What Is the Tech Missing? Nutrition Reporting in Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Q1: Have you been diagnosed with Type 1 Diabetes?
|
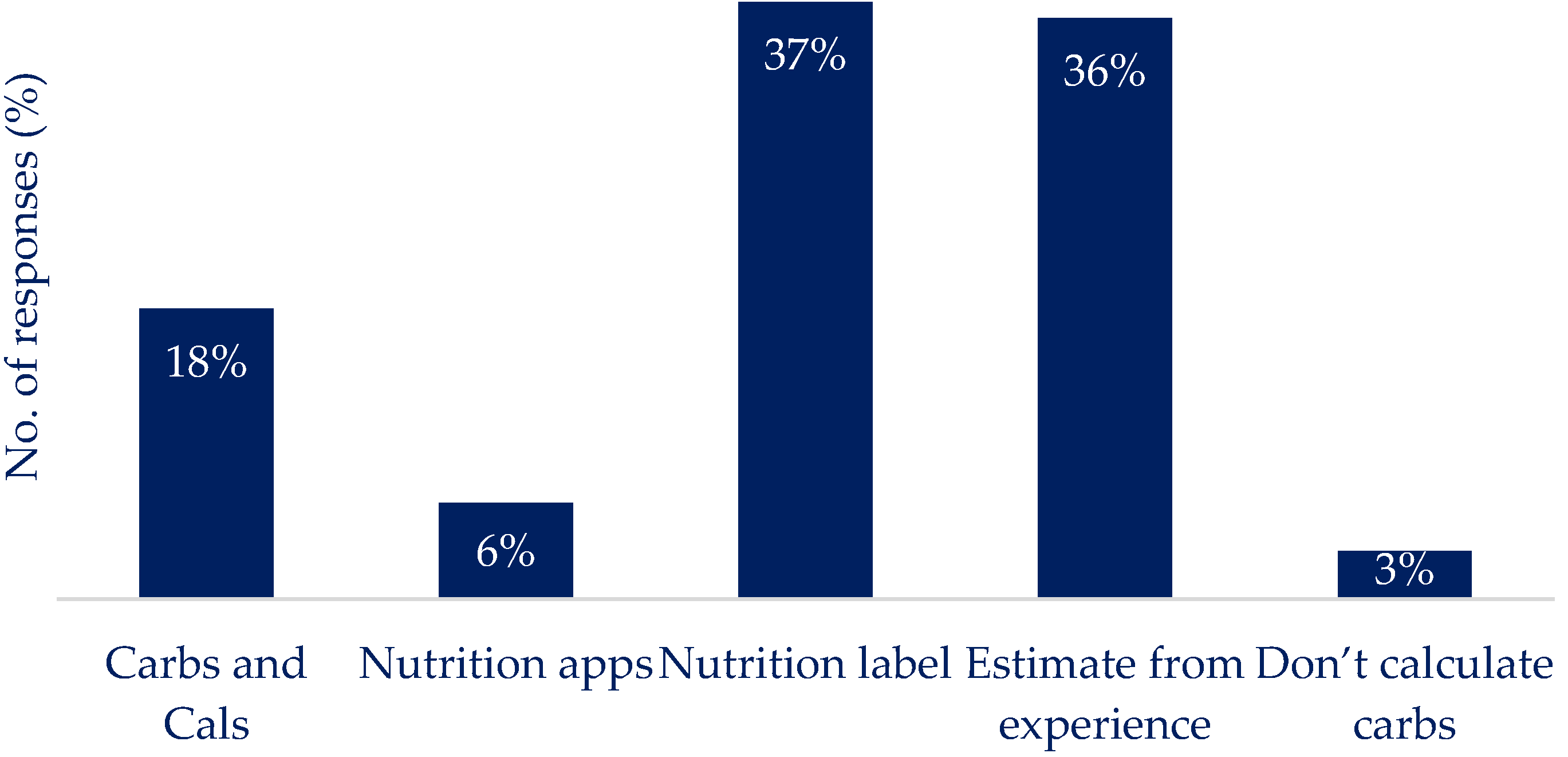
Q2: How do you currently work out the amount of carbohydrates you are eating in a meal? (select all that apply)
|
Q3: When thinking about calculating the carbohydrates present in a meal, how do you measure your food? (select all that apply)
|
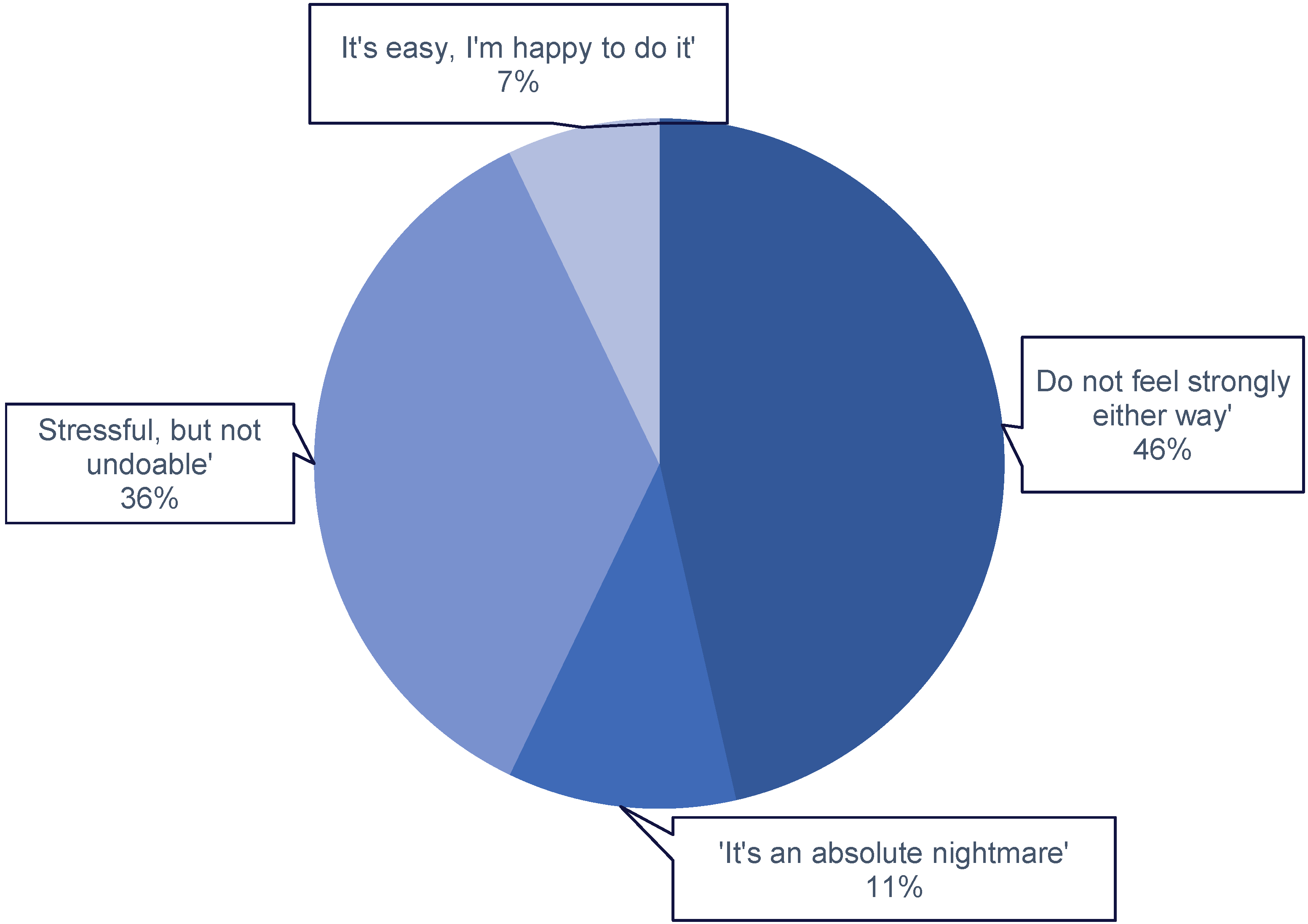
Q4: If you record your food intake, how do you feel about it? (Likert Scale)
|
Q5: Do you track/report any other nutrition information (i.e., total energy or vitamins and minerals)?
|
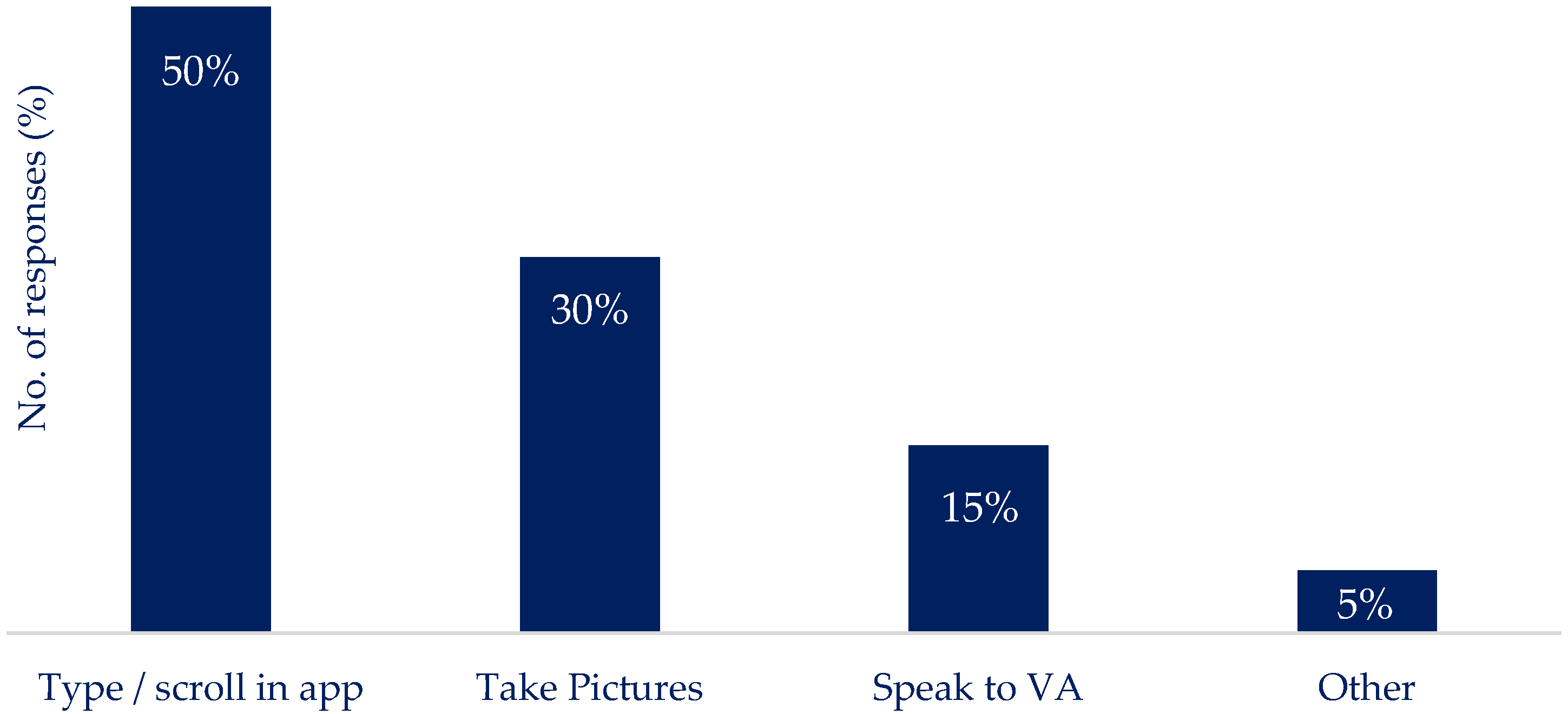
Q6: If you think about engaging with technology around your nutritional intake, what would be the most practical way for you to do this?
|
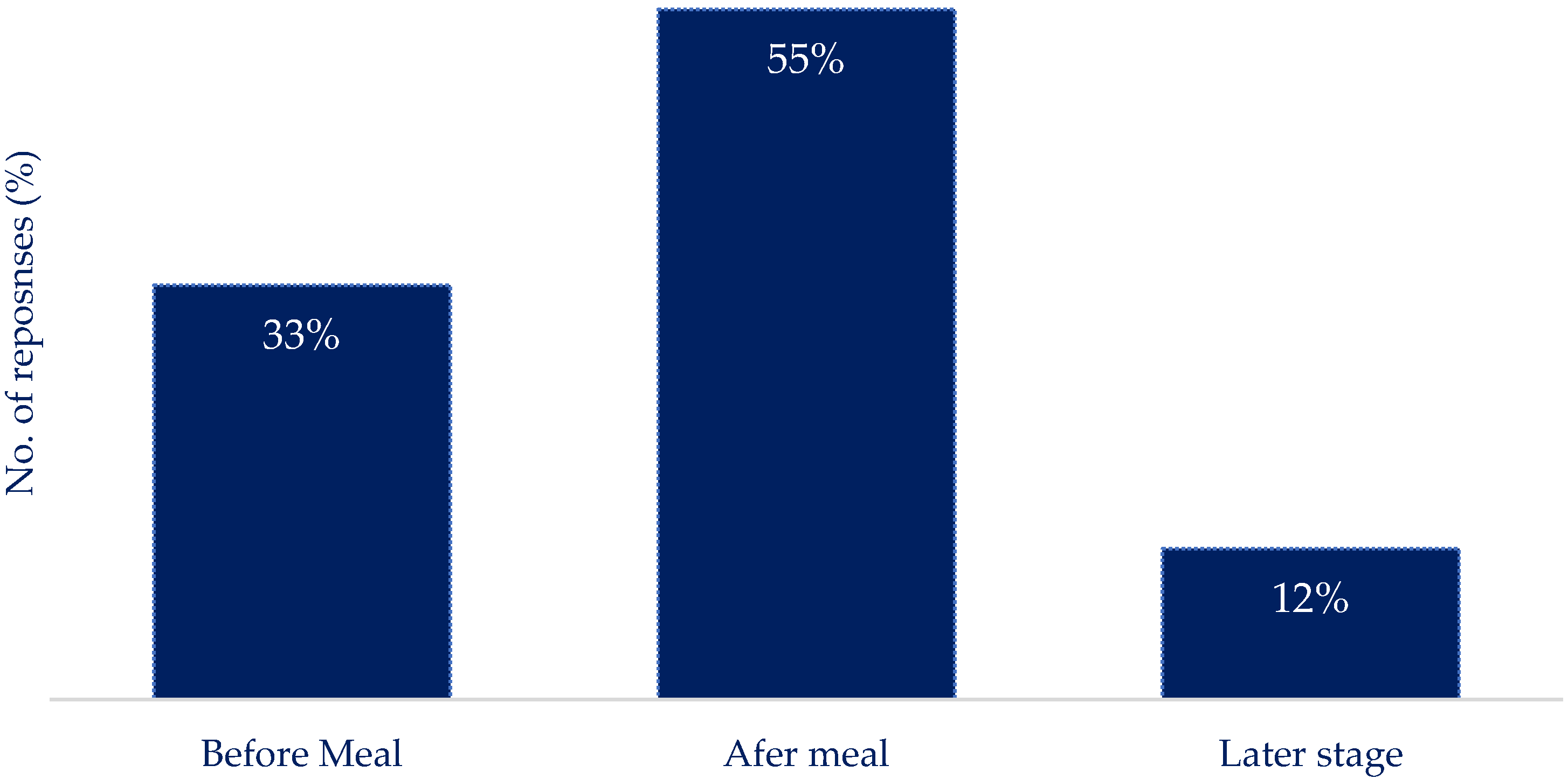
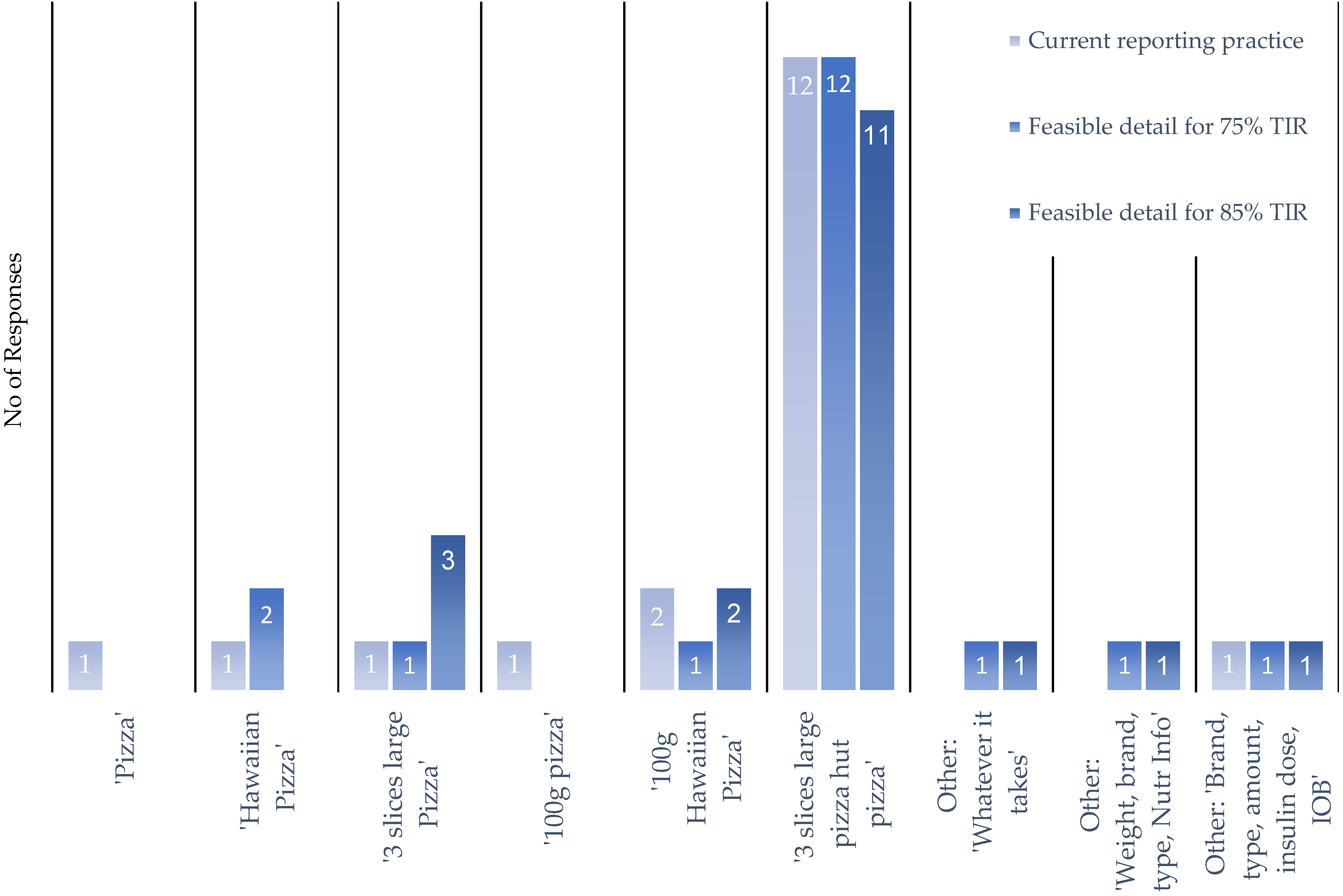
Q7: Following on from the above scenario, to what level of detail would you record your nutritional intake if you were to report it by typing/scrolling or using a voice- activated interface (let us use a Hawaiian pizza as an example)? You can choose from the below options:
|
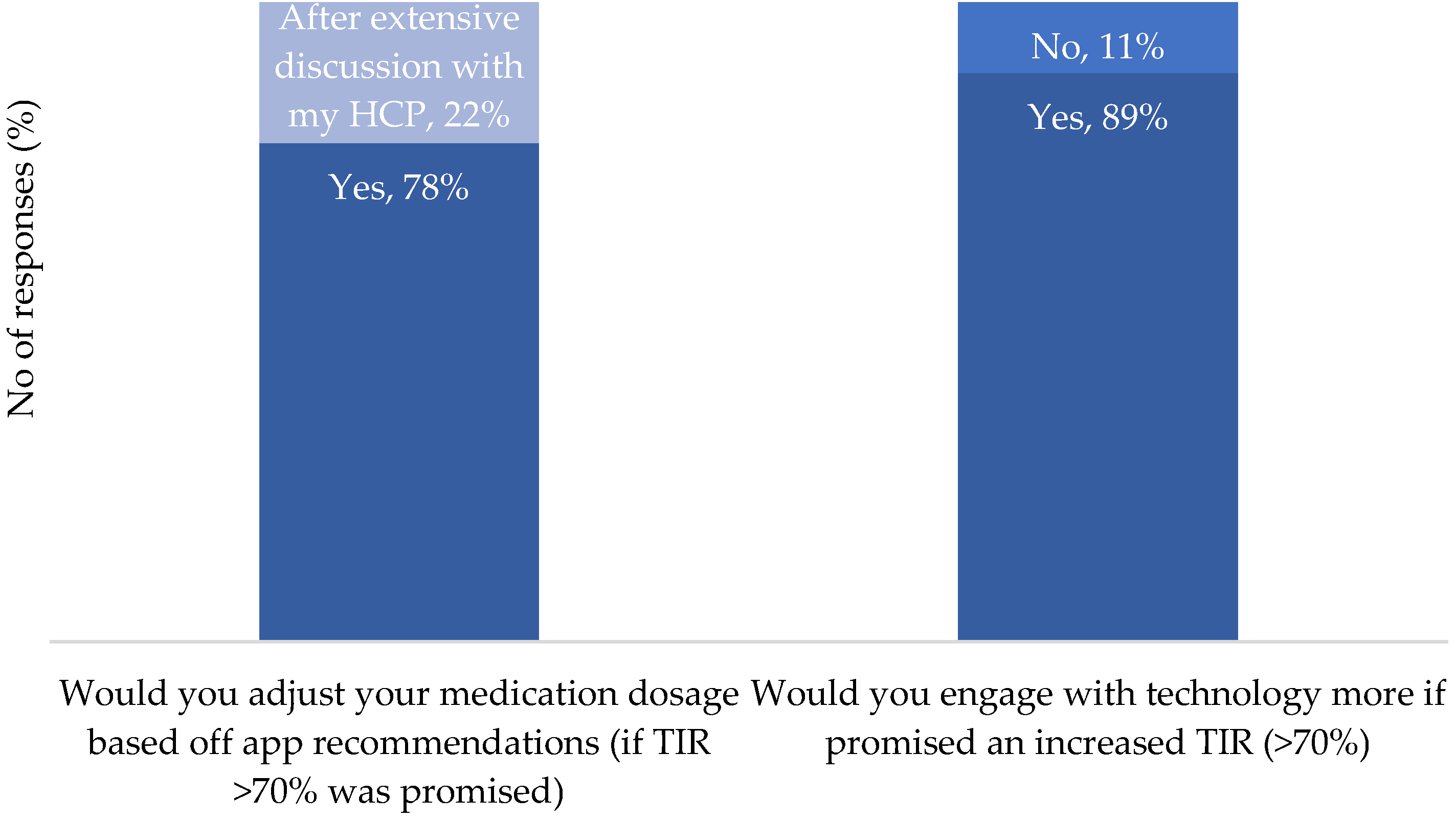
Q8: If providing more detail of the food you eat would allow an application to offer improved blood glucose control. Ensuring an HbA1C of 7% and at least 75% time in blood glucose target range. To what level of detail would you be willing to record your nutritional intake.
|
Q9: If providing more detail of the food you eat would allow an application to offer improved blood glucose control. Ensuring an HbA1C of below 6.5% and at least 80% time in blood glucose target range. To what level of detail would you be willing to record your nutritional intake.
|
Q10: If you were to make use of an application to report your nutritional intake, what would be the most effective way to report the information?
|
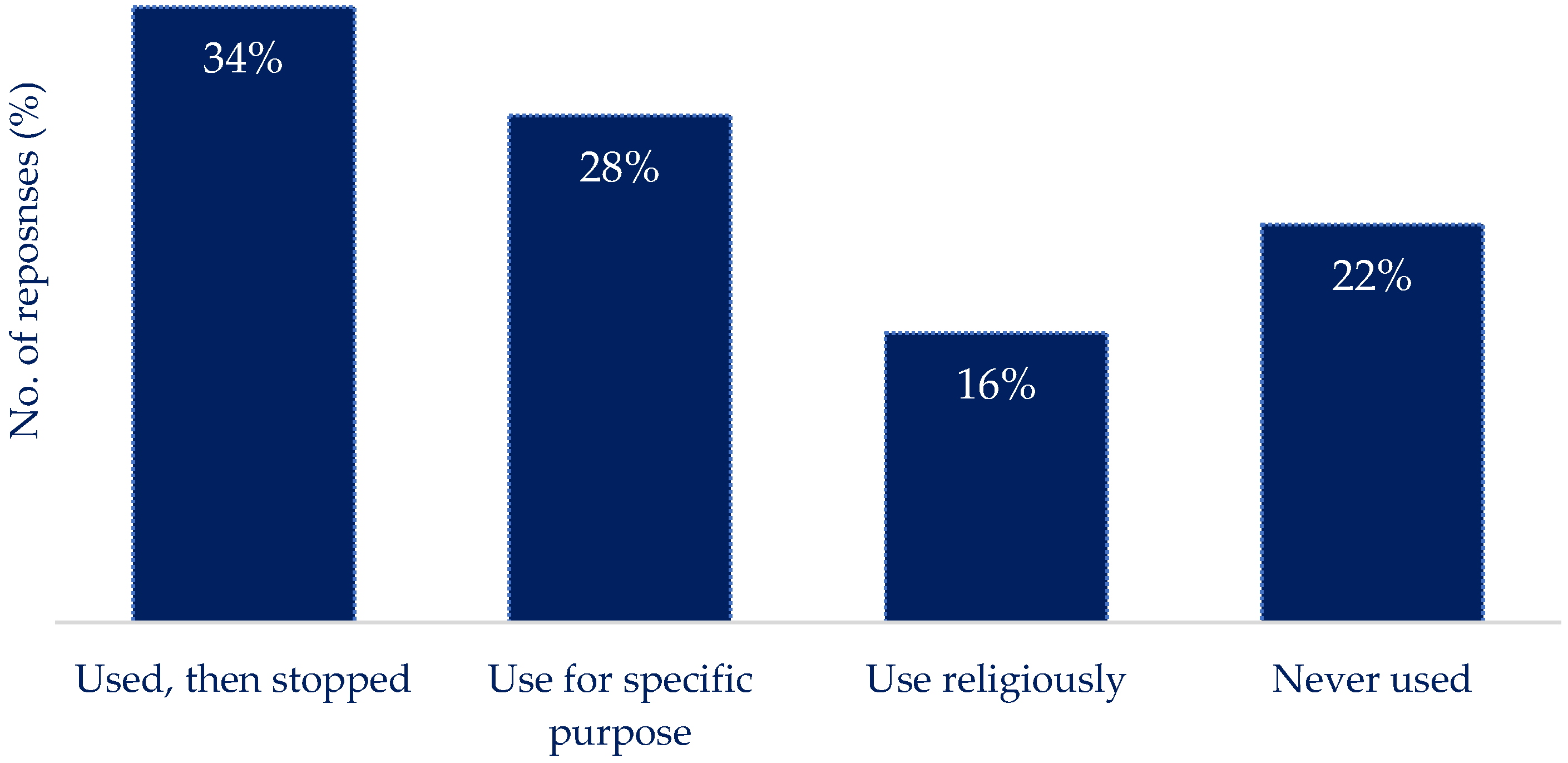
Q11: If you currently use/have used technology to track nutritional intake. What was your experience?
|
| Q12: Thinking about the foods you eat most often (no need to fill in all the spaces). List them below in the amount of detail you would naturally use. For example, this can be as straightforward as a sandwich or as detailed as ham and cheese sandwich on whole wheat with margarine and mayo or anything in between. |
Q13: Are you willing to share your HbA1c results with us and your current treatment plan? If yes, please answer the below:
|
Q14: How old were you, or your family member, when diagnosed with Type 1 Diabetes?
|
References
- Smith, T.A.; Blowes, A.A.; King, B.R.; Howley, P.P.; Smart, C.E. Families’ Reports of Problematic Foods, Management Strategies and Continuous Glucose Monitoring in Type 1 Diabetes: A Cross-sectional Study. Nutr. Diet. 2021, 78, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- JDRF: Type 1 Diabetes Facts and Figures. Available online: https://jdrf.org.uk/information-support/about-type-1-diabetes/facts-and-figures/ (accessed on 6 October 2022).
- Prahalad, P.; Tanenbaum, M.; Hood, K.; Maahs, D.M. Diabetes Technology: Improving Care, Improving Patient- Reported Outcomes and Preventing Complications in Young People with Type 1 Diabetes. Diabet. Med. 2018, 35, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, J.E.; Choi, E.E.Y.; Arshad, T.; Sammott, S.-A.S.; Pais, V.; Nguyen, C.; Maguire, B.R.; Stinson, J.N.; Palmert, M.R. Carbohydrate Counting App Using Image Recognition for Youth With Type 1 Diabetes: Pilot Randomized Control Trial. JMIR mHealth uHealth 2020, 8, e22074. [Google Scholar] [CrossRef] [PubMed]
- JDRF: Apps for Managing Type 1 Diabetes. Available online: https://jdrf.org.uk/knowledge-support/managing-type-1-diabetes/guide-to-type-1-diabetes-technology/apps-for-managing-type-1-diabetes/ (accessed on 12 April 2024).
- Chen, J.; Lieffers, J.; Bauman, A.; Hanning, R.; Allman-Farinelli, M. The Use of Smartphone Health Apps and Other Mobile Health (mHealth) Technologies in Dietetic Practice: A Three Country Study. J. Hum. Nutr. Diet. 2017, 30, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.; Gallagher, S.; Andrews, T.; Ashall-Payne, L.; Humphreys, L.; Leigh, S. The Effectiveness of Digital Health Technologies for Patients with Diabetes Mellitus: A Systematic Review. Front. Clin. Diabetes Healthc. 2022, 3, 936752. [Google Scholar] [CrossRef] [PubMed]
- Rankin, D.; Harden, J.; Barnard, K.; Bath, L.; Noyes, K.; Stephen, J.; Lawton, J. Barriers and Facilitators to Taking on Diabetes Self-Management Tasks in Pre-Adolescent Children with Type 1 Diabetes: A Qualitative Study. BMC Endocr. Disord. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.; Wilson, R.; Corsica, J.; Bradley, L.; Chirinos, D.; Vivo, A. What Do We Know about Mobile Applications for Diabetes Self-Management? A Review of Reviews. J. Behav. Med. 2016, 39, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.S.; Price, B.A.; Holland, S.; Dalton, N.S. Data, Data Everywhere, and Still Too Hard to Link: Insights from User Interactions with Diabetes Apps. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21 April 2018; ACM: New York, NY, USA; pp. 1–12. [Google Scholar]
- Lucassen, D.A.; Willemsen, R.F.; Geelen, A.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. The Accuracy of Portion Size Estimation Using Food Images and Textual Descriptions of Portion Sizes: An Evaluation Study. J. Hum. Nutr. Diet. 2021, 34, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The Relative Validity of a Food Record Using the Smartphone Application MyFitnessPal: Relative Validity of a Smartphone Dietary Record. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef]
- Brazeau, A.S.; Mircescu, H.; Desjardins, K.; Leroux, C.; Strychar, I.; Ekoé, J.M.; Rabasa-Lhoret, R. Carbohydrate Counting Accuracy and Blood Glucose Variability in Adults with Type 1 Diabetes. Diabetes Res. Clin. Pract. 2013, 99, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tascini, G.; Berioli, M.; Cerquiglini, L.; Santi, E.; Mancini, G.; Rogari, F.; Toni, G.; Esposito, S. Carbohydrate Counting in Children and Adolescents with Type 1 Diabetes. Nutrients 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Gabbay, M.A.L.; Rodacki, M.; Calliari, L.E.; Vianna, A.G.D.; Krakauer, M.; Pinto, M.S.; Reis, J.S.; Puñales, M.; Miranda, L.G.; Ramalho, A.C.; et al. Time in Range: A New Parameter to Evaluate Blood Glucose Control in Patients with Diabetes. Diabetol. Metab. Syndr. 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Buck, S.; Krauss, C.; Waldenmaier, D.; Liebing, C.; Jendrike, N.; Högel, J.; Pfeiffer, B.M.; Haug, C.; Freckmann, G. Evaluation of Meal Carbohydrate Counting Errors in Patients with Type 1 Diabetes. Exp. Clin. Endocrinol. Diabetes 2022, 130, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.M.; Pischke, C.R. Popular Diabetes Apps and the Impact of Diabetes App Use on Self-Care Behaviour: A Survey Among the Digital Community of Persons With Diabetes on Social Media. Front. Endocrinol. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.W.; Mok, M.; Kam, G.; Jung, M.; Tang, A.; Chan, U. Improvement in Diabetes Control with a Monitoring System Based on a Hand-Held, Touch-Screen Electronic Diary. J. Telemed. Telecare 2001, 7, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Agiostratidou, G.; Anhalt, H.; Ball, D.; Blonde, L.; Gourgari, E.; Harriman, K.N.; Kowalski, A.J.; Madden, P.; McAuliffe-Fogarty, A.H.; McElwee-Malloy, M.; et al. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017, 40, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Aleppo, G.; Gal, R.L.; Raghinaru, D.; Kruger, D.; Beck, R.W.; Bergenstal, R.M.; Cushman, T.; Hood, K.K.; Johnson, M.L.; McArthur, T.; et al. Comprehensive Telehealth Model to Support Diabetes Self-Management. JAMA Netw. Open 2023, 6, e2336876. [Google Scholar] [CrossRef] [PubMed]
- Qualtrics 2022. Available online: https://www.qualtrics.com/uk (accessed on 16 August 2022).
- Likert, R.; Roslow, S.; Murphy, G. A Simple and Reliable Method of Scoring the Thurstone Attitude Scales. J. Soc. Psychol. 1934, 5, 228–238. [Google Scholar] [CrossRef]
- Carbs&Cals. Available online: https://carbsandcals.com (accessed on 11 April 2024).
- Martinez-Millana, A.; Jarones, E.; Fernandez-Llatas, C.; Hartvigsen, G.; Traver, V. App Features for Type 1 Diabetes Support and Patient Empowerment: Systematic Literature Review and Benchmark Comparison. JMIR mHealth uHealth 2018, 6, e12237. [Google Scholar] [CrossRef] [PubMed]
- Caburnay, C.; Graff, K.; Harris, J.; McQueen, A.; Smith, M.; Fairchild, M.; Kreuter, M. Evaluating Diabetes Mobile Applications for Health Literate Designs and Functionality. Prev. Chronic Dis. 2015, 12, E61. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of Attrition and Dropout in App-Based Interventions for Chronic Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e20283. [Google Scholar] [CrossRef] [PubMed]
- Amagai, S.; Pila, S.; Kaat, A.J.; Nowinski, C.J.; Gershon, R.C. Challenges in Participant Engagement and Retention Using Mobile Health Apps: Literature Review. J. Med. Internet Res. 2022, 24, e35120. [Google Scholar] [CrossRef]
- Trawley, S.; Baptista, S.; Browne, J.L.; Pouwer, F.; Speight, J. The Use of Mobile Applications Among Adults with Type 1 and Type 2 Diabetes: Results from the Second MILES—Australia (MILES-2) Study. Diabetes Technol. Ther. 2017, 19, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.J.; König, M.; Wiesbauer, F. Evaluating Success for Behavioral Change in Diabetes via MHealth and Gamification: MySugr’s Keys to Retention and Patient Engagement. In Proceedings of the Advanced Technologies & Treatments for Diabetes (ATTD), Paris, France, 27 February–2 March 2013. [Google Scholar]
- Debong, F.; Mayer, H.; Kober, J. Real-World Assessments of mySugr Mobile Health App. Diabetes Technol. Ther. 2019, 21, S2-35–S2-40. [Google Scholar] [CrossRef] [PubMed]
- Vasiloglou, M.; Christodoulidis, S.; Reber, E.; Stathopoulou, T.; Ya, L.; Stanga, Z.; Mougiakakou, S. Perspectives and Preferences of Adult Smartphone Users Regarding Nutrition and Diet Apps: Web-Based Survey Study. JMIR mHealth uHealth 2021, 9, e27885. [Google Scholar] [CrossRef]
- Andrew, A.; Borriello, G.; Fogarty, J. Simplifying Mobile Phone Food Diaries. In Proceedings of the ICTs for improving Patients Rehabilitation Research Techniques, Venice, Italy, 5 May 2013. [Google Scholar]
- Nansel, T.R.; Gellar, L.; McGill, A. Effect of Varying Glycemic Index Meals on Blood Glucose Control Assessed With Continuous Glucose Monitoring in Youth With Type 1 Diabetes on Basal-Bolus Insulin Regimens. Diabetes Care 2008, 31, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Priesterroth, L.; Grammes, J.; Holtz, K.; Reinwarth, A.; Kubiak, T. Gamification and Behavior Change Techniques in Diabetes Self-Management Apps. J. Diabetes Sci. Technol. 2019, 13, 954–958. [Google Scholar] [CrossRef]
- Davison, B.; Quigg, R.; Skidmore, P. Pilot Testing a Photo-Based Food Diary in Nine- to Twelve- Year Old- Children from Dunedin, New Zealand. Nutrients 2018, 10, 240. [Google Scholar] [CrossRef]
- Rusin, M.; Årsand, E.; Hartvigsen, G. Functionalities and Input Methods for Recording Food Intake: A Systematic Review. Int. J. Med. Inform. 2013, 82, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, L.; Vanhala, M.; Korpelainen, R.; Tossavainen, P. Agreement between an Image-Based Dietary Assessment Method and a Written Food Diary among Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Han, H.; Coulon, S.M.; Allen, H.R.; Champagne, C.M.; Anton, S.D. A Novel Method to Remotely Measure Food Intake of Free-Living Individuals in Real Time: The Remote Food Photography Method. Br. J. Nutr. 2008, 101, 446–456. [Google Scholar] [CrossRef]
- Franco, R.Z.; Fallaize, R.; Lovegrove, J.A.; Hwang, F. Popular Nutrition-Related Mobile Apps: A Feature Assessment. JMIR mHealth uHealth 2016, 4, e85. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, P.; Sazawal, S.; Menon, V.P.; Dhingra, U.; Black, R.E. Validation of Visual Estimation of Portion Size Consumed as a Method for Estimating Food Intake by Young Indian Children. J. Health Popul. Nutr. 2007, 25, 112. [Google Scholar] [PubMed]
- Hernández, T.; Wilder, L.; Kuehn, D.; Rubotzky, K.; Moser-Veillon, P.; Godwin, S.; Thompson, C.; Wang, C. Portion Size Estimation and Expectation of Accuracy. J. Food Compos. Anal. 2006, 19, S14–S21. [Google Scholar] [CrossRef]
- Sharp, D.B.; Allman-Farinelli, M. Feasibility and Validity of Mobile Phones to Assess Dietary Intake. Nutrition 2014, 30, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.; Wu, L. Should You “Picture” This? Effects of Picture-Taking Features of Food Diary Apps on Memory, Liking, and Wanting. Appetite 2022, 168, 105682. [Google Scholar] [CrossRef] [PubMed]
- König, L.M.; Attig, C.; Franke, T.; Renner, B. Barriers to and Facilitators for Using Nutrition Apps: Systematic Review and Conceptual Framework. JMIR mHealth uHealth 2021, 9, e20037. [Google Scholar] [CrossRef] [PubMed]
- Ladyzynski, P.; Krzymien, J.; Foltynski, P.; Rachuta, M.; Bonalska, B. Accuracy of Automatic Carbohydrate, Protein, Fat and Calorie Counting Based on Voice Descriptions of Meals in People with Type 1 Diabetes. Nutrients 2018, 10, 518. [Google Scholar] [CrossRef]
- Andrade, C. The Limitations of Online Surveys. Indian J. Psychol. Med. 2020, 42, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Yang, D.; Luo, S.; Ding, Y.; Xu, W.; Zheng, X.; Weng, J.; Yan, J. High Engagement in Mobile Peer Support Is Associated with Better Glycemic Control in Type 1 Diabetes: A Real-world Study. J. Diabetes Investig. 2022, 13, 1914–1924. [Google Scholar] [CrossRef] [PubMed]

| No. of Responses | |
|---|---|
| Diagnosis of Type 1 Diabetes: | |
| Self | 33 |
| I support a family member with T1D | 6 |
| Duration of diabetes: | |
| 0–5 years | 5 |
| 5–10 years | 0 |
| 10–15 years | 0 |
| 15+ years | 28 |
| Not reported | 6 |
| Average HbA1c | 6.8 ± 1.6% |
| Age of PwT1D at the time of the questionnaire: | |
| 0–9 | 3 |
| 10–14 | 0 |
| 15–24 | 0 |
| 25–34 | 7 |
| 35–44 | 6 |
| 45+ | 18 |
| Not reported | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubasinski, N.; Thabit, H.; Nutter, P.W.; Harper, S. What Is the Tech Missing? Nutrition Reporting in Type 1 Diabetes. Nutrients 2024, 16, 1690. https://doi.org/10.3390/nu16111690
Lubasinski N, Thabit H, Nutter PW, Harper S. What Is the Tech Missing? Nutrition Reporting in Type 1 Diabetes. Nutrients. 2024; 16(11):1690. https://doi.org/10.3390/nu16111690
Chicago/Turabian StyleLubasinski, Nicole, Hood Thabit, Paul W. Nutter, and Simon Harper. 2024. "What Is the Tech Missing? Nutrition Reporting in Type 1 Diabetes" Nutrients 16, no. 11: 1690. https://doi.org/10.3390/nu16111690
APA StyleLubasinski, N., Thabit, H., Nutter, P. W., & Harper, S. (2024). What Is the Tech Missing? Nutrition Reporting in Type 1 Diabetes. Nutrients, 16(11), 1690. https://doi.org/10.3390/nu16111690






