Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot
Abstract
1. Introduction
2. Materials and Methods
3. Standard Versus Individualized Parenteral Nutrition
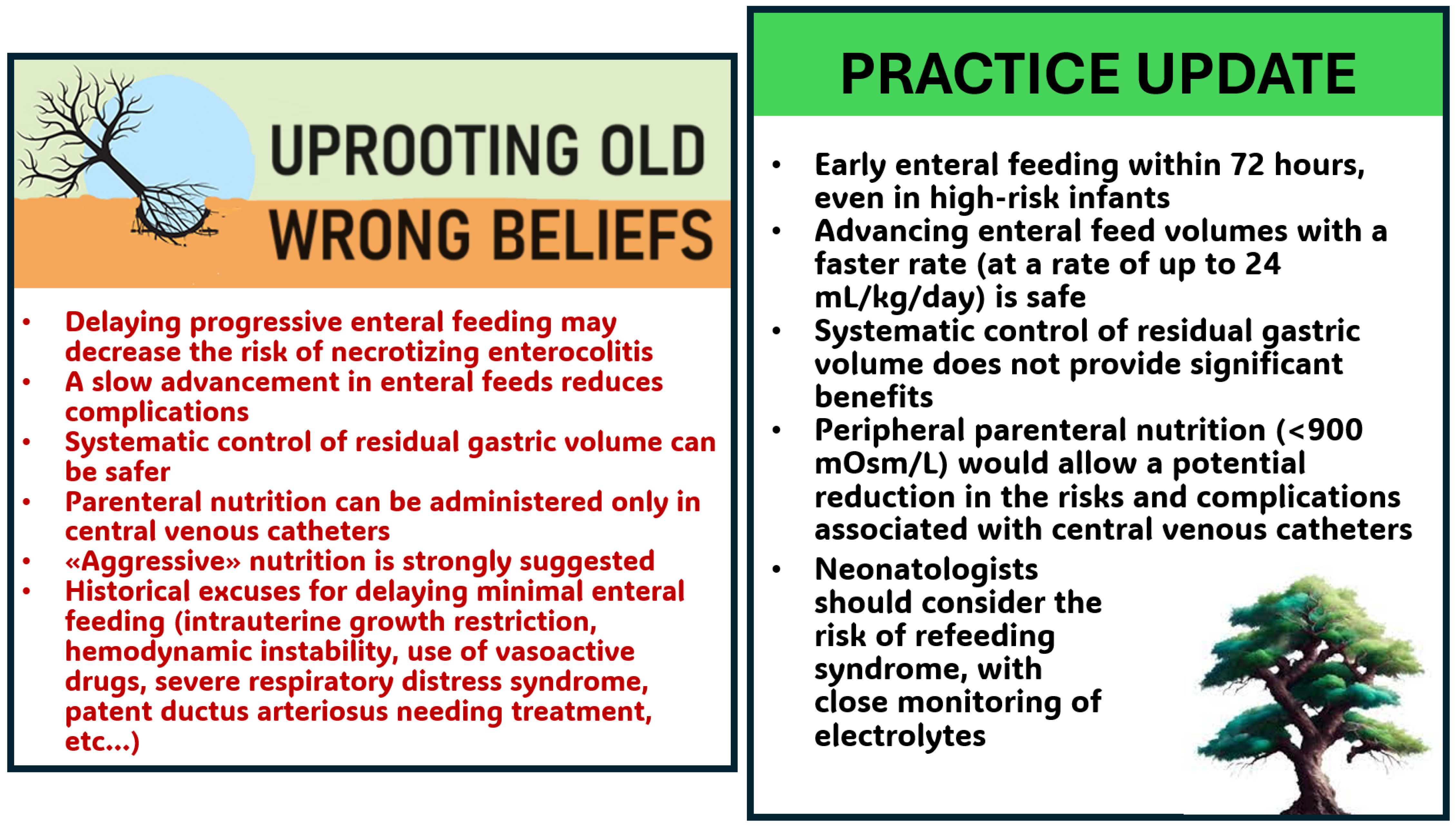
4. Early Enteral Feeding and Fast Advancement in the Preterm Infant
5. Early Enteral Feeding and Fast Advancement
6. The Crucial Role of Human Milk
7. The Importance of Fortification of Human Milk
7.1. Available Fortifiers
7.2. Fortification of Human Milk in Preterm Infants
7.3. Fortification of Human Milk in Preterm Infants Undergoing Surgery
8. The Role of Fetal Growth Restriction and Prenatal Doppler Anomalies
9. Routine Monitoring of Gastric Residual for Prevention of NEC in Preterm Infants
10. Peripheral Parenteral Nutrition
11. Refeeding Syndrome in Preterm Infants
12. The Relationship between Respiratory Distress Syndrome and Enteral Nutrition Tolerance
13. Higher Volume versus Standard-Volume Enteral Feeds
14. Antibiotics and Acid Suppressants Can Impact the Development of Gut Microbiome
15. New Monitoring Tools
16. Extrauterine Growth Restriction
17. New Frontiers: Artificial Intelligence and Nutrition
18. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silveira, R.C.; Corso, A.L.; Procianoy, R.S. The Influence of Early Nutrition on Neurodevelopmental Outcomes in Preterm Infants. Nutrients 2023, 15, 4644. [Google Scholar] [CrossRef] [PubMed]
- Morniroli, D.; Tiraferri, V.; Maiocco, G.; De Rose, D.U.; Cresi, F.; Coscia, A.; Mosca, F.; Giannì, M.L. Beyond Survival: The Lasting Effects of Premature Birth. Front. Pediatr. 2023, 11, 1213243. [Google Scholar] [CrossRef] [PubMed]
- Gallini, F.; Coppola, M.; De Rose, D.U.; Maggio, L.; Arena, R.; Romano, V.; Cota, F.; Ricci, D.; Romeo, D.M.; Mercuri, E.M.; et al. Neurodevelopmental Outcomes in Very Preterm Infants: The Role of Severity of Bronchopulmonary Dysplasia. Early Hum. Dev. 2021, 152, 105275. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Cota, F.; Gallini, F.; Bottoni, A.; Fabrizio, G.C.; Ricci, D.; Romeo, D.M.; Mercuri, E.; Vento, G.; Maggio, L. Extra-Uterine Growth Restriction in Preterm Infants: Neurodevelopmental Outcomes According to Different Definitions. Eur. J. Paediatr. Neurol. 2021, 33, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rozé, J.C.; Morel, B.; Lapillonne, A.; Marret, S.; Guellec, I.; Darmaun, D.; Bednarek, N.; Moyon, T.; Marchand-Martin, L.; Benhammou, V.; et al. Association Between Early Amino Acid Intake and Full-Scale IQ at Age 5 Years Among Infants Born at Less Than 30 Weeks’ Gestation. JAMA Netw. Open 2021, 4, E2135452. [Google Scholar] [CrossRef]
- Robinson, D.T.; Calkins, K.L.; Chen, Y.; Cober, M.P.; Falciglia, G.H.; Church, D.D.; Mey, J.; McKeever, L.; Sentongo, T. Guidelines for Parenteral Nutrition in Preterm Infants: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2023, 47, 830–858. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S. Feeding Intolerance in the Preterm Infant. Early Hum. Dev. 2013, 89, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.K.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.S.; et al. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef]
- Escobar, M.A.; Caty, M.G. Complications in Neonatal Surgery. Semin. Pediatr. Surg. 2016, 25, 347–370. [Google Scholar] [CrossRef]
- Moltu, S.J.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; De Koning, B.; Lapillonne, A.; Norsa, L.; Verduci, E.; et al. Nutritional Management of the Critically Ill Neonate: A Position Paper of the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 274–289. [Google Scholar] [CrossRef]
- Mo, I.; Lapillonne, A.; Khashu, M.; Johnson, M.J.; McElroy, S.J.; Van Den Akker, C.H.P.; Zachariassen, G. ESPR Nutrition Council Members Nutritional Management after Medical and Surgical Necrotizing Enterocolitis in Preterm Infants. Pediatr. Res. 2024, in press. [Google Scholar]
- Nagel, E.M.; Elgersma, K.M.; Gallagher, T.T.; Johnson, K.E.; Demerath, E.; Gale, C.A. Importance of Human Milk for Infants in the Clinical Setting: Updates and Mechanistic Links. Nutr. Clin. Pract. 2023, 38, S39–S55. [Google Scholar] [CrossRef] [PubMed]
- Riera, P.; Garrido-Alejos, G.; Cardenete, J.; Moliner, E.; Zapico-Muñiz, E.; Cardona, D.; Garin, N. Physicochemical Stability and Sterility of Standard Parenteral Nutrition Solutions and Simulated Y-Site Admixtures for Neonates. Nutr. Clin. Pract. 2018, 33, 694–700. [Google Scholar] [CrossRef] [PubMed]
- De Cloet, J.; Van Biervliet, S.; Van Winckel, M. Physicochemical stable standard all-in-one parenteral nutrition admix-tures for infants and children in accordance with the ESPGHAN/ESPEN guidelines. Nutrition 2018, 49, 41–47. [Google Scholar] [CrossRef]
- Yeung, M.Y.; Smyth, J.P.; Maheshwari, R.; Shah, S. Evaluation of Standardized versus Individualized Total Parenteral Nutrition Regime for Neonates Less than 33 Weeks Gestation. J. Paediatr. Child. Health 2003, 39, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Lenclen, R.; Crauste-Manciet, S.; Narcy, P.; Boukhouna, S.; Geffray, A.; Guerrault, M.N.; Bordet, F.; Brossard, D. Assessment of Implementation of a Standardized Parenteral Formulation for Early Nutritional Support of Very Preterm Infants. Eur. J. Pediatr. 2006, 165, 512–518. [Google Scholar] [CrossRef]
- Morgan, C.; Tan, M. Attainment Targets for Protein Intake Using Standardised, Concentrated and Individualised Neonatal Parenteral Nutrition Regimens. Nutrients 2019, 11, 2167. [Google Scholar] [CrossRef]
- Immeli, L.; Mäkelä, P.M.; Leskinen, M.; Rinta-Koski, O.P.; Sund, R.; Andersson, S.; Luukkainen, P. A Triple-Chamber Parenteral Nutrition Solution Was Associated with Improved Protein Intake in Very Low Birthweight Infants. Acta Paediatr. 2020, 109, 1588–1594. [Google Scholar] [CrossRef]
- Riskin, A.; Picaud, J.C.; Shamir, R.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Standard versus Individualized Parenteral Nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef]
- Dice, J.E.; Burckart, G.J.; Woo, J.T.; Helms, R.A. Standardized versus Pharmacist-Monitored Individualized Parenteral Nutrition in Low-Birth-Weight Infants. Am. J. Hosp. Pharm. 1981, 38, 1487–1489. [Google Scholar] [CrossRef]
- Smolkin, T.; Diab, G.; Shohat, I.; Jubran, H.; Blazer, S.; Rozen, G.S.; Makhoul, I.R. Standardized versus Individualized Parenteral Nutrition in Very Low Birth Weight Infants: A Comparative Study. Neonatology 2010, 98, 170–178. [Google Scholar] [CrossRef]
- Mihatsch, W.; Jiménez Varas, M.Á.; Diehl, L.L.; Carnielli, V.; Schuler, R.; Gebauer, C.; Sáenz de Pipaón Marcos, M. Systematic Review on Individualized Versus Standardized Parenteral Nutrition in Preterm Infants. Nutrients 2023, 15, 1224. [Google Scholar] [CrossRef]
- Thoene, M.; Anderson-Berry, A. Early Enteral Feeding in Preterm Infants: A Narrative Review of the Nutritional, Metabolic, and Developmental Benefits. Nutrients 2021, 13, 2289. [Google Scholar] [CrossRef]
- Wildhaber, B.E.; Yang, H.; Spencer, A.U.; Drongowski, R.A.; Teitelbaum, D.H. Lack of Enteral Nutrition—Effects on the Intestinal Immune System. J. Surg. Res. 2005, 123, 8–16. [Google Scholar] [CrossRef]
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal Changes in the Gut Microbiome of Infants on Total Parenteral Nutrition. Pediatr. Res. 2019, 86, 107–114. [Google Scholar] [CrossRef]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; Van Den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; Van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Konnikova, Y.; Zaman, M.M.; Makda, M.; D’Onofrio, D.; Freedman, S.D.; Martin, C.R. Late Enteral Feedings Are Associated with Intestinal Inflammation and Adverse Neonatal Outcomes. PLoS ONE 2015, 10, e0132924. [Google Scholar] [CrossRef]
- Young, L.; Oddie, S.J.; McGuire, W. Delayed Introduction of Progressive Enteral Feeds to Prevent Necrotising Enterocolitis in Very Low Birth Weight Infants. Cochrane Database Syst. Rev. 2022, 1, CD001970. [Google Scholar] [CrossRef]
- Salas, A.A.; Travers, C.P. The Practice of Enteral Nutrition: Clinical Evidence for Feeding Protocols. Clin. Perinatol. 2023, 50, 607–623. [Google Scholar] [CrossRef]
- Rozé, J.C.; Ancel, P.Y.; Lepage, P.; Martin-Marchand, L.; Nabhani, Z.A.; Delannoy, J.; Picaud, J.C.; Lapillonne, A.; Aires, J.; Durox, M.; et al. Nutritional Strategies and Gut Microbiota Composition as Risk Factors for Necrotizing Enterocolitis in Very-Preterm Infants. Am. J. Clin. Nutr. 2017, 106, 821–830. [Google Scholar] [CrossRef]
- Oddie, S.J.; Young, L.; Mcguire, W. Slow Advancement of Enteral Feed Volumes to Prevent Necrotising Enterocolitis in Very Low Birth Weight Infants. Cochrane Database Syst. Rev. 2021, 8, CD001241. [Google Scholar] [CrossRef]
- Itriago, E.; Trahan, K.F.; Massieu, L.A.; Garg, P.M.; Premkumar, M.H. Current Practices, Challenges, and Recommendations in Enteral Nutrition After Necrotizing Enterocolitis. Clin. Perinatol. 2023, 50, 683–698. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.U.; Santisi, A.; Martini, L.; Ronchetti, M.P.; Ravà, L.; Antenucci, V.; Bernaschi, P.; Serafini, L.; Catarzi, S.; et al. Incidence and Risk Factors of Bacterial Sepsis and Invasive Fungal Infection in Neonates and Infants Requiring Major Surgery: An Italian Multicentre Prospective Study. J. Hosp. Infect. 2022, 130, 122–130. [Google Scholar] [CrossRef]
- Ahmad, I.; Premkumar, M.H.; Hair, A.B.; Sullivan, K.M.; Zaniletti, I.; Sharma, J.; Nayak, S.P.; Reber, K.M.; Padula, M.; Brozanski, B.; et al. Variability in Antibiotic Duration for Necrotizing Enterocolitis and Outcomes in a Large Multicenter Cohort. J. Perinatol. 2022, 42, 1458–1464. [Google Scholar] [CrossRef]
- Bohnhorst, B.; Müller, S.; Dördelmann, M.; Peter, C.S.; Petersen, C.; Poets, C.F. Early Feeding after Necrotizing Enterocolitis in Preterm Infants. J. Pediatr. 2003, 143, 484–487. [Google Scholar] [CrossRef]
- Patel, E.U.; Wilson, D.A.; Brennan, E.A.; Lesher, A.P.; Ryan, R.M. Earlier Re-Initiation of Enteral Feeding after Necrotizing Enterocolitis Decreases Recurrence or Stricture: A Systematic Review and Meta-Analysis. J. Perinatol. 2020, 40, 1679–1687. [Google Scholar] [CrossRef]
- Hock, A.M.; Chen, Y.; Miyake, H.; Koike, Y.; Seo, S.; Pierro, A. Initiation of Enteral Feeding after Necrotizing Enterocolitis. Eur. J. Pediatr. Surg. 2018, 28, 44–50. [Google Scholar] [CrossRef]
- Norsa, L.; Goulet, O.; Alberti, D.; Dekooning, B.; Domellöf, M.; Haiden, N.; Hill, S.; Indrio, F.; Kglmeier, J.; Lapillonne, A.; et al. Nutrition and Intestinal Rehabilitation of Children with Short Bowel Syndrome: A Position Paper of the ESPGHAN Committee on Nutrition. Part 1: From Intestinal Resection to Home Discharge. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 281–297. [Google Scholar] [CrossRef]
- Savoie, K.B.; Bachier-Rodriguez, M.; Jones, T.L.; Jeffreys, K.; Papraniku, D.; Sevilla, W.M.A.; Tillman, E.; Huang, E.Y. Standardization of Feeding Advancement after Neonatal Gastrointestinal Surgery: Does It Improve Outcomes? Nutr. Clin. Pract. 2016, 31, 810–818. [Google Scholar] [CrossRef]
- Passaro, R.C.; Savoie, K.B.; Huang, E.Y. Use of a Gastroschisis Feeding Guideline to Improve Standardization of Care and Patient Outcomes at an Urban Children’s Hospital. Nutr. Clin. Pract. 2018, 33, 545–552. [Google Scholar] [CrossRef]
- Gosselin, K.B.; Duggan, C. Enteral Nutrition in the Management of Pediatric Intestinal Failure. J. Pediatr. 2014, 165, 1085–1090. [Google Scholar] [CrossRef]
- Mayer, O.; Kerner, J.A. Management of Short Bowel Syndrome in Postoperative Very Low Birth Weight Infants. Semin. Fetal Neonatal Med. 2017, 22, 49–56. [Google Scholar] [CrossRef]
- Chandra, R.; Kesavan, A. Current Treatment Paradigms in Pediatric Short Bowel Syndrome. Clin. J. Gastroenterol. 2018, 11, 103–112. [Google Scholar] [CrossRef]
- Kim, J.H. Providing Optimal Nutrition to Very Low Birthweight Infants in the NICU. Neoreviews 2023, 24, E271–E284. [Google Scholar] [CrossRef]
- Salvatori, G.; Foligno, S.; Occasi, F.; Pannone, V.; Valentini, G.B.; Dall’oglio, I.; Bagolan, P.; Dotta, A. Human Milk and Breastfeeding in Surgical Infants. Breastfeed. Med. 2014, 9, 491–493. [Google Scholar] [CrossRef]
- Gialeli, G.; Panagopoulou, O.; Liosis, G.; Siahanidou, T. Potential Epigenetic Effects of Human Milk on Infants’ Neurodevelopment. Nutrients 2023, 15, 3614. [Google Scholar] [CrossRef]
- Mitguard, S.; Doucette, O.; Miklavcic, J. Human Milk Polyunsaturated Fatty Acids Are Related to Neurodevelopmental, Anthropometric, and Allergic Outcomes in Early Life: A Systematic Review. J. Dev. Orig. Health Dis. 2024, 14, 1–10. [Google Scholar] [CrossRef]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in Nutrition of the Newborn Infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827. [Google Scholar] [CrossRef]
- Abrams, S.A.; Landers, S.; Noble, L.M.; Poindexter, B.B. Donor Human Milk for the High- Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Walsh, V.; McGuire, W. Formula versus Maternal Breast Milk for Feeding Preterm or Low Birth Weight Infants. Cochrane Database Syst. Rev. 2019, 8, CD002972. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Cavallarin, L.; Cresi, F.; Tonetto, P.; Peila, C.; Ansaldi, G.; Raia, M.; Varalda, A.; Giribaldi, M.; Conti, A.; et al. A Novel Donkey Milk-Derived Human Milk Fortifier in Feeding Preterm Infants: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Golombek, S.G. Evolution of Preterm Infant Nutrition from Breastfeeding to an Exclusive Human Milk Diet: A Review. Neoreviews 2022, 23, e558–e571. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Gunawan, E.; Nguyen, K.; Reeves, A.; Argent, V.; Finck, A.; Carlo, W.A. Early Human Milk Fortification in Infants Born Extremely Preterm: A Randomized Trial. Pediatrics 2023, 152, e2023061603. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.R.; Becker, A.; Fox, J.; Horgan, M.; Moores, R.; Pardalos, J.; Pinheiro, J.; Stewart, D.; Robinson, T. Implementing an Exclusive Human Milk Diet for Preterm Infants: Real-World Experience in Diverse NICUs. BMC Pediatr. 2023, 23, 237. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Ziegler, E.E. WAPM Working Group on Nutrition Optimization of Human Milk Fortification for Preterm Infants: New Concepts and Recommendations. J. Perinat. Med. 2010, 38, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Burnham, L.A.; Kerr, S.; Belfort, M.B.; Perrin, M.; Corwin, M.; Heeren, T. Prevalence and Predictors of Donor Milk Programs among U.S. Advanced Neonatal Care Facilities. J. Perinatol. 2020, 40, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Kreins, N.; Buffin, R.; Michel-Molnar, D.; Chambon, V.; Pradat, P.; Picaud, J.C. Individualized Fortification Influences the Osmolality of Human Milk. Front. Pediatr. 2018, 6, 322. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Lin, L.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-Nutrient Fortification of Human Milk for Preterm Infants. Cochrane Database Syst. Rev. 2020, 6, CD000343. [Google Scholar] [CrossRef]
- Adhisivam, B.; Kohat, D.; Tanigasalam, V.; Bhat, V.; Plakkal, N.; Palanivel, C. Does Fortification of Pasteurized Donor Human Milk Increase the Incidence of Necrotizing Enterocolitis among Preterm Neonates? A Randomized Controlled Trial. J. Matern. Fetal Neonatal Med. 2019, 32, 3232–3237. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.B.; Domellöf, M.; Ahlsson, F.; Elfvin, A.; Navér, L.; Abrahamsson, T. Effect of Human Milk-Based Fortification in Extremely Preterm Infants Fed Exclusively with Breast Milk: A Randomised Controlled Trial. EClinicalMedicine 2024, 68, 102375. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Veldman, A.; Menahem, S. Does Milk Fortification Increase the Risk of Necrotising Enterocolitis in Preterm Infants with Congenital Heart Disease? Cardiol. Young 2013, 23, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.L.; Hair, A.; Justice, L.B.; Roddy, D.; Bonagurio, K.; Williams, P.K.; Machado, D.; Marino, B.S.; Chi, A.; Takao, C.; et al. A Randomized Trial of an Exclusive Human Milk Diet in Neonates with Single Ventricle Physiology. J. Pediatr. 2023, 256, 105–112.e4. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.R.; Xie, W.P.; Liu, J.F.; Wang, L.W.; Cao, H.; Chen, Q. Effect of the Addition of Human Milk Fortifier to Breast Milk on the Early Recovery of Infants After Congenital Cardiac Surgery. Front. Pediatr. 2021, 9, 661927. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.P.; Kirkham, E.N.; Hawton, K.A.; Mannix, P.A. Feeding Growth Restricted Premature Neonates: A Challenging Perspective. Sudan. J. Paediatr. 2018, 18, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, V.; Tagliabue, P.E. Enteral Feeding of Intrauterine Growth Restriction Preterm Infants: Theoretical Risks and Practical Implications. Pediatr. Med. Chir. 2017, 39, 160. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.; Kempley, S.; Leaf, A. Feeding Growth Restricted Preterm Infants with Abnormal Antenatal Doppler Results. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, 359–364. [Google Scholar] [CrossRef]
- Martini, S.; Annunziata, M.; Della Gatta, A.N.; Aceti, A.; Brunetti, M.; Pilu, G.; Simonazzi, G.; Corvaglia, L. Association between Abnormal Antenatal Doppler Characteristics and Gastrointestinal Outcomes in Preterm Infants. Nutrients 2022, 14, 5121. [Google Scholar] [CrossRef]
- Tewari, V.V.; Dubey, S.K.; Kumar, R.; Vardhan, S.; Sreedhar, C.M.; Gupta, G. Early versus Late Enteral Feeding in Preterm Intrauterine Growth Restricted Neonates with Antenatal Doppler Abnormalities: An Open-Label Randomized Trial. J. Trop. Pediatr. 2018, 64, 4–14. [Google Scholar] [CrossRef]
- Li, Y.F.; Lin, H.C.; Torrazza, R.M.; Parker, L.; Talaga, E.; Neu, J. Gastric Residual Evaluation in Preterm Neonates: A Useful Monitoring Technique or a Hindrance? Pediatr. Neonatol. 2014, 55, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Thanigainathan, S.; Ramaswamy, V.V.; Rajaiah, B.; Ramakrishnan, S. Routine Monitoring of Gastric Residual for Prevention of Necrotising Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2023, 6, CD012937. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kler, N.; Saluja, S.; Modi, M.; Soni, A.; Thakur, A.; Garg, P. Abdominal Circumference or Gastric Residual Volume as Measure of Feed Intolerance in VLBW Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Torrazza, R.M.; Parker, L.A.; Li, Y.; Shuster, J.; Neu, J. The Value of Routine Evaluation of Gastric Residuals in Very Low Birth Weight Infants. J. Perinatol. 2015, 35, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Cohen, K.; Kugelman, A.; Toropine, A.; Said, W.; Bader, D. The Impact of Routine Evaluation of Gastric Residual Volumes on the Time to Achieve Full Enteral Feeding in Preterm Infants. J. Pediatr. 2017, 189, 128–134. [Google Scholar] [CrossRef]
- Parker, L.A.; Weaver, M.; Murgas Torrazza, R.J.; Shuster, J.; Li, N.; Krueger, C.; Neu, J. Effect of Gastric Residual Evaluation on Enteral Intake in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Thanigainathan, S.; Ramaswamy, V.V.; Rajaiah, B.; Ramakrishnan, S. Re-Feeding versus Discarding Gastric Residuals to Improve Growth in Preterm Infants. Cochrane Database Syst. Rev. 2023, 6, CD012940. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, H.; Bonney, D.; Andersen, C.C.; McPhee, A.J.; Sullivan, T.R.; Gibson, R.A.; Collins, C.T. The Efficacy and Safety of Peripheral Intravenous Parenteral Nutrition vs 10% Glucose in Preterm Infants Born 30 to 33 Weeks’ Gestation: A Randomised Controlled Trial. BMC Pediatr. 2020, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Shamir, R.; Simchowitz, V.; Lohner, S.; Cai, W.; Decsi, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Complications. Clin. Nutr. 2018, 37, 2418–2429. [Google Scholar] [CrossRef]
- Dugan, S.; Le, J.; Jew, R.K. Maximum Tolerated Osmolarity for Peripheral Administration of Parenteral Nutrition in Pediatric Patients. JPEN J. Parenter. Enteral Nutr. 2014, 38, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Fessler, A.G.; Rejrat, C.E. Re-Evaluating Safe Osmolarity for Peripheral Parenteral Nutrition in Neonatal Intensive Care Patients. J. Pediatr. Pharmacol. Ther. 2021, 26, 632–637. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.T.; Taylor, S.N.; Moya, F. Preterm Infant Nutrition: Considerations for Infants at Risk of Refeeding Syndrome. J. Perinatol. 2023, 43, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.D.; Btaiche, I.F.; Sacks, G.S. Review of the Refeeding Syndrome. Nutr. Clin. Pract. 2005, 20, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.V.; Cober, M.P.; Miller, J.L. Refeeding Syndrome in the Neonatal Intensive Care Unit. J. Pediatr. Pharmacol. Ther. 2021, 26, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.R.; Finch, C.; Ebeling, M.; Taylor, S.N. Refeeding Syndrome in Very-Low-Birth-Weight Intrauterine Growth-Restricted Neonates. J. Perinatol. 2013, 33, 717–720. [Google Scholar] [CrossRef]
- Bonsante, F.; Iacobelli, S.; Latorre, G.; Rigo, J.; de Felice, C.; Robillard, P.Y.; Gouyon, J.B. Initial Amino Acid Intake Influences Phosphorus and Calcium Homeostasis in Preterm Infants—It Is Time to Change the Composition of the Early Parenteral Nutrition. PLoS ONE 2013, 8, e72880. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced Feeding in Very-Low-Birth-Weight Infants May Cause Electrolyte Disturbances and Septicemia—A Randomized, Controlled Trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H. Neonatal Refeeding Syndrome and Clinical Outcome in Extremely Low-Birth-Weight Babies: Secondary Cohort Analysis From the ProVIDe Trial. JPEN J. Parenter. Enteral Nutr. 2021, 45, 65–78. [Google Scholar] [CrossRef]
- Bozzetti, V.; De Angelis, C.; Tagliabue, P.E. Nutritional Approach to Preterm Infants on Noninvasive Ventilation: An Update. Nutrition 2017, 37, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Cresi, F.; Maggiora, E.; Lista, G.; Dani, C.; Borgione, S.M.; Spada, E.; Ferroglio, M.; Bertino, E.; Coscia, A. Effect of Nasal Continuous Positive Airway Pressure vs Heated Humidified High-Flow Nasal Cannula on Feeding Intolerance in Preterm Infants with Respiratory Distress Syndrome: The ENTARES Randomized Clinical Trial. JAMA Netw. Open 2023, 6, E2323052. [Google Scholar] [CrossRef] [PubMed]
- Amendolia, B.; Fisher, K.; Wittmann-Price, R.A.; Bloch, J.R.; Gardner, M.; Basit, M.; Aghai, Z.H. Feeding Tolerance in Preterm Infants on Noninvasive Respiratory Support. J. Perinat. Neonatal Nurs. 2014, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Controzzi, T.; Chesi, F.; Scaramuzzo, R.T.; Giampietri, M.; Morganti, R.; Fiori, S.; Moretti, E.; Gargani, L.; Filippi, L. Lung Ultrasound Supports Clinical Evaluation of Feeding Competence Development in Preterm Neonates. Front. Pediatr. 2023, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Thomas, N.; Thanigainathan, S. High versus Standard Volume Enteral Feeds to Promote Growth in Preterm or Low Birth Weight Infants. Cochrane Database Syst. Rev. 2021, 3, CD012413. [Google Scholar] [CrossRef] [PubMed]
- Prusakov, P.; Goff, D.A.; Wozniak, P.S.; Cassim, A.; Scipion, C.E.A.; Urzúa, S.; Ronchi, A.; Zeng, L.; Ladipo-Ajayi, O.; Aviles-Otero, N.; et al. A Global Point Prevalence Survey of Antimicrobial Use in Neonatal Intensive Care Units: The No-More-Antibiotics and Resistance (NO-MAS-R) Study. EClinicalMedicine 2021, 32, 100727. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Pyle, A.K.; Wozniak, P.S.; Hynan, L.S.; Sánchez, P.J. Early Antibiotic Exposure and Adverse Outcomes in Preterm, Very Low Birth Weight Infants. J. Pediatr. 2018, 203, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Morowitz, M.J.; Sampath, V. Early Antibiotics and Risk for Necrotizing Enterocolitis in Premature Infants: A Narrative Review. Front. Pediatr. 2023, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bernabè, G.; Shalata, M.E.M.; Zatta, V.; Bellato, M.; Porzionato, A.; Castagliuolo, I.; Brun, P. Antibiotic Treatment Induces Long-Lasting Effects on Gut Microbiota and the Enteric Nervous System in Mice. Antibiotics 2023, 12, 1000. [Google Scholar] [CrossRef]
- Reyes-García, D.V.; Canul-Euan, A.A.; Rivera-Rueda, M.A.; Cruz-Alvarado, C.E.; Bermejo-Martínez, L.B.; Arreola-Ramírez, G.; Cordero-González, G.; Carrera-Muiños, S.; Diaz-Valencia, J.D.; Estrada-Gutiérrez, G.; et al. Neonatal Antibiotic Treatment Can Affect Stool Pattern and Oral Tolerance in Preterm Infants. Life 2022, 12, 1043. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Wu, F.; Mao, J.; Liu, L.; Zhang, R.; Shen, W.; Tang, L.; Chang, Y.; Ye, X.; et al. The Impact of Early Empirical Antibiotics Treatment on Clinical Outcome of Very Preterm Infants: A Nationwide Multicentre Study in China. Ital. J. Pediatr. 2023, 49, 14. [Google Scholar] [CrossRef]
- Terrin, G.; Passariello, A.; De Curtis, M.; Manguso, F.; Salvia, G.; Lega, L.; Messina, F.; Paludetto, R.; Canani, R.B. Ranitidine Is Associated with Infections, Necrotizing Enterocolitis, and Fatal Outcome in Newborns. Pediatrics 2012, 129, e40–e45. [Google Scholar] [CrossRef]
- Aschenbrenner, D.S. Ranitidine Withdrawn from the Market. Am. J. Nurs. 2020, 120, 83. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.I.; Hoang, D.M.; Vandenplas, Y. The Effects of Proton Pump Inhibitors on the Microbiome in Young Children. Acta Paediatr. 2020, 109, 1531–1538. [Google Scholar] [CrossRef]
- Patil, U.P.; Bailey, S.M.; Wachtel, E.V.; Orosz, E.; Zarchin, R.; Mally, P.V. Efficacy of and Potential Morbidities Associated with the Use of Antacid Medications in Preterm Neonates. J. Perinat. Med. 2017, 45, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Hartz, L.E.; Bradshaw, W.; Brandon, D.H. Potential NICU Environmental Influences on the Neonate’s Microbiome: A Systematic Review. Adv. Neonatal Care 2015, 15, 324–335. [Google Scholar] [CrossRef]
- De Rose, D.U.; Santisi, A.; Ronchetti, M.P.; Martini, L.; Serafini, L.; Betta, P.; Maino, M.; Cavigioli, F.; Giuffré, M.; Bonanno, E.; et al. Decreased Incidence of Late-Onset Sepsis during the SARS-CoV-2 Pandemic in Italy: A Multicentric Study on a Cohort of Infants Requiring Major Surgery. Eur. J. Pediatr. 2023, 182, 4859–4866. [Google Scholar] [CrossRef]
- Pavlek, L.R.; Mueller, C.; Jebbia, M.R.; Kielt, M.J.; Fathi, O. Near-Infrared Spectroscopy in Extremely Preterm Infants. Front. Pediatr. 2021, 8, 624113. [Google Scholar] [CrossRef] [PubMed]
- Corvaglia, L.; Martini, S.; Battistini, B.; Rucci, P.; Faldella, G.; Aceti, A. Splanchnic Oxygenation at First Enteral Feeding in Preterm Infants: Correlation with Feeding Intolerance. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 550–554. [Google Scholar] [CrossRef]
- Martini, S.; Aceti, A.; Beghetti, I.; Faldella, G.; Corvaglia, L. Feed-Related Splanchnic Oxygenation in Preterm Infants with Abnormal Antenatal Doppler Developing Gut Complications. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 755–759. [Google Scholar] [CrossRef]
- Kuik, S.J.; Van Zoonen, A.G.J.F.; Bos, A.F.; Van Braeckel, K.N.J.A.; Hulscher, J.B.F.; Kooi, E.M.W. The Effect of Enteral Bolus Feeding on Regional Intestinal Oxygen Saturation in Preterm Infants Is Age-Dependent: A Longitudinal Observational Study. BMC Pediatr. 2019, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Sirota, G.L.; Litmanovitz, I.; Vider, C.; Arnon, S.; Moore, S.S.; Grinblatt, E.; Levkovitz, O.; Rusek, S.B. Regional Splanchnic Oxygenation during Continuous versus Bolus Feeding among Stable Preterm Infants. Children 2022, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Spada, C.; Aceti, A.; Rucci, P.; Gibertoni, D.; Battistini, B.; Arcuri, S.; Faldella, G.; Corvaglia, L. Red Blood Cell Transfusions Alter Splanchnic Oxygenation Response to Enteral Feeding in Preterm Infants: An Observational Pilot Study. Transfusion 2020, 60, 1669–1675. [Google Scholar] [CrossRef]
- Chock, V.Y.; Kirpalani, H.; Bell, E.F.; Tan, S.; Hintz, S.R.; Ball, M.B.; Smith, E.; Das, A.; Loggins, Y.C.; Sood, B.G.; et al. Tissue Oxygenation Changes after Transfusion and Outcomes in Preterm Infants: A Secondary Near-Infrared Spectroscopy Study of the Transfusion of Prematures Randomized Clinical Trial (TOP NIRS). JAMA Netw. Open 2023, 6, E2334889. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Mally, P.V. Near-Infrared Spectroscopy to Guide and Understand Effects of Red Blood Cell Transfusion. Clin. Perinatol. 2023, 50, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Chock, V.Y.; Rose, L.A.; Mante, J.V.; Punn, R. Near-Infrared Spectroscopy for Detection of a Significant Patent Ductus Arteriosus. Pediatr. Res. 2016, 80, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Corvaglia, L.; Aceti, A.; Vitali, F.; Faldella, G.; Galletti, S. Effect of Patent Ductus Arteriosus on Splanchnic Oxygenation at Enteral Feeding Introduction in Very Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 493–497. [Google Scholar] [CrossRef]
- Dani, C.; Corsini, I.; Generoso, M.; Gozzini, E.; Bianconi, T.; Pratesi, S. Splanchnic Tissue Oxygenation for Predicting Feeding Tolerance in Preterm Infants. JPEN J. Parenter. Enteral Nutr. 2015, 39, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Bouhellec, J.L.; Prodhomme, O.; Mura, T.; Jacquot, A.; Combes, C.; Gamon, L.; Durand, S.; Filleron, A.; Cambonie, G. Near-Infrared Spectroscopy: A Tool for Diagnosing Necrotizing Enterocolitis at Onset of Symptoms in Preterm Neonates with Acute Gastrointestinal Symptoms? Am. J. Perinatol. 2021, 38, e299–e308. [Google Scholar] [CrossRef]
- Zozaya, C.; Díaz, C.; De Pipaón, M.S. How Should We Define Postnatal Growth Restriction in Preterm Infants? Neonatology 2018, 114, 177–180. [Google Scholar] [CrossRef]
- Saigal, S.; Doyle, L.W. An Overview of Mortality and Sequelae of Preterm Birth from Infancy to Adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O’Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A. Nutritional Practices and Growth Velocity in the First Month of Life in Extremely Premature Infants. Pediatrics 2009, 124, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Giannì, M.L.; Orsi, A.; Amato, O.; Piemontese, P.; Liotto, N.; Morlacchi, L.; Taroni, F.; Garavaglia, E.; Bracco, B.; et al. Implementation of Nutritional Strategies Decreases Postnatal Growth Restriction in Preterm Infants. PLoS ONE 2012, 7, e51166. [Google Scholar] [CrossRef] [PubMed]
- Maiocco, G.; Migliaretti, G.; Cresi, F.; Peila, C.; Deantoni, S.; Trapani, B.; Giuliani, F.; Bertino, E.; Coscia, A. Evaluation of Head Circumference Extrauterine Growth with Longitudinal Intergrowth-21 St Charts: A New Approach to Identify Very Preterm Infants at Risk of Long-Term Neurodevelopmental Adverse Outcomes. Front. Pediatr. 2020, 8, 572930. [Google Scholar] [CrossRef] [PubMed]
- Chioma, R.; Sbordone, A.; Patti, M.L.; Perri, A.; Vento, G.; Nobile, S. Applications of Artificial Intelligence in Neonatology. Appl. Sci. 2023, 13, 3211. [Google Scholar] [CrossRef]
- Solis-Garcia, G.; Pierro, A.; Jasani, B. Laparotomy versus Peritoneal Drainage as Primary Treatment for Surgical Necrotizing Enterocolitis or Spontaneous Intestinal Perforation in Preterm Neonates: A Systematic Review and Meta-Analysis. Children 2023, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Prgomet, S.; Lukšić, B.; Pogorelić, Z.; Jurić, I.; Čapkun, V.; Arapović, A.; Boban, N. Perinatal Risk Factors in Newborns with Gastrointestinal Perforation. World J. Gastrointest. Surg. 2017, 9, 46. [Google Scholar] [CrossRef]
- Son, J.; Kim, D.; Na, J.Y.; Jung, D.; Ahn, J.H.; Kim, T.H.; Park, H.K. Development of Artificial Neural Networks for Early Prediction of Intestinal Perforation in Preterm Infants. Sci. Rep. 2022, 12, 12112. [Google Scholar] [CrossRef] [PubMed]
- Irles, C.; González-Pérez, G.; Muiños, S.C.; Macias, C.M.; Gómez, C.S.; Martínez-Zepeda, A.; González, G.C.; Servitje, E.L. Estimation of Neonatal Intestinal Perforation Associated with Necrotizing Enterocolitis by Machine Learning Reveals New Key Factors. Int. J. Environ. Res. Public Health 2018, 15, 2509. [Google Scholar] [CrossRef]
- Wong, R.K.; Pitino, M.A.; Mahmood, R.; Zhu, I.Y.; Stone, D.; O’Connor, D.L.; Unger, S.; Chan, T.C.Y. Predicting Protein and Fat Content in Human Donor Milk Using Machine Learning. J. Nutr. 2021, 151, 2075–2083. [Google Scholar] [CrossRef]
- Greenbury, S.F.; Ougham, K.; Wu, J.; Battersby, C.; Gale, C.; Modi, N.; Angelini, E.D. Identification of Variation in Nutritional Practice in Neonatal Units in England and Association with Clinical Outcomes Using Agnostic Machine Learning. Sci. Rep. 2021, 11, 7178. [Google Scholar] [CrossRef] [PubMed]
| Birth Weight | Start Rate (mL/kg/Day) | How to Advance |
|---|---|---|
| ≤1000 g | 20 within the first 72 h after birth | Minimal enteral feeding for 3 days, then, if feeds are tolerated, +20 |
| 1001–1500 g | 20 | Minimal enteral feeding for 3 days, then, if feeds are tolerated, +30 |
| >1500 g | 30 | Advance daily +30 |
| >1500 g with congenital heart disorders *, prenatal Doppler abnormalities **, or cardiorespiratory instability | 20 within the first 72 h after birth | Minimal enteral feeding for about 3 days, then, if feeds are tolerated, +10–20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rose, D.U.; Lapillonne, A.; Iacobelli, S.; Capolupo, I.; Dotta, A.; Salvatori, G. Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot. Nutrients 2024, 16, 1719. https://doi.org/10.3390/nu16111719
De Rose DU, Lapillonne A, Iacobelli S, Capolupo I, Dotta A, Salvatori G. Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot. Nutrients. 2024; 16(11):1719. https://doi.org/10.3390/nu16111719
Chicago/Turabian StyleDe Rose, Domenico Umberto, Alexandre Lapillonne, Silvia Iacobelli, Irma Capolupo, Andrea Dotta, and Guglielmo Salvatori. 2024. "Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot" Nutrients 16, no. 11: 1719. https://doi.org/10.3390/nu16111719
APA StyleDe Rose, D. U., Lapillonne, A., Iacobelli, S., Capolupo, I., Dotta, A., & Salvatori, G. (2024). Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot. Nutrients, 16(11), 1719. https://doi.org/10.3390/nu16111719






