The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice
Abstract
1. Introduction
2. Methods
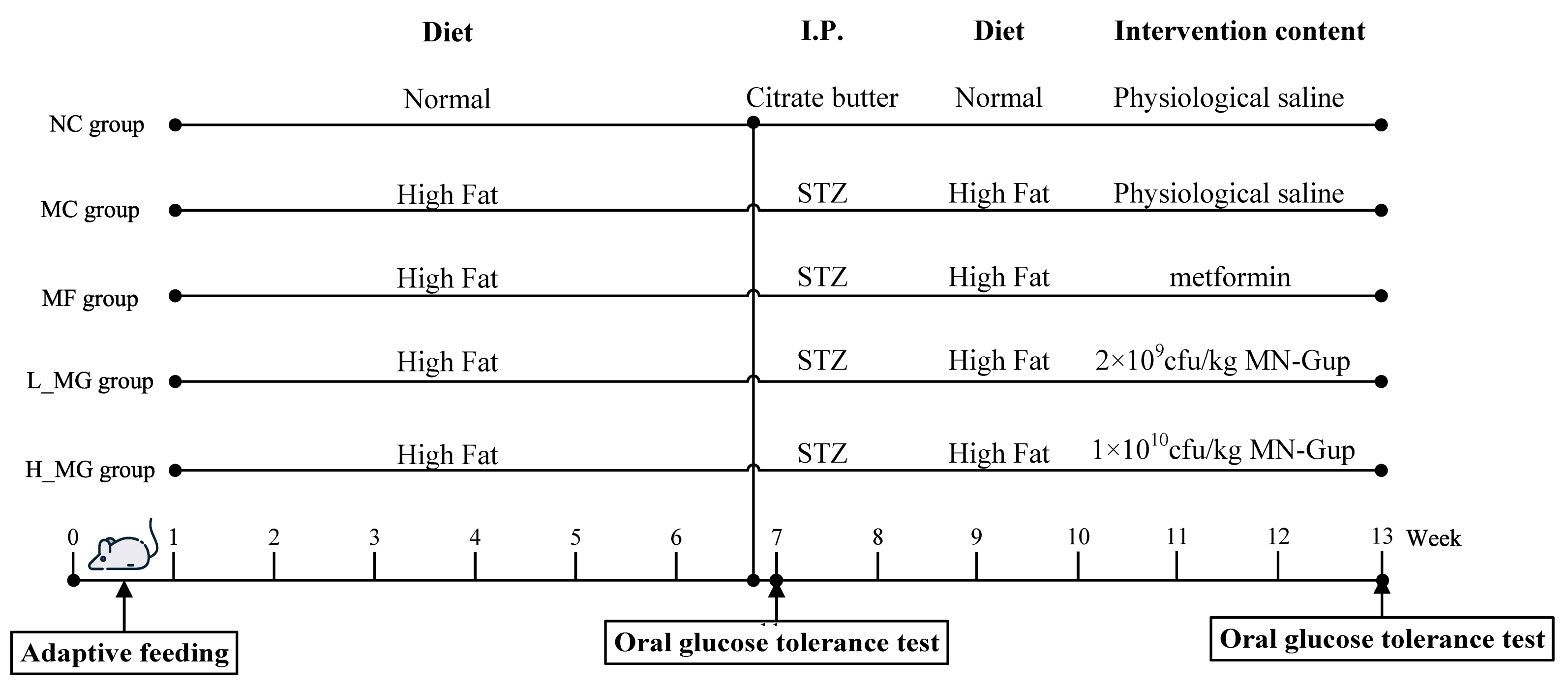
2.1. Animals and Treatment
2.2. The Detection of Glucose Metabolism Indicators
2.3. Histological Analysis
2.4. Gut Microbiota Analysis
2.5. Short-Chain Fatty Acid Analysis
3. Statistical Analysis
4. Results
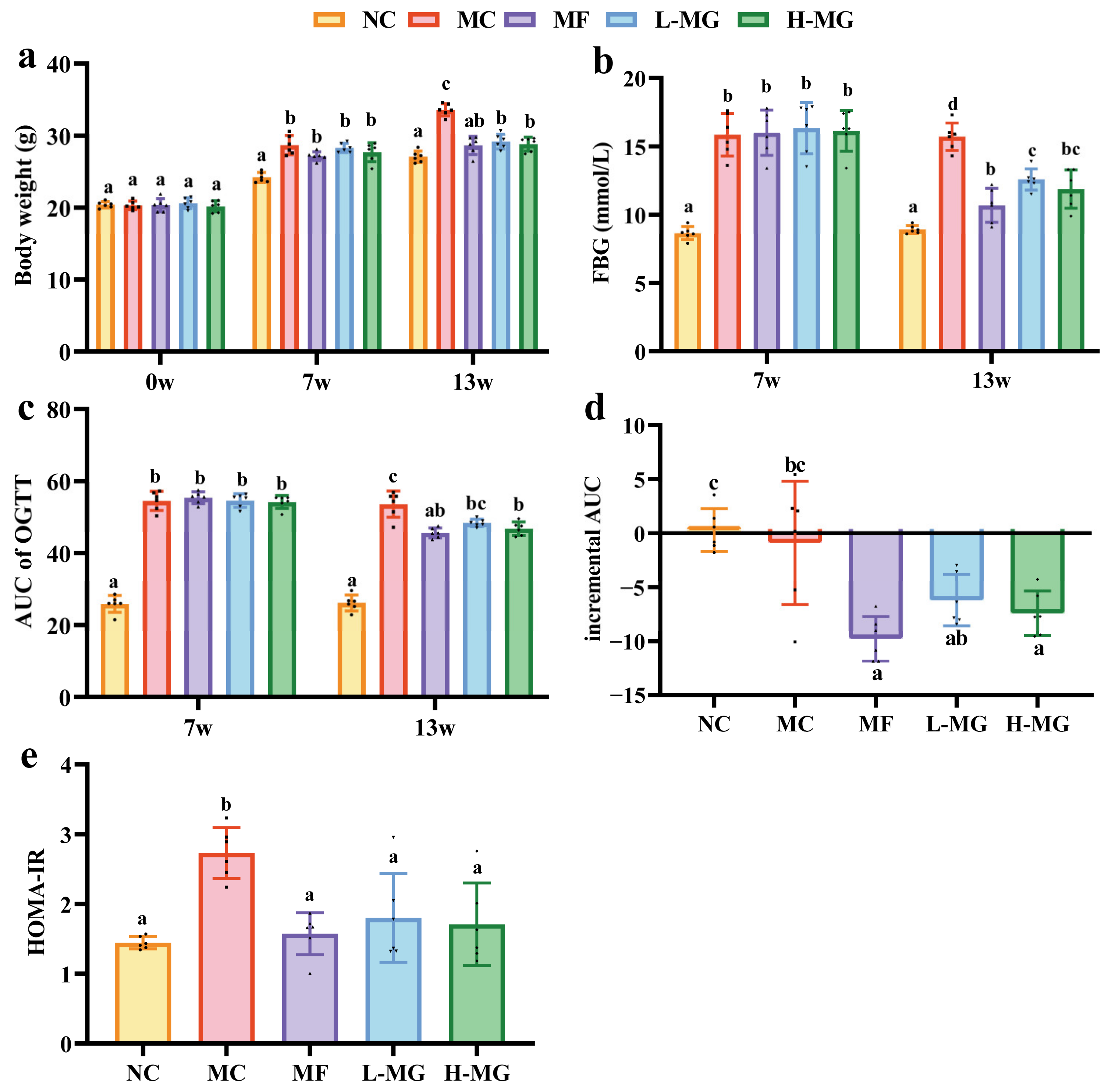
4.1. Effect of MN-Gup on Glucose Metabolism in T2DM Mice
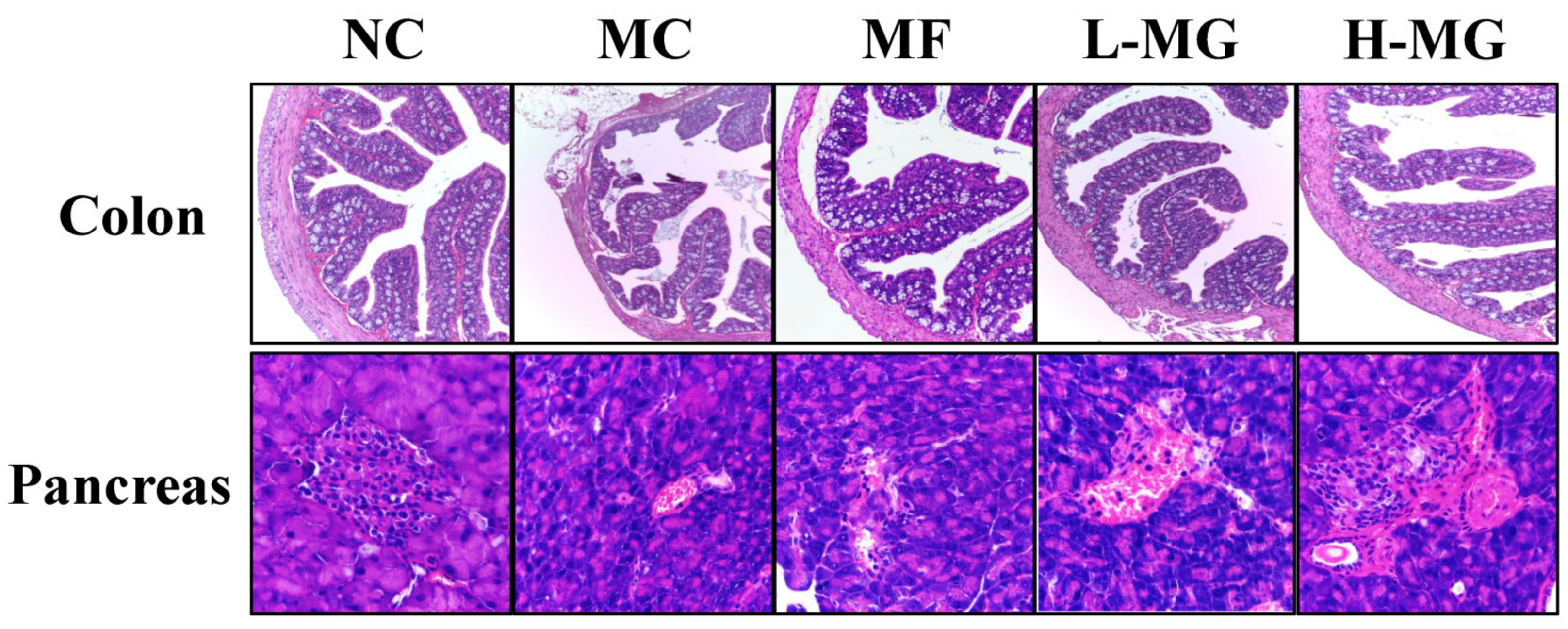
4.2. Effects of MN-Gup on Colon and Pancreatic Tissue in T2DM Mice
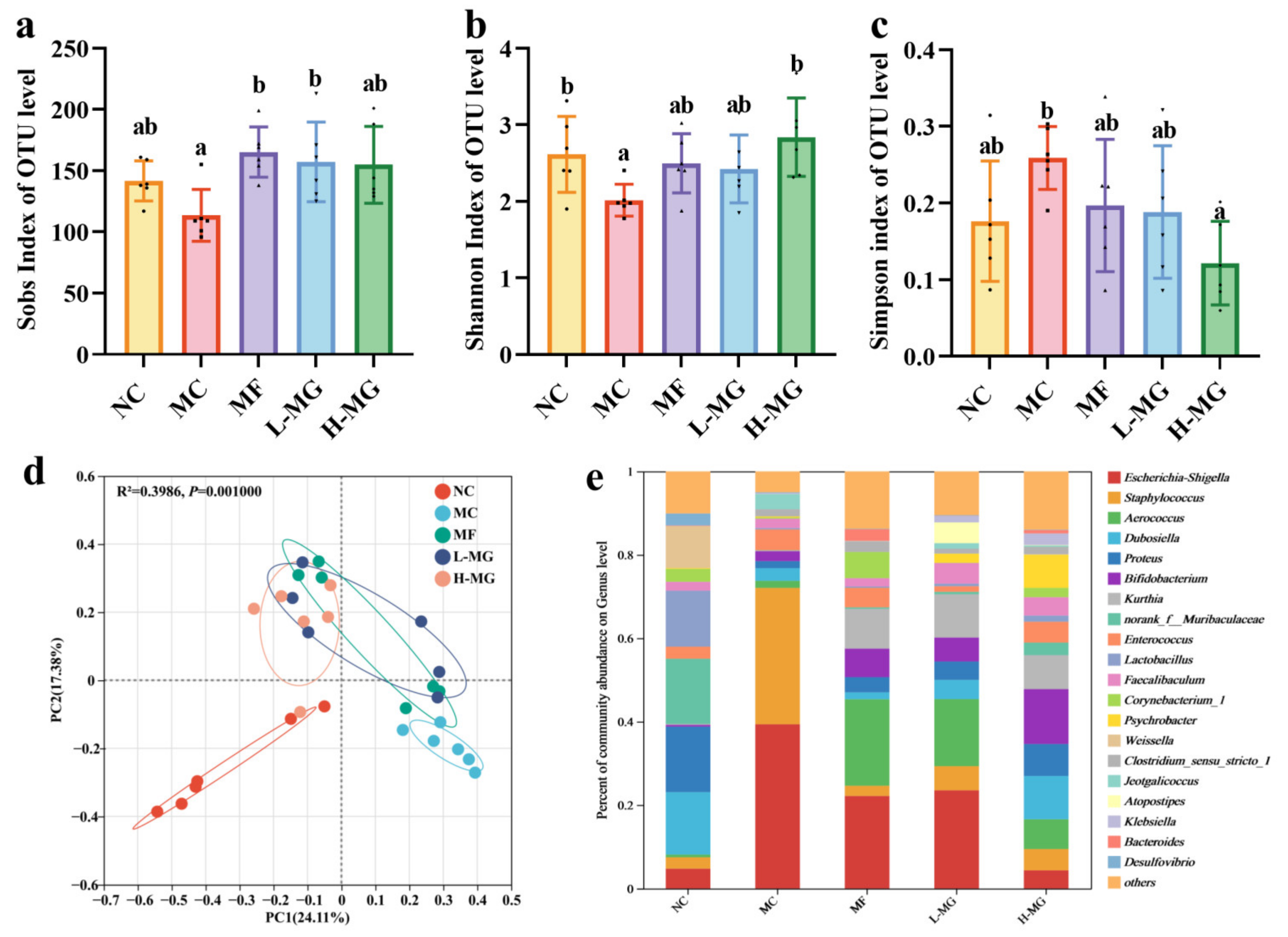
4.3. Effects of MN-Gup on Gut Microbiota Composition and Structure in T2DM Mice
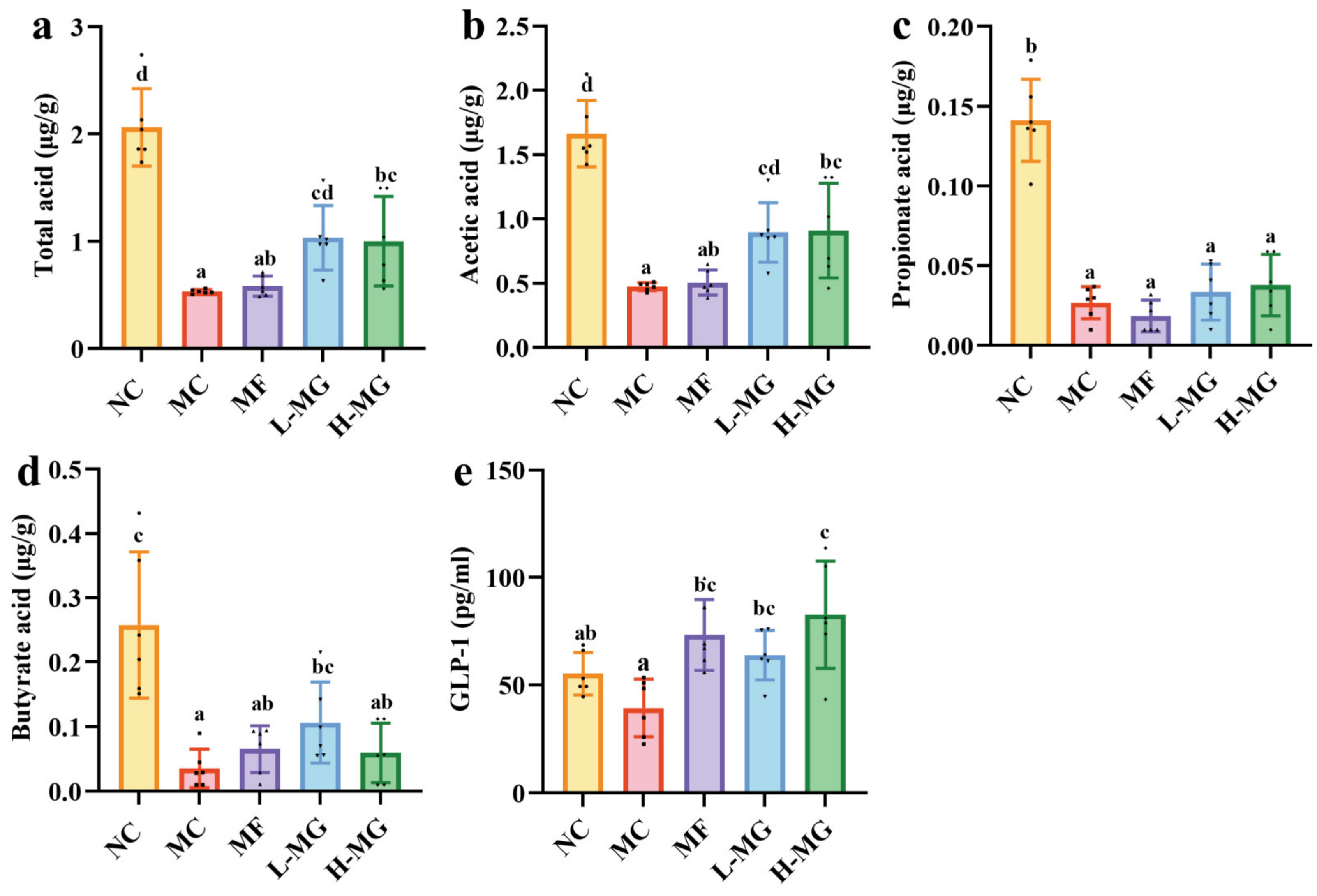
4.4. Effect of MN-Gup on the Composition of SCFAs and GLP-1 in T2DM Mice
4.5. Correlation between Gut Microbiota and Environmental Factors in T2DM Mice
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, L.; Cheng, S.; Zhang, Y.; Yang, M.; Fang, R.; Li, H.; Man, C.; Jiang, Y. A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef. Microbes 2021, 12, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, N.; Li, S.; Li, N.; Wang, R.; Zhang, Q.; He, J.; Sun, E.; Kang, X.; Zhan, J. Bifidobacterium animalis subsp. lactis MN-Gup protects mice against gut microbiota-related obesity and endotoxemia induced by a high fat diet. Front. Nutr. 2022, 9, 992947. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, R.; Li, S.; Kang, X.; Ren, Y.; Sun, E.; Wang, C.; He, J.; Zhan, J. Preliminary Evaluation of Potential Properties of Three Probiotics and Their Combination with Prebiotics on GLP-1 Secretion and Type 2 Diabetes Alleviation. J. Food Qual. 2022, 2022, 8586843. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. American journal of physiology. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, G.; Zhao, W.; Wang, X.; He, J.; Zhou, L.; Zhang, X.; An, P.; Liu, Y.; Zhang, C.; et al. Efficacy of Bifidobacterium animalis subsp. lactis BL-99 in the treatment of functional dyspepsia: A randomized placebo-controlled clinical trial. Nat. Commun. 2024, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, D.; Xu, X.; Dai, J.; Lao, G.; Zhang, S.; Xu, X.; Dinnyés, A.; Xiong, Y.; Sun, Q. Myofibrillar protein-chlorogenic acid complexes ameliorate glucose metabolism via modulating gut microbiota in a type 2 diabetic rat model. Food Chem. 2023, 409, 135195. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; He, S.; Dang, C. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. J. Vis. Exp. 2022, 183, e61715. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef] [PubMed]
- Salgaço, M.K.; Oliveira, L.G.S.; Costa, G.N.; Bianchi, F.; Sivieri, K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl. Microbiol. Biotechnol. 2019, 103, 9229–9238. [Google Scholar] [CrossRef] [PubMed]
- Borse, S.P.; Chhipa, A.S.; Sharma, V.; Singh, D.P.; Nivsarkar, M. Management of Type 2 Diabetes: Current Strategies, Unfocussed Aspects, Challenges, and Alternatives. Med. Princ. Pract. 2021, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Mouse models of insulin dependent diabetes: Low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes/Metab. Rev. 1987, 3, 751–778. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Luo, J.; Liu, Y.; Li, H.; Jin, R.; Li, S.; Wei, J.; Wei, H.; Chen, T. Improvement effect of a next-generation probiotic L. plantarum-pMG36e-GLP-1 on type 2 diabetes mellitus via the gut-pancreas-liver axis. Food Funct. 2023, 14, 3179–3195. [Google Scholar] [CrossRef]
- Van Syoc, E.P.; Damani, J.; DiMattia, Z.; Ganda, E.; Rogers, C.J. The Effects of Bifidobacterium Probiotic Supplementation on Blood Glucose: A Systematic Review and Meta-Analysis of Animal Models and Clinical Evidence. Adv. Nutr. 2024, 15, 100137. [Google Scholar] [CrossRef]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. Drug Des. Dev. Ther. 2023, 17, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Sanschagrin, S.; Yergeau, E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J. Vis. Exp. 2014, 90, e51709. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Rezazadah, A.; Farahbod, K.; Gerts, A.; Clunes, L.A.; Kollias, T.F. Type-2 Diabetes Mellitus and the Gut Microbiota: Systematic Review. Cureus 2023, 15, e49740. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M. Aerococcus: An increasingly acknowledged human pathogen. Clin. Microbiol. Infect. 2016, 22, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lozica, L.; Maurić Maljković, M.; Mazić, M.; Gottstein, Ž. Kurthia gibsonii, a novel opportunistic pathogen in poultry. Avian Pathol. 2022, 51, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gou, X.; Ding, Y.; Liu, J.; Wang, Y.; Wang, Y.; Zhang, J.; Du, L.; Peng, W.; Fan, G. The interplay between herbal medicines and gut microbiota in metabolic diseases. Front. Pharmacol. 2023, 14, 1105405. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Visai, L.; Rindi, S.; Pietrocola, G.; Provenza, G.; Provenzano, M. Prevention and treatment of Staphylococcus biofilms. Curr. Med. Chem. 2008, 15, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Long, X.S.; Liao, S.T.; Li, E.N.; Pang, D.R.; Li, Q.; Liu, S.C.; Hu, T.G.; Zou, Y.X. The hypoglycemic effect of freeze-dried fermented mulberry mixed with soybean on type 2 diabetes mellitus. Food Sci. Nutr. 2021, 9, 3641–3654. [Google Scholar] [CrossRef]
- Zhao, R.; Li, N.; Liu, W.; Liu, Q.; Zhang, L.; Peng, X.; Zhao, R.; Hu, H. Low glycemic index potato biscuits alleviate physio-histological damage and gut dysbiosis in rats with type-2 diabetes mellitus induced by high-sugar and high-fat diet and streptozotocin. J. Nutr. Biochem. 2023, 119, 109401. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Wei, C.; Wang, X.; Li, R.; Xu, X.; Zhang, Y.; Geng, G.; Dang, K.; Ming, Z.; et al. Vitamin K2 supplementation improves impaired glycemic homeostasis and insulin sensitivity for type 2 diabetes through gut microbiome and fecal metabolites. BMC Med. 2023, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Weng, J. Hepatic functions of GLP-1 and its based drugs: Current disputes and perspectives. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E620–E627. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Fang, B.; Zhang, N.; Zhang, Q.; Niu, T.; Zhao, L.; Sun, E.; Wang, J.; Xiao, R.; He, J.; et al. The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice. Nutrients 2024, 16, 1691. https://doi.org/10.3390/nu16111691
Zhang C, Fang B, Zhang N, Zhang Q, Niu T, Zhao L, Sun E, Wang J, Xiao R, He J, et al. The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice. Nutrients. 2024; 16(11):1691. https://doi.org/10.3390/nu16111691
Chicago/Turabian StyleZhang, Chao, Bing Fang, Nana Zhang, Qi Zhang, Tianjiao Niu, Liang Zhao, Erna Sun, Jian Wang, Ran Xiao, Jingjing He, and et al. 2024. "The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice" Nutrients 16, no. 11: 1691. https://doi.org/10.3390/nu16111691
APA StyleZhang, C., Fang, B., Zhang, N., Zhang, Q., Niu, T., Zhao, L., Sun, E., Wang, J., Xiao, R., He, J., Li, S., Chen, J., Guo, J., Xiong, W., & Wang, R. (2024). The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice. Nutrients, 16(11), 1691. https://doi.org/10.3390/nu16111691






