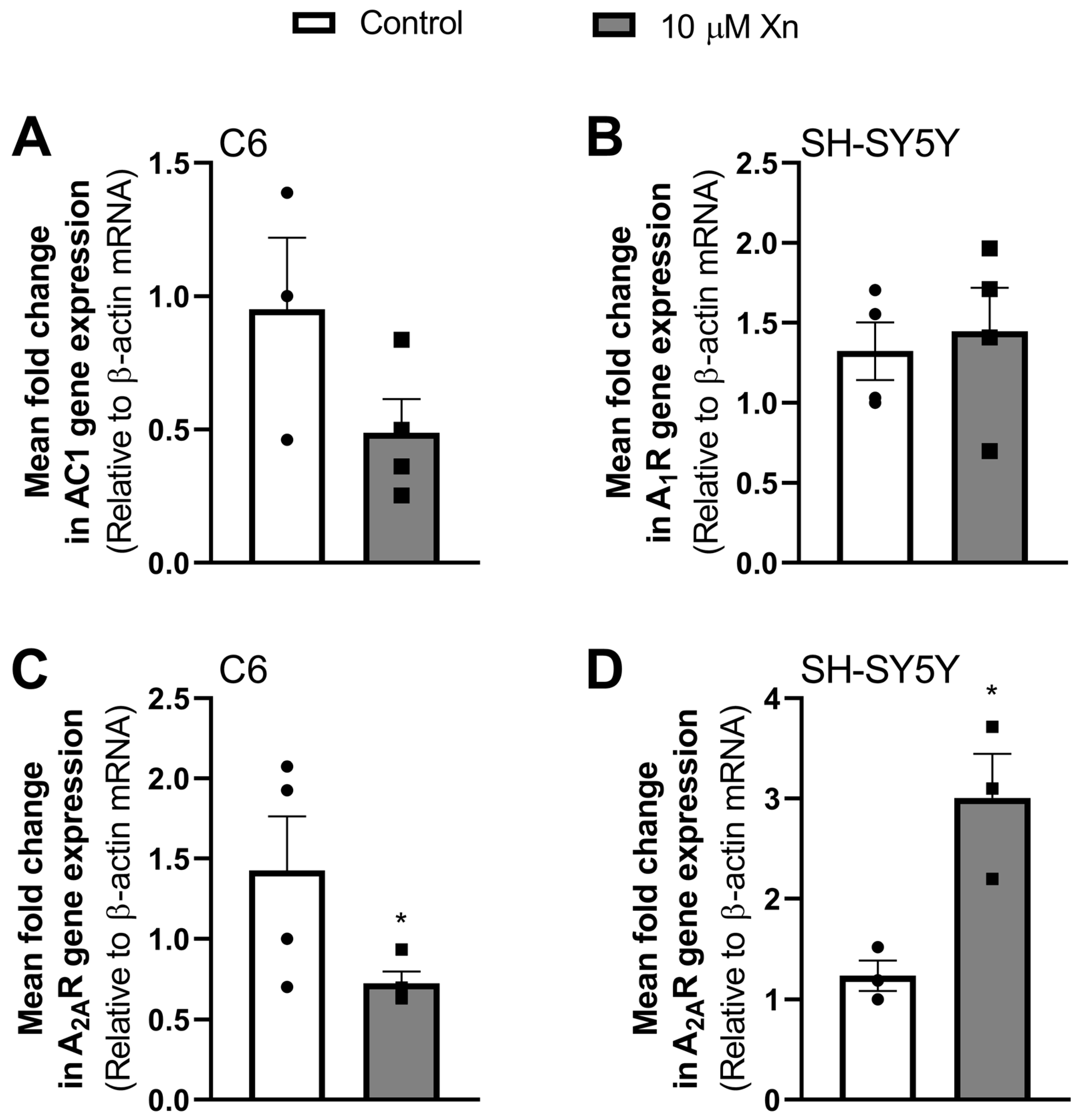
3.2. Effect of Xn on Adenosine Receptor Gene Expression
3.3. Effect of Xn on Adenosine Receptors Levels in Plasma Membrane
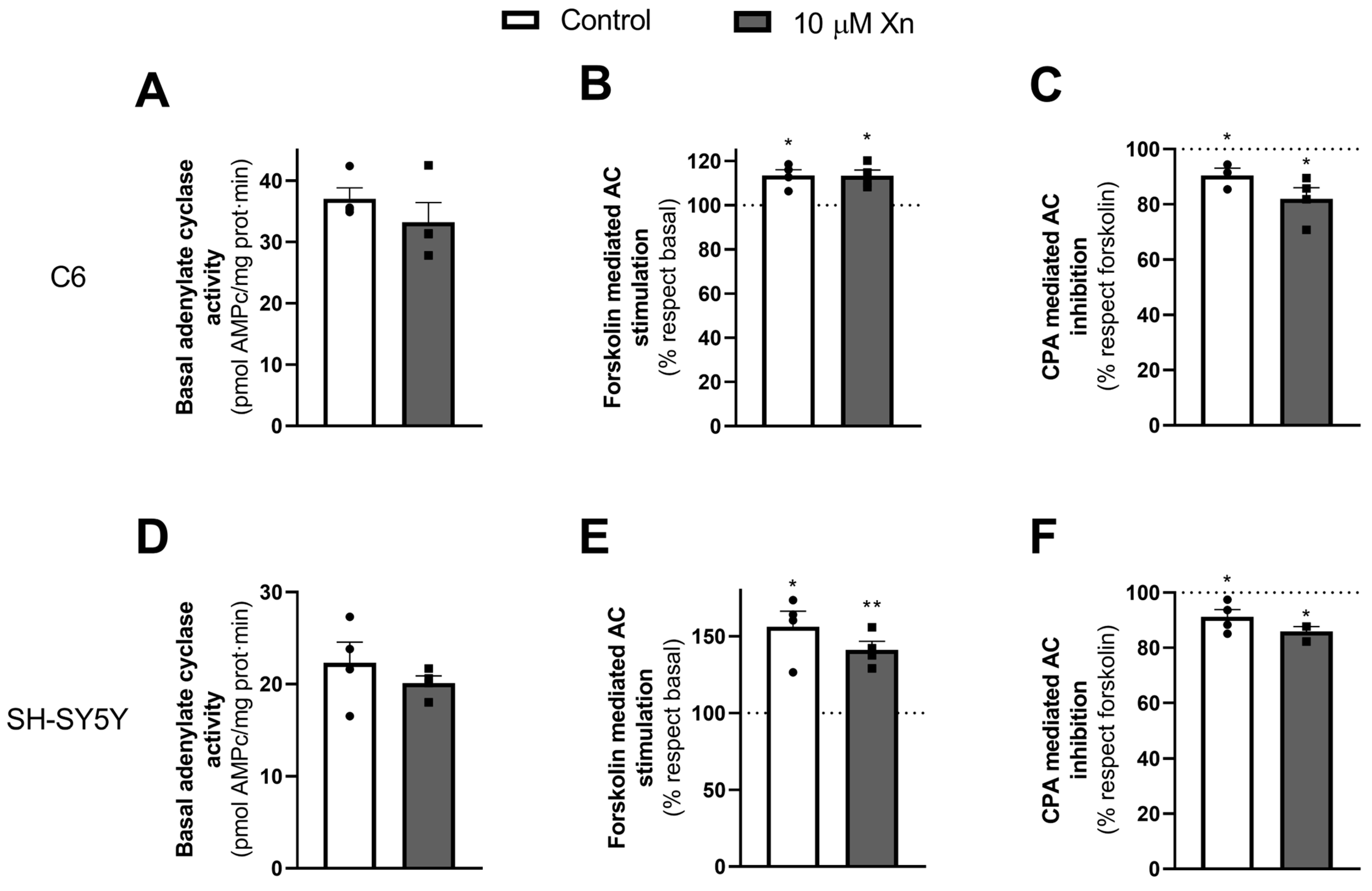
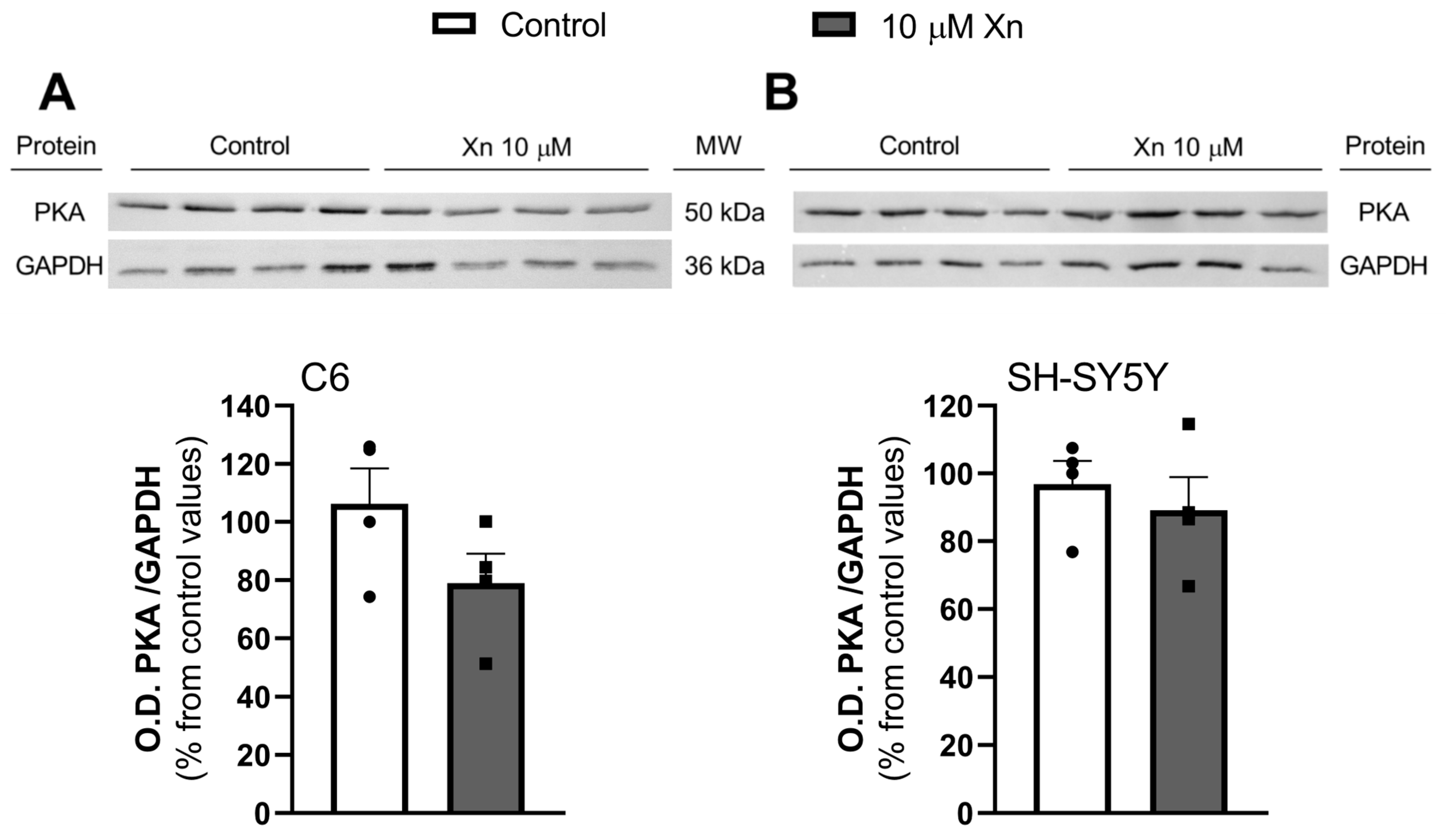
3.4. Effect of Xn on Adenylate Cyclase Activity and PKA Levels
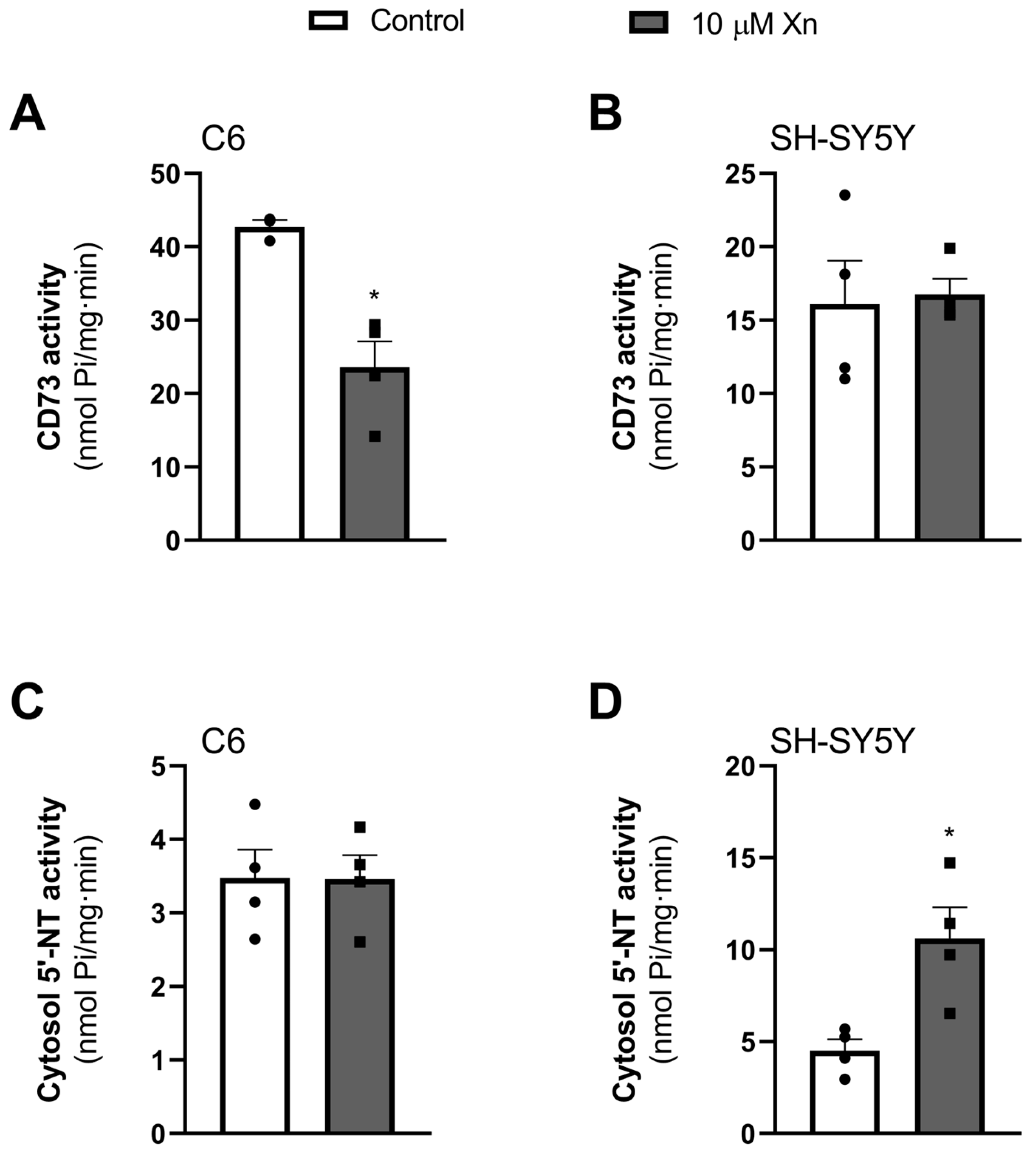
3.5. Effect of Xn in 5′-Nucleotidase Activity (CD73)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phukan, B.C.; Roy, R.; Gahatraj, I.; Bhattacharya, P.; Borah, A. Therapeutic Considerations of Bioactive Compounds in Alzheimer’s Disease and Parkinson’s Disease: Dissecting the Molecular Pathways. Phytother. Res. 2023, 37, 5657–5699. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M. An Overview of Bioactive Phenolic Molecules and Antioxidant Properties of Beer: Emerging Trends. Molecules 2023, 28, 3221. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, C.; Rancan, L.; Paredes, S.D.; Montero, C.; de la Fuente, M.; Vara, E.; Tresguerres, J.A.F. Xanthohumol Exerts Protective Effects in Liver Alterations Associated with Aging. Eur. J. Nutr. 2019, 58, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, T.; Shuqing, Z.; Yu, L.; Chen, S.; Lu, H.; Zhu, H.; Min, X.; Li, X.; Liu, L. Xanthohumol Relieves Arthritis Pain in Mice by Suppressing Mitochondrial-Mediated Inflammation. Mol. Pain 2023, 19, 17448069231204051. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Oliveira, R.; Johansson, B.; Guido, L.F. Dose-Dependent Protective and Inductive Effects of Xanthohumol on Oxidative DNA Damage in Saccharomyces Cerevisiae. Food Technol. Biotechnol. 2016, 54, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wongchum, N.; Dechakhamphu, A. Xanthohumol Prolongs Lifespan and Decreases Stress-Induced Mortality in Drosophila Melanogaster. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 244, 108994. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes to Confer Protection against Oxidative Damage in PC12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for Cancer Prevention and Treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Chen, X.; Liu, P.; Wang, S.; Song, F.; Zhang, Z.; Zhu, F.; Huang, X.; Liu, J.; et al. The Prenylflavonoid Xanthohumol Reduces Alzheimer-Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 2018, 9, 199. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Dhapola, R.; Sarma, P.; Medhi, B.; Prakash, A.; Reddy, D.H. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Tanaka, K.; Dawe, G.S.; Ittner, L.M.; Farooqui, A.A. Slow Excitotoxicity in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 35, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, A.; Lukens, J.R. How Neurons Die in Alzheimer’s Disease: Implications for Neuroinflammation. Curr. Opin. Neurobiol. 2022, 75, 102575. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? Journal of Neurochemistry 2016, 139, 1019–1055. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Sromek, S.; Wilcox, B.J.; Unnerstall, J.R. Hippocampal Adenosine A1 Receptors Are Decreased in Alzheimer’s Disease. Neurosci. Lett. 1990, 118, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Ishii, K.; Kimura, Y.; Oda, K.; Hashimoto, M.; Suzuki, M.; Ishiwata, K. Adenosine A(1) Receptors Using 8-Dicyclopropylmethyl-1-[(11)C]Methyl-3-Propylxanthine PET in Alzheimer’s Disease. Ann. Nucl. Med. 2008, 22, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine Consumption Prevents Memory Impairment, Neuronal Damage, and Adenosine A2A Receptors Upregulation in the Hippocampus of a Rat Model of Sporadic Dementia. J. Alzheimers Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Viana da Silva, S.; Haberl, M.G.; Zhang, P.; Bethge, P.; Lemos, C.; Gonçalves, N.; Gorlewicz, A.; Malezieux, M.; Gonçalves, F.Q.; Grosjean, N.; et al. Early Synaptic Deficits in the APP/PS1 Mouse Model of Alzheimer’s Disease Involve Neuronal Adenosine A2A Receptors. Nat. Commun. 2016, 7, 11915. [Google Scholar] [CrossRef]
- Silva, A.C.; Lemos, C.; Gonçalves, F.Q.; Pliássova, A.V.; Machado, N.J.; Silva, H.B.; Canas, P.M.; Cunha, R.A.; Lopes, J.P.; Agostinho, P. Blockade of Adenosine A2A Receptors Recovers Early Deficits of Memory and Plasticity in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2018, 117, 72–81. [Google Scholar] [CrossRef]
- Lopes, C.R.; Silva, A.C.; Silva, H.B.; Canas, P.M.; Agostinho, P.; Cunha, R.A.; Lopes, J.P. Adenosine A2A Receptor Up-Regulation Pre-Dates Deficits of Synaptic Plasticity and of Memory in Mice Exposed to Aβ1–42 to Model Early Alzheimer’s Disease. Biomolecules 2023, 13, 1173. [Google Scholar] [CrossRef] [PubMed]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-Related Shift in LTD Is Dependent on Neuronal Adenosine A2A Receptors Interplay with mGluR5 and NMDA Receptors. Mol. Psychiatry 2020, 25, 1876–1900. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.Q.; Lopes, J.P.; Silva, H.B.; Lemos, C.; Silva, A.C.; Gonçalves, N.; Tomé, Â.R.; Ferreira, S.G.; Canas, P.M.; Rial, D.; et al. Synaptic and Memory Dysfunction in a β-Amyloid Model of Early Alzheimer’s Disease Depends on Increased Formation of ATP-Derived Extracellular Adenosine. Neurobiol. Dis. 2019, 132, 104570. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Andrés, P.; Albasanz, J.L.; Ferrer, I.; Martín, M. Purine-Related Metabolites and Their Converting Enzymes Are Altered in Frontal, Parietal and Temporal Cortex at Early Stages of Alzheimer’s Disease Pathology. Brain Pathol. 2018, 28, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Gonçalves, F.Q.; Canas, P.M.; Oses, J.-P.; Fernandes, F.D.; Duarte, F.V.; Palmeira, C.M.; Tomé, A.R.; Agostinho, P.; Andrade, G.M.; et al. Enhanced ATP Release and CD73-Mediated Adenosine Formation Sustain Adenosine A2A Receptor over-Activation in a Rat Model of Parkinson’s Disease. Br. J. Pharmacol. 2019, 176, 3666–3680. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Fedele, D.; Millner, D.; Alharfoush, E.; Vegunta, G.; Boison, D. Adenosine Kinase: An Epigenetic Modulator in Development and Disease. Neurochem. Int. 2021, 147, 105054. [Google Scholar] [CrossRef] [PubMed]
- Boison, D. Modulators of Nucleoside Metabolism in the Therapy of Brain Diseases. Curr. Top Med. Chem. 2011, 11, 1068–1086. [Google Scholar] [CrossRef]
- Alonso, P.; Albasanz, J.L.; Martín, M. Modulation of Adenosine Receptors by Hops and Xanthohumol in Cell Cultures. ACS Chem. Neurosci. 2021, 12, 2373–2384. [Google Scholar] [CrossRef]
- Niederau, C.; Bhargava, S.; Schneider-Kramman, R.; Jankowski, J.; Craveiro, R.B.; Wolf, M. Xanthohumol Exerts Anti-Inflammatory Effects in an in Vitro Model of Mechanically Stimulated Cementoblasts. Sci. Rep. 2022, 12, 14970. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Alorda-Clara, M.; Martínez-Vigara, M.; Roca, P.; Sastre-Serra, J.; Oliver, J.; Pons, D.G. Xanthohumol Reduces Inflammation and Cell Metabolism in HT29 Primary Colon Cancer Cells. Int. J. Food Sci. Nutr. 2022, 73, 471–479. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The Impact of Hop Bitter Acid and Polyphenol Profiles on the Perceived Bitterness of Beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Blanco Ayala, T.; Pérez de la Cruz, V.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Deng, T.; Zhai, Z.; Sun, T.; Xu, Y. The Cellular Model for Alzheimer’s Disease Research: PC12 Cells. Front. Mol. Neurosci. 2022, 15, 1016559. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Choi, E.M. Cytoprotective Effects of Xanthohumol against Methylglyoxal-Induced Cytotoxicity in MC3T3-E1 Osteoblastic Cells. J. Appl. Toxicol. 2018, 38, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Chen, J.-F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.-M. Adenosine and Brain Function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, A.F.; Malva, J.O.; Carvalho, A.P.; Carvalho, C.M. Inhibition of N-,P/Q- and Other Types of Ca2+ Channels in Rat Hippocampal Nerve Terminals by the Adenosine A1 Receptor. Eur. J. Pharmacol. 1997, 340, 301–310. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Perez, S.; Barrachina, M.; Ferrer, I.; Martín, M. Up-Regulation of Adenosine Receptors in the Frontal Cortex in Alzheimer’s Disease. Brain Pathol. 2008, 18, 211–219. [Google Scholar] [CrossRef]
- Muñoz-López, S.; Sánchez-Melgar, A.; Martín, M.; Albasanz, J.L. Resveratrol Enhances A1 and Hinders A2A Adenosine Receptors Signaling in Both HeLa and SH-SY5Y Cells: Potential Mechanism of Its Antitumoral Action. Front. Endocrinol. 2022, 13, 1007801. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Albasanz, J.L.; Palomera-Ávalos, V.; Pallàs, M.; Martín, M. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol. Neurobiol. 2019, 56, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Oliver, M.; Díaz-Ríos, M. Using Caffeine and Other Adenosine Receptor Antagonists and Agonists as Therapeutic Tools against Neurodegenerative Diseases: A Review. Life Sci. 2014, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.A.; Albasanz, J.L.; León, D.; Jordán, J.; Pallàs, M.; Camins, A.; Martín, M. Age-Related Expression of Adenosine Receptors in Brain from the Senescence-Accelerated Mouse. Exp. Gerontol. 2009, 44, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lezmy, J.; Arancibia-Cárcamo, I.L.; Quintela-López, T.; Sherman, D.L.; Brophy, P.J.; Attwell, D. Astrocyte Ca2+-Evoked ATP Release Regulates Myelinated Axon Excitability and Conduction Speed. Science 2021, 374, eabh2858. [Google Scholar] [CrossRef] [PubMed]
- Sobolczyk, M.; Boczek, T. Astrocytic Calcium and cAMP in Neurodegenerative Diseases. Front. Cell Neurosci. 2022, 16, 889939. [Google Scholar] [CrossRef] [PubMed]
- Zyuz’kov, G.N.; Miroshnichenko, L.A.; Kotlovskaya, L.Y.; Chaikovskii, A.V. The Role of cAMP-Dependent Intracellular Signaling Pathways in the Regulation of the Functions of Neural Stem Cells and Neuroglial Cells in Amyloid-β-Induced Neurodegeneration. Bull. Exp. Biol. Med. 2023, 175, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Schnecko, A.; Witte, K.; Bohl, J.; Ohm, T.; Lemmer, B. Adenylyl Cyclase Activity in Alzheimer’s Disease Brain: Stimulatory and Inhibitory Signal Transduction Pathways Are Differently Affected. Brain Res. 1994, 644, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular Function and Molecular Structure of Ecto-Nucleotidases. Purinergic Signal 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Grković, I.; Drakulić, D.; Martinović, J.; Mitrović, N. Role of Ectonucleotidases in Synapse Formation During Brain Development: Physiological and Pathological Implications. Curr. Neuropharmacol. 2019, 17, 84–98. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Ribeiro, J.A. Neuromodulation and Metamodulation by Adenosine: Impact and Subtleties upon Synaptic Plasticity Regulation. Brain Res. 2015, 1621, 102–113. [Google Scholar] [CrossRef]
- Coppi, E.; Cellai, L.; Maraula, G.; Dettori, I.; Melani, A.; Pugliese, A.M.; Pedata, F. Role of Adenosine in Oligodendrocyte Precursor Maturation. Front. Cell Neurosci. 2015, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Guo, Z.; Hu, Y.; Mai, W.; Zhang, Z.; Zhang, B.; Ge, Q.; Lou, H.; Guo, F.; Chen, J.; et al. CD73-Derived Adenosine Controls Inflammation and Neurodegeneration by Modulating Dopamine Signalling. Brain 2019, 142, 700–718. [Google Scholar] [CrossRef] [PubMed]
- Augusto, E.; Matos, M.; Sévigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.-F. Ecto-5′- Nucleotidase (CD73)-Mediated Formation of Adenosine Is Critical for the Striatal Adenosine A2A Receptor Functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef]
- Augusto, E.; Gonçalves, F.Q.; Real, J.E.; Silva, H.B.; Pochmann, D.; Silva, T.S.; Matos, M.; Gonçalves, N.; Tomé, Â.R.; Chen, J.-F.; et al. Increased ATP Release and CD73-Mediated Adenosine A2A Receptor Activation Mediate Convulsion-Associated Neuronal Damage and Hippocampal Dysfunction. Neurobiol. Dis. 2021, 157, 105441. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Pochmann, D.; Lemos, C.; Silva, H.B.; Real, J.I.; Gonçalves, F.Q.; Rial, D.; Gonçalves, N.; Simões, A.P.; Ferreira, S.G.; et al. Increased Synaptic ATP Release and CD73-Mediated Formation of Extracellular Adenosine in the Control of Behavioral and Electrophysiological Modifications Caused by Chronic Stress. ACS Chem. Neurosci. 2023, 14, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Alçada-Morais, S.; Gonçalves, N.; Moreno-Juan, V.; Andres, B.; Ferreira, S.; Marques, J.M.; Magalhães, J.; Rocha, J.M.M.; Xu, X.; Partidário, M.; et al. Adenosine A2A Receptors Contribute to the Radial Migration of Cortical Projection Neurons through the Regulation of Neuronal Polarization and Axon Formation. Cereb. Cortex 2021, 31, 5652–5663. [Google Scholar] [CrossRef]
- Colopi, A.; Fuda, S.; Santi, S.; Onorato, A.; Cesarini, V.; Salvati, M.; Balistreri, C.R.; Dolci, S.; Guida, E. Impact of Age and Gender on Glioblastoma Onset, Progression, and Management. Mech. Ageing Dev. 2023, 211, 111801. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejero, A.; León-Navarro, D.A.; Martín, M. Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines. Nutrients 2024, 16, 1792. https://doi.org/10.3390/nu16111792
Tejero A, León-Navarro DA, Martín M. Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines. Nutrients. 2024; 16(11):1792. https://doi.org/10.3390/nu16111792
Chicago/Turabian StyleTejero, Adrián, David Agustín León-Navarro, and Mairena Martín. 2024. "Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines" Nutrients 16, no. 11: 1792. https://doi.org/10.3390/nu16111792
APA StyleTejero, A., León-Navarro, D. A., & Martín, M. (2024). Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines. Nutrients, 16(11), 1792. https://doi.org/10.3390/nu16111792






