The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice
Abstract
1. Introduction
2. Materials and Methods
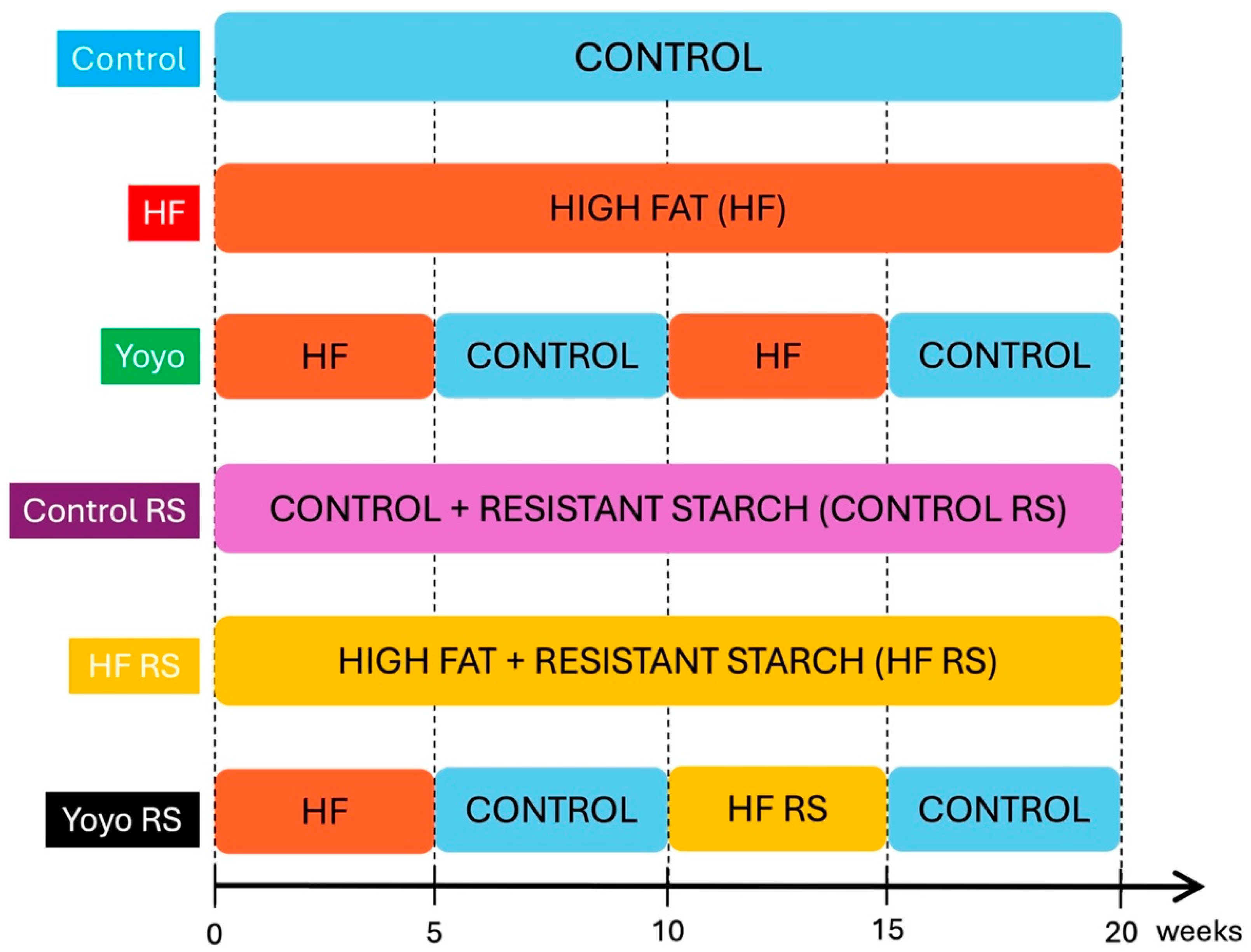
2.1. Animals and Diets
2.2. Oral Glucose Tolerance Test
2.3. Insulin Tolerance Test
2.4. Liver Histology
2.5. Liver Triglyceride Determination
2.6. Short-Chain Fatty Acids (SCFAs)
2.7. Metabolic Statistical Analysis
2.8. Taxonomic Microbiota Analysis
3. Results
3.1. Body Weight Change, Rate of Weight Regain, and Tissue Mass
3.1.1. Body Weight Change
3.1.2. Rates of Body Weight Gain during High-Fat Feeding Periods
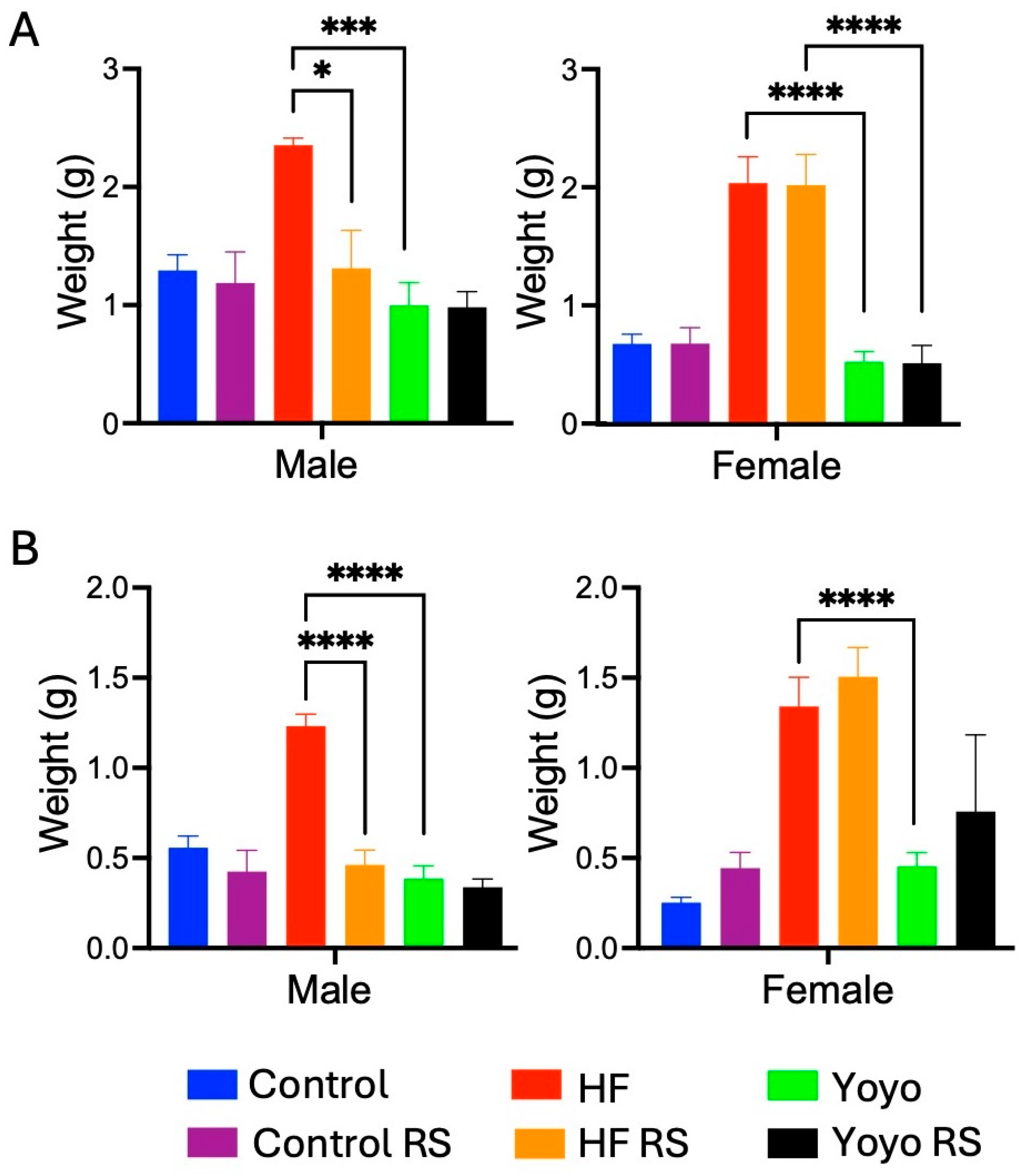
3.1.3. Fat Mass
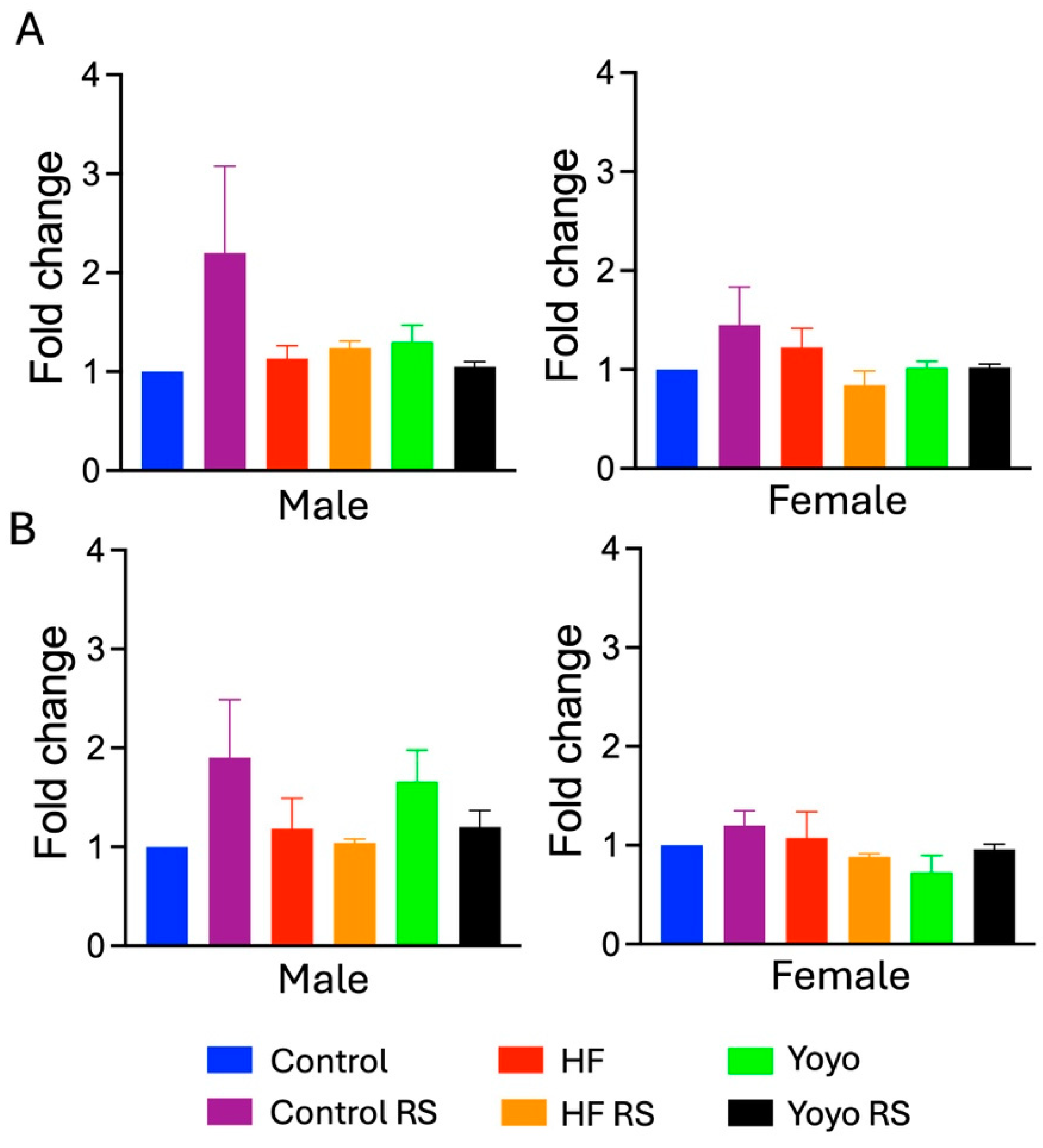
3.2. Liver Health
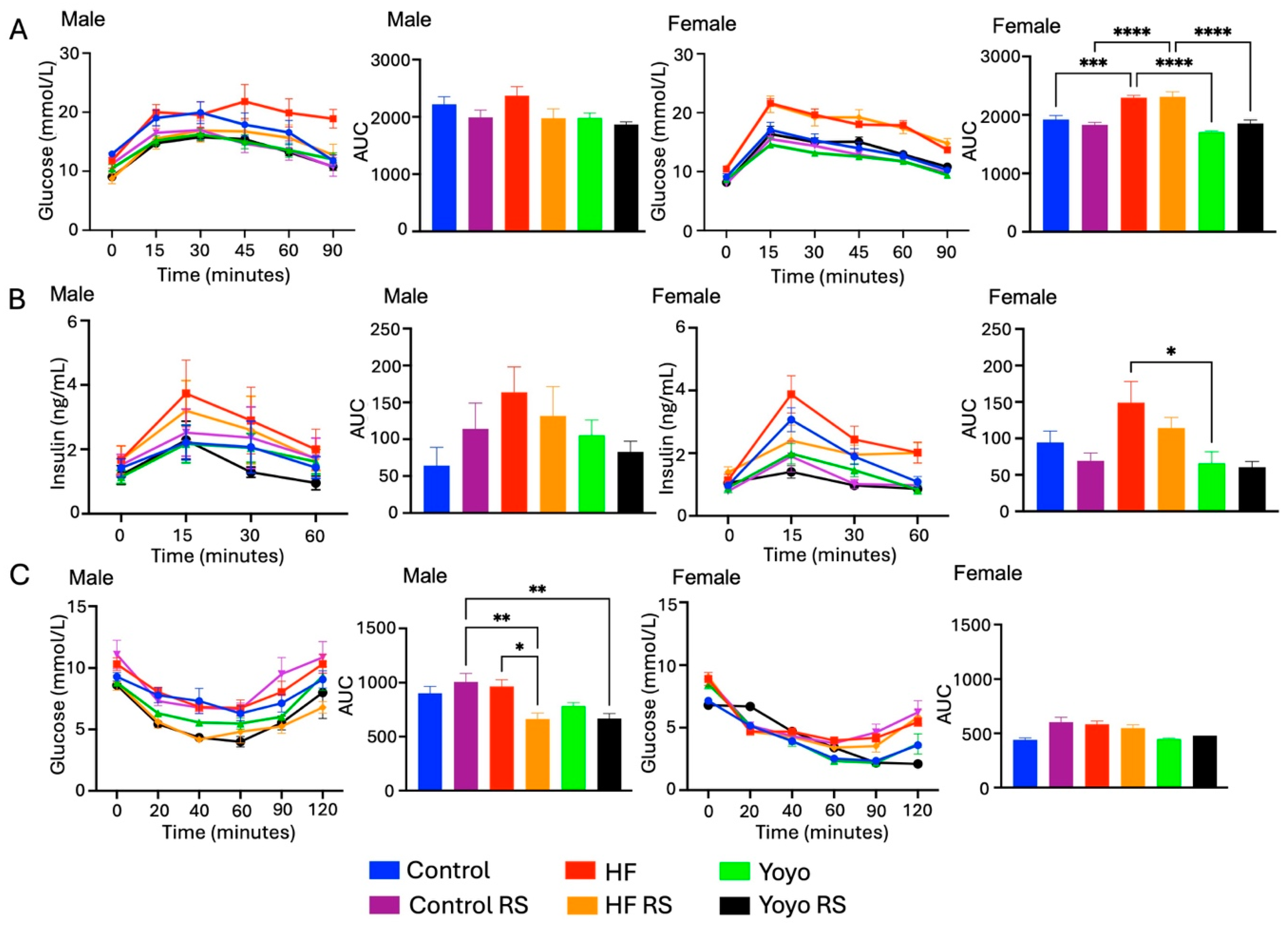
3.3. Blood Glucose Metabolism
3.4. Short-Chain Fatty Acid Concentration
3.5. Resistant Starch and Yoyo Dieting Reshaped Gut Microbiota
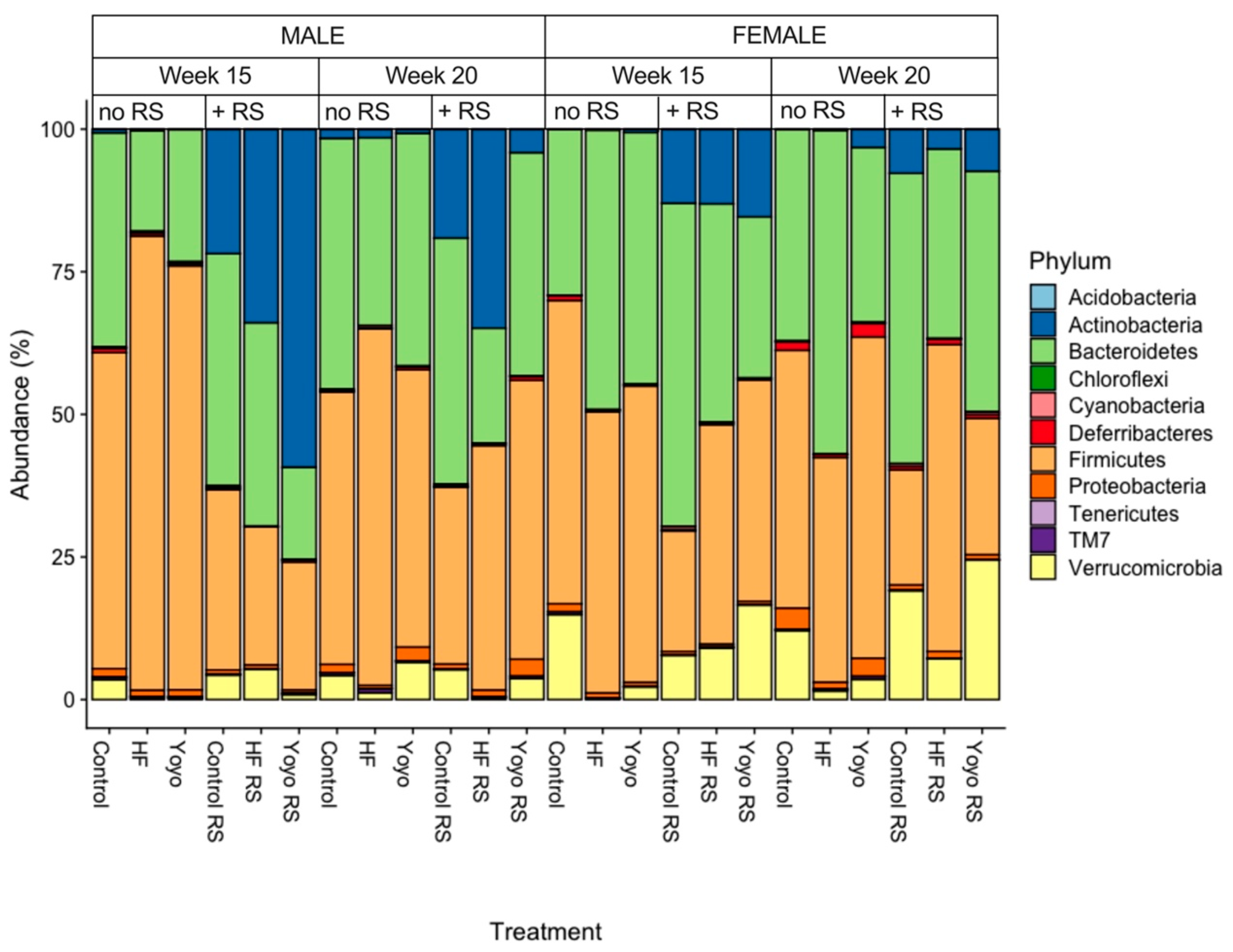
3.5.1. Relative Abundance by Phylum
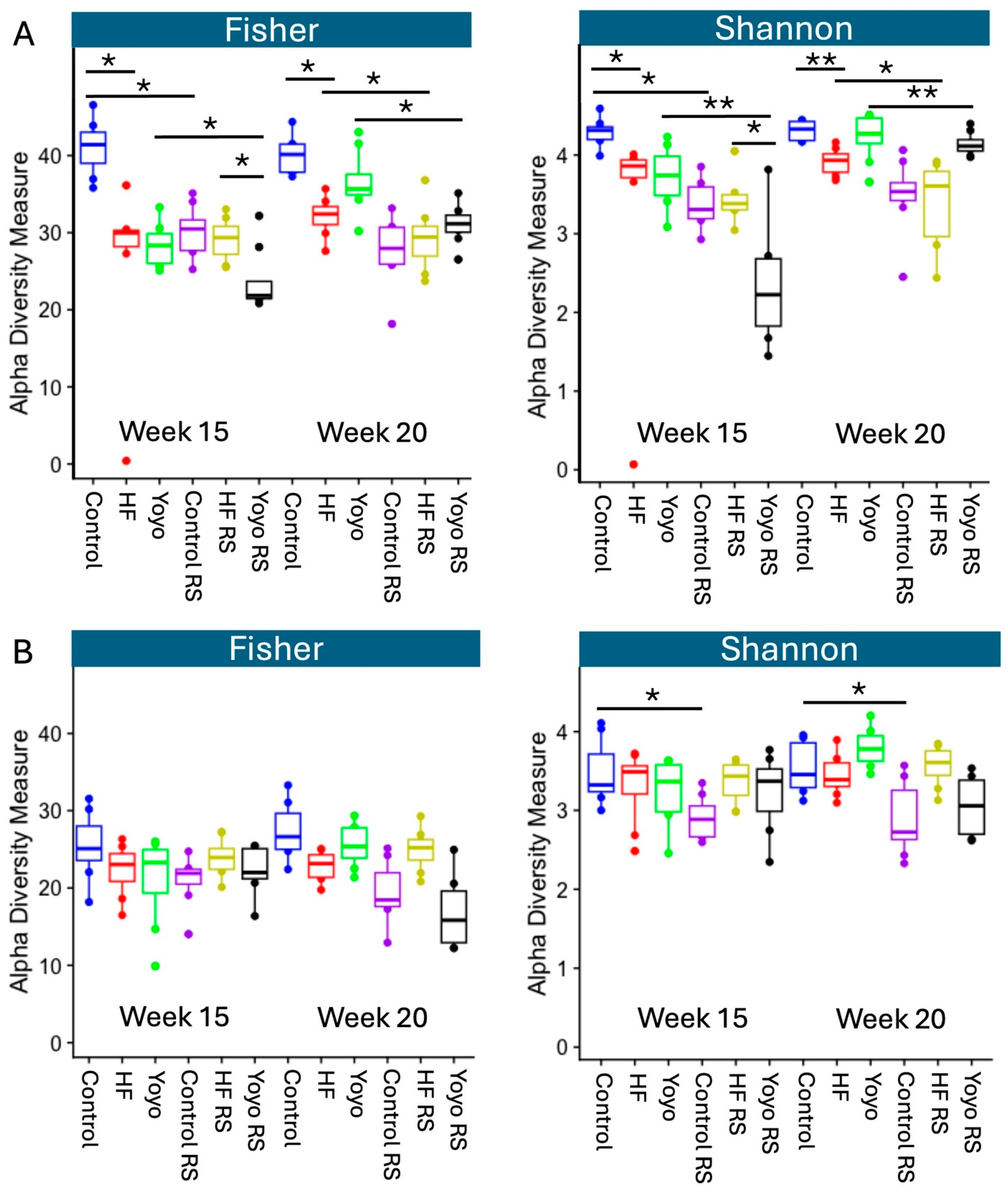
3.5.2. Alpha Diversity
3.5.3. Beta Diversity
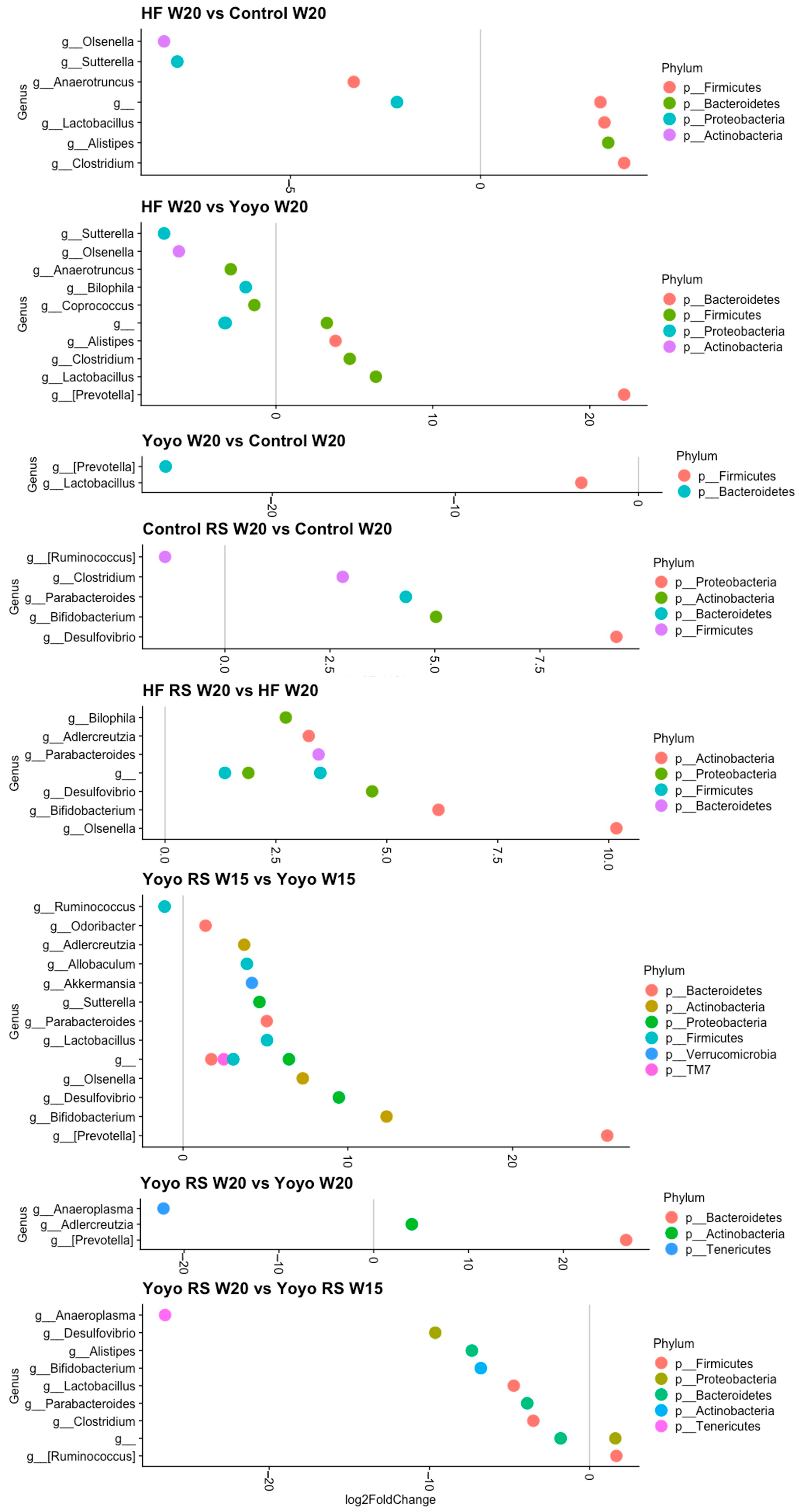
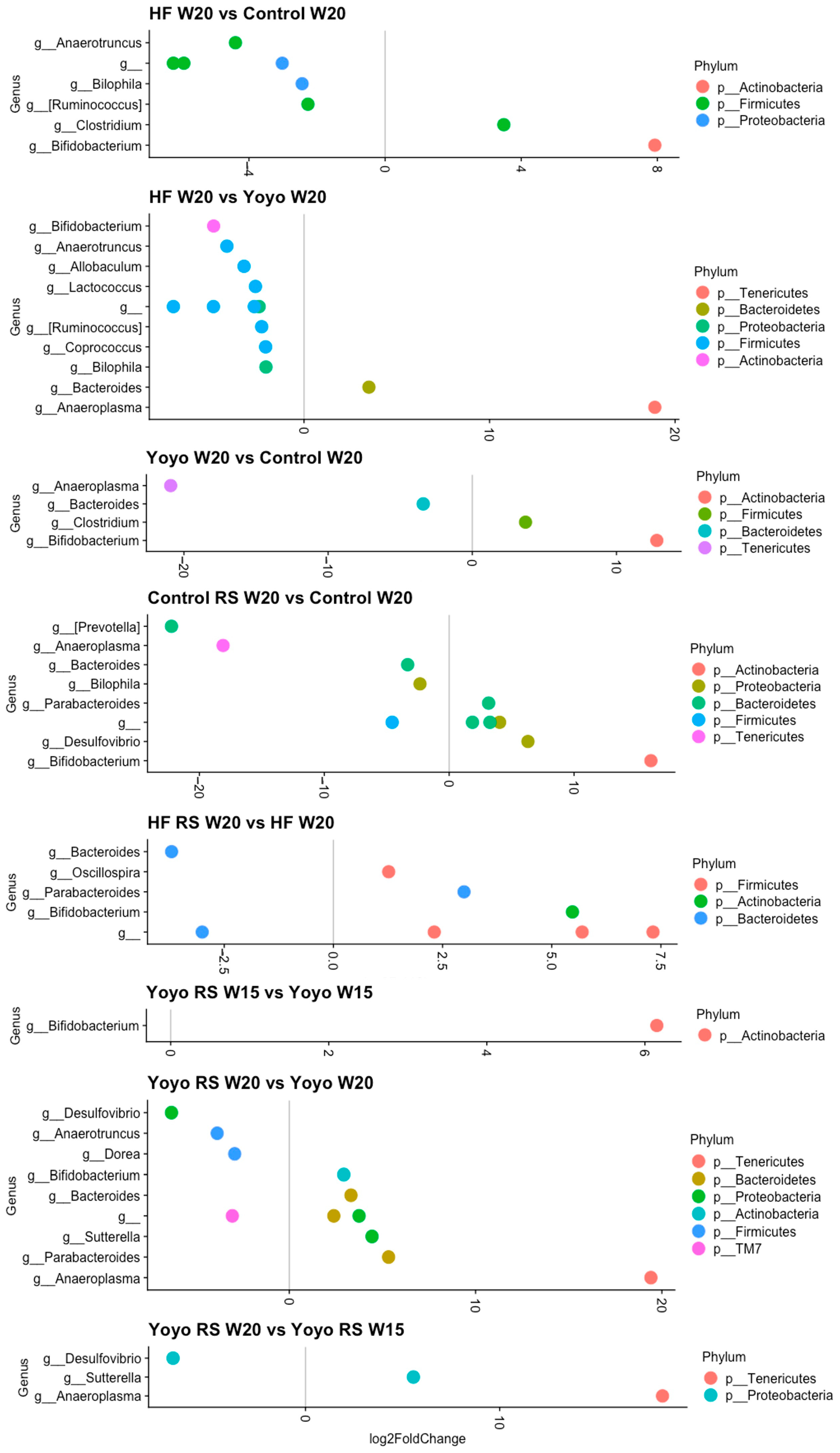
3.5.4. Differential Abundance Analysis
4. Discussion
4.1. Yoyo Dieting Appears to Be a Double-Edged Sword: Beneficial and Deleterious Effects of Yoyo Dieting on Metabolic and Gut Health
4.2. Beneficial Effects of Resistant Starch on Metabolic Health
4.3. Beneficial Effects of Resistant Starch on Gut Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Smith, C.M.; Kearns, B.; Haywood, A.; Bissell, P. The association between obesity and quality of life: A retrospective analysis of a large-scale population-based cohort study. BMC Public Health 2021, 21, 1990. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. Millwood 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Griffith, R. Obesity, Poverty and Public Policy. Econ. J. 2022, 132, 1235–1258. [Google Scholar] [CrossRef]
- Australian Institute of Health Welfare (AIHW). Overweight and Obesity among Australian Children and Adolescents; AIHW: Canberra, Australia, 2020.
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain after Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; Sherwood, N.E.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Rock, C.L.; Thornquist, M.D.; Cheskin, L.J.; Neuhouser, M.L.; Barnett, M.J. Weight-control behaviors among adults and adolescents: Associations with dietary intake. Prev. Med. 2000, 30, 381–391. [Google Scholar]
- Jeffery, R.W.; Adlis, S.A.; Forster, J.L. Prevalence of dieting among working men and women: The healthy worker project. Health Psychol. 1991, 10, 274. [Google Scholar]
- Anderson, J.W.; Konz, E.C.; Frederich, R.C.; Wood, C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001, 74, 579–584. [Google Scholar] [CrossRef]
- Weiss, E.C.; Galuska, D.A.; Khan, L.K.; Gillespie, C.; Serdula, M.K. Weight regain in US adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 2007, 33, 34–40. [Google Scholar] [PubMed]
- Bacon, L.; Aphramor, L. Weight Science: Evaluating the Evidence for a Paradigm Shift. Nutr. J. 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.-M.; Samuels, B.; Chatman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psychol. 2007, 62, 220. [Google Scholar] [PubMed]
- Anastasiou, C.A.; Karfopoulou, E.; Yannakoulia, M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism 2015, 64, 1395–1407. [Google Scholar] [CrossRef]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Jimenez, L.S.; Mendonça Chaim, F.H.; Mendonça Chaim, F.D.; Utrini, M.P.; Gestic, M.A.; Chaim, E.A.; Cazzo, E. Impact of Weight Regain on the Evolution of Non-alcoholic Fatty Liver Disease after Roux-en-Y Gastric Bypass: A 3-Year Follow-up. Obes. Surg. 2018, 28, 3131–3135. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar]
- Lien, L.F.; Haqq, A.M.; Arlotto, M.; Slentz, C.A.; Muehlbauer, M.J.; McMahon, R.L.; Rochon, J.; Gallup, D.; Bain, J.R.; Ilkayeva, O. The STEDMAN project: Biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS J. Integr. Biol. 2009, 13, 21–35. [Google Scholar]
- Wang, P.; Holst, C.; Wodzig, W.; Andersen, M.; Astrup, A.; Van Baak, M.; Larsen, T.; Jebb, S.; Kafatos, A.; Pfeiffer, A. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int. J. Obes. 2012, 36, 1545–1551. [Google Scholar]
- Linna, M.; Borg, P.; Kukkonen-Harjula, K.; Fogelholm, M.; Nenonen, A.; Ahotupa, M.; Vasankari, T. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int. J. Obes. 2007, 31, 245–253. [Google Scholar]
- Thomas, T.R.; Warner, S.O.; Dellsperger, K.C.; Hinton, P.S.; Whaley-Connell, A.T.; Rector, R.S.; Liu, Y.; Linden, M.A.; Chockalingam, A.; Thyfault, J.P. Exercise and the metabolic syndrome with weight regain. J. Appl. Physiol. 2010, 109, 3–10. [Google Scholar] [PubMed]
- Delbridge, E.A.; Prendergast, L.A.; Pritchard, J.E.; Proietto, J. One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: Does diet composition matter? Am. J. Clin. Nutr. 2009, 90, 1203–1214. [Google Scholar] [PubMed]
- Matsuo, T.; Kato, Y.; Murotake, Y.; Kim, M.; Unno, H.; Tanaka, K. An increase in high-density lipoprotein cholesterol after weight loss intervention is associated with long-term maintenance of reduced visceral abdominal fat. Int. J. Obes. 2010, 34, 1742–1751. [Google Scholar]
- Brownell, K.D.; Rodin, J. Medical, Metabolic, and Psychological Effects of Weight Cycling. Arch. Intern. Med. 1994, 154, 1325–1330. [Google Scholar] [CrossRef]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef]
- Hallberg, S.J.; Gershuni, V.M.; Hazbun, T.L.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. [Google Scholar]
- Kakinami, L.; Knäuper, B.; Brunet, J. Weight cycling is associated with adverse cardiometabolic markers in a cross-sectional representative US sample. J. Epidemiol. Community Health 2020, 74, 662–667. [Google Scholar] [CrossRef]
- Mackie, G.M.; Samocha-Bonet, D.; Tam, C.S. Does weight cycling promote obesity and metabolic risk factors? Obes. Res. Clin. Pract. 2017, 11, 131–139. [Google Scholar] [CrossRef]
- Korkeila, M.; Rissanen, A.; Kaprio, J.; Sørensen, T.I.; Koskenvuo, M. Weight-loss attempts and risk of major weight gain: A prospective study in Finnish adults. Am. J. Clin. Nutr. 1999, 70, 965–975. [Google Scholar]
- Pietiläinen, K.; Saarni, S.; Kaprio, J.; Rissanen, A. Does dieting make you fat? A twin study. Int. J. Obes. 2012, 36, 456–464. [Google Scholar]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Humblot, C.; Seyoum, Y.; Turpin, W.; Mrabt, R.; List, E.O.; Berryman, D.E.; Jensen, E.A.; Sustarsic, E.G.; Kopchick, J.J.; Ricort, J.-M. Long Term Weight Cycling Affects Fecal Microbiota of Mice. Mol. Nutr. Food Res. 2022, 66, 2200439. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Kim, M.H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.L.; Kim, H.N. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic(®) on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2017, 4, 260. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [PubMed]
- Qian, Y.; Zhao, X.; Kan, J. Preventive effect of resistant starch on activated carbon-induced constipation in mice. Exp. Ther. Med. 2013, 6, 228–232. [Google Scholar] [PubMed]
- Wang, J.; Huang, J.H.; Cheng, Y.F.; Yang, G.M. Banana resistant starch and its effects on constipation model mice. J. Med. Food 2014, 17, 902–907. [Google Scholar]
- Clarke, J.M.; Topping, D.L.; Bird, A.R.; Young, G.P.; Cobiac, L. Effects of high-amylose maize starch and butyrylated high-amylose maize starch on azoxymethane-induced intestinal cancer in rats. Carcinogenesis 2008, 29, 2190–2194. [Google Scholar]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Nofrarías, M.; Martínez-Puig, D.; Pujols, J.; Majó, N.; Pérez, J.F. Long-term intake of resistant starch improves colonic mucosal integrity and reduces gut apoptosis and blood immune cells. Nutrition 2007, 23, 861–870. [Google Scholar]
- Qin, S.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Tian, G.; Xuan, Y.; Su, Z.; Zeng, Q. Microbiome-metabolomics analysis insight into the effects of dietary resistant starch on intestinal integrity. Food Chem. 2023, 401, 134148. [Google Scholar]
- Zhao, Y.; Hasjim, J.; Li, L.; Jane, J.-L.; Hendrich, S.; Birt, D.F. Inhibition of azoxymethane-induced preneoplastic lesions in the rat colon by a cooked stearic acid complexed high-amylose cornstarch. J. Agric. Food Chem. 2011, 59, 9700–9708. [Google Scholar] [PubMed]
- Cray, N.; Zhao, Y.; Fang, Y.; Liu, P.; Pollak, L.; Duvick, S.; Birt, D.F.; Whitley, E.M. Effects of dietary resistant starch on the wnt signaling pathway and preneoplastic cells in the colons of azoxymethane-treated rats. Nutr. Cancer 2017, 69, 632–642. [Google Scholar] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [PubMed]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health beneficial effects of resistant starch on diabetes and obesity via regulation of gut microbiota: A review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [PubMed]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar]
- Higgins, J.A.; Jackman, M.R.; Brown, I.L.; Johnson, G.C.; Steig, A.; Wyatt, H.R.; Hill, J.O.; MacLean, P.S. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutr. Metab. 2011, 8, 49. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar]
- Roediger, W. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [PubMed]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Chen, Y.; Wang, Y.; Shi, N. Resistant starch and the gut microbiome: Exploring beneficial interactions and dietary impacts. Food Chem. X 2024, 21, 101118. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. WJG 2011, 17, 1519. [Google Scholar]
- Haub, M.D.; Hubach, K.L.; Al-Tamimi, E.K.; Ornelas, S.; Seib, P.A. Different types of resistant starch elicit different glucose reponses in humans. J. Nutr. Metab. 2010, 2010, 230501. [Google Scholar]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar]
- Si, X.; Zhou, Z.; Strappe, P.; Blanchard, C. A comparison of RS4-type resistant starch to RS2-type resistant starch in suppressing oxidative stress in high-fat-diet-induced obese rats. Food Funct. 2017, 8, 232–240. [Google Scholar] [CrossRef]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Bird, A.R.; Jackson, M.; Esterman, A.; Young, G.P. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J. Nutr. 2005, 135, 996–1001. [Google Scholar] [CrossRef]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.; Kuwahara, A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 2008, 59, 251–262. [Google Scholar]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar] [PubMed]
- Todesco, T.; Rao, A.V.; Bosello, O.; Jenkins, D. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am. J. Clin. Nutr. 1991, 54, 860–865. [Google Scholar]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Valeur, J.; Aas, A.-M. Short-chain fatty acids as a link between diet and cardiometabolic risk: A narrative review. Lipids Health Dis. 2023, 22, 40. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar]
- Li, Q.; Cao, L.; Tian, Y.; Zhang, P.; Ding, C.; Lu, W.; Jia, C.; Shao, C.; Liu, W.; Wang, D. Butyrate suppresses the proliferation of colorectal cancer cells via targeting pyruvate kinase M2 and metabolic reprogramming. Mol. Cell. Proteom. 2018, 17, 1531–1545. [Google Scholar]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor–dependent repression of claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Liu, S.; Reimer, M.; Ai, Y. In vitro digestibility of different types of resistant starches under high-temperature cooking conditions. Food Hydrocoll. 2020, 107, 105927. [Google Scholar]
- Gutiérrez, T.J.; Tovar, J. Update of the concept of type 5 resistant starch (RS5): Self-assembled starch V-type complexes. Trends Food Sci. Technol. 2021, 109, 711–724. [Google Scholar]
- Wang, Z.; Wang, S.; Xu, Q.; Kong, Q.; Li, F.; Lu, L.; Xu, Y.; Wei, Y. Synthesis and functions of resistant starch. Adv. Nutr. 2023, 14, 1131–1144. [Google Scholar] [PubMed]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Chapter 20—Mouse Models in Aging Research. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Burlington, VT, USA, 2007; pp. 637–672. [Google Scholar]
- Fox, J.; Barthold, S.W.; Davisson, M.T.; Newcomer, C.E.; Quimby, F.; Smith, A.L. The Mouse in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Lackner, C.; Gogg-Kamerer, M.; Zatloukal, K.; Stumptner, C.; Brunt, E.M.; Denk, H. Ballooned hepatocytes in steatohepatitis: The value of keratin immunohistochemistry for diagnosis. J. Hepatol. 2008, 48, 821–828. [Google Scholar] [CrossRef]
- Caldwell, S.; Ikura, Y.; Dias, D.; Isomoto, K.; Yabu, A.; Moskaluk, C.; Pramoonjago, P.; Simmons, W.; Scruggs, H.; Rosenbaum, N.; et al. Hepatocellular ballooning in NASH. J. Hepatol. 2010, 53, 719–723. [Google Scholar] [CrossRef]
- Jouihan, H. Measurement of liver triglyceride content. Bio-Protocol 2012, 2, e223. [Google Scholar]
- Tomcik, K.; Ibarra, R.A.; Sadhukhan, S.; Han, Y.; Tochtrop, G.P.; Zhang, G.F. Isotopomer enrichment assay for very short chain fatty acids and its metabolic applications. Anal. Biochem. 2011, 410, 110–117. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Smith, D.L., Jr.; Yang, Y.; Nagy, T.R.; Patki, A.; Vasselli, J.R.; Zhang, Y.; Dickinson, S.L.; Allison, D.B. Weight Cycling Increases Longevity Compared with Sustained Obesity in Mice. Obesity 2018, 26, 1733–1739. [Google Scholar] [CrossRef]
- Rossi, A.P.; Rubele, S.; Calugi, S.; Caliari, C.; Pedelini, F.; Soave, F.; Chignola, E.; Vittoria Bazzani, P.; Mazzali, G.; Dalle Grave, R. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity 2019, 27, 1068–1075. [Google Scholar]
- Zamarron, B.F.; Porsche, C.E.; Luan, D.; Lucas, H.R.; Mergian, T.A.; Martinez-Santibanez, G.; Cho, K.W.; DelProposto, J.L.; Geletka, L.M.; Muir, L.A. Weight regain in formerly obese mice hastens development of hepatic steatosis due to impaired adipose tissue function. Obesity 2020, 28, 1086–1097. [Google Scholar] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar]
- Seo, K.; Cho, H.-W.; Chun, J.L.; So, K.M.; Kim, K.H. Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet. Animals 2022, 12, 3440. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato resistant starch inhibits diet-induced obesity by modifying the composition of intestinal microbiota and their metabolites in obese mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of Banana Resistant Starch on the Biochemical Indexes and Intestinal Flora of Obese Rats Induced by a High-Fat Diet and Their Correlation Analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory function of buckwheat-resistant starch supplementation on lipid profile and gut microbiota in mice fed with a high-fat diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar]
- Rosado, C.P.; Rosa, V.H.C.; Martins, B.C.; Soares, A.C.; Santos, I.B.; Monteiro, E.B.; Moura-Nunes, N.; da Costa, C.A.; Mulder, A.d.R.P.; Daleprane, J.B. Resistant starch from green banana (Musa sp.) attenuates non-alcoholic fat liver accumulation and increases short-chain fatty acids production in high-fat diet-induced obesity in mice. Int. J. Biol. Macromol. 2020, 145, 1066–1072. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- CSIRO. Understanding Resistant Starch and Its Role in Gut Health. Available online: https://www.csiro.au/en/research/health-medical/nutrition/resistant-starch (accessed on 17 June 2024).
- Xu, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food Funct. 2020, 11, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef]
- Peterson, C.M.; Beyl, R.A.; Marlatt, K.L.; Martin, C.K.; Aryana, K.J.; Marco, M.L.; Martin, R.J.; Keenan, M.J.; Ravussin, E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Wutzke, K.D.; Schmidek, K.V. The effect of resistant starches on fat oxidation in healthy adults as measured by a 13CO2-breath test. Isot. Environ. Health Stud. 2017, 53, 553–562. [Google Scholar]
- Jiménez-Domínguez, G.; Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Ble-Castillo, D.J.; García-Vázquez, C.; Olvera-Hernández, V.; Pérez-Pimienta, B.; Diaz-Zagoya, J.C. Effects of acute ingestion of native banana starch on glycemic response evaluated by continuous glucose monitoring in obese and lean subjects. Int. J. Environ. Res. Public Health 2015, 12, 7491–7505. [Google Scholar] [CrossRef] [PubMed]
- Gargari, B.P.; Namazi, N.; Khalili, M.; Sarmadi, B.; Jafarabadi, M.A.; Dehghan, P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement. Ther. Med. 2015, 23, 810–815. [Google Scholar] [CrossRef]
- Goldsmith, F.; Guice, J.; Page, R.; Welsh, D.A.; Taylor, C.M.; Blanchard, E.E.; Luo, M.; Raggio, A.M.; Stout, R.W.; Carvajal-Aldaz, D.; et al. Obese ZDF rats fermented resistant starch with effects on gut microbiota but no reduction in abdominal fat. Mol. Nutr. Food Res. 2017, 61, 1501025. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar]
- Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Francisco-Luria, M.U.; Córdova-Uscanga, R.; Rodríguez-Hernández, A.; Méndez, J.D.; Díaz-Zagoya, J.C. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int. J. Environ. Res. Public Health 2010, 7, 1953–1962. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Wright, J.; Frost, G.S.; Robertson, M.D. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE 2012, 7, e40834. [Google Scholar]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [PubMed]
- Robertson, M.D.; Wright, J.W.; Loizon, E.; Debard, C.; Vidal, H.; Shojaee-Moradie, F.; Russell-Jones, D.; Umpleby, A.M. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3326–3332. [Google Scholar]
- Karimi, P.; Farhangi, M.A.; Sarmadi, B.; Gargari, B.; Zare Javid, A.; Pouraghaei, M.; Dehghan, P. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: A randomized controlled clinical trial. Ann. Nutr. Metab. 2016, 68, 85–93. [Google Scholar] [PubMed]
- Bindels, L.B.; Segura Munoz, R.R.; Gomes-Neto, J.C.; Mutemberezi, V.; Martínez, I.; Salazar, N.; Cody, E.A.; Quintero-Villegas, M.I.; Kittana, H.; de Los Reyes-Gavilán, C.G. Resistant starch can improve insulin sensitivity independently of the gut microbiota. Microbiome 2017, 5, 1–16. [Google Scholar]
- Nichenametla, S.N.; Weidauer, L.A.; Wey, H.E.; Beare, T.M.; Specker, B.L.; Dey, M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol. Nutr. Food Res. 2014, 58, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef]
- Ding, Y.; Xiao, Y.; Ouyang, Q.; Luo, F.; Lin, Q. Modulating the in vitro digestibility of chemically modified starch ingredient by a non-thermal processing technology of ultrasonic treatment. Ultrason. Sonochem. 2021, 70, 105350. [Google Scholar] [CrossRef]
- Toden, S.; Bird, A.R.; Topping, D.L.; Conlon, M.A. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer Biol. Ther. 2007, 6, 253–258. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef]
- Wang, A.; Liu, M.; Shang, W.; Liu, J.; Dai, Z.; Strappe, P.; Zhou, Z. Attenuation of metabolic syndrome in the ob/ob mouse model by resistant starch intervention is dose dependent. Food Funct. 2019, 10, 7940–7951. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Martínez, L.; Paz Lorenzo, M.; Martos-Moreno, G.Á.; Graell, M.; Barbas, C.; Rupérez, F.J.; Argente, J.; García, A. Short-chain fatty acids in plasma and feces: An optimized and validated LC-QqQ-MS method applied to study anorexia nervosa. Microchem. J. 2024, 200, 110255. [Google Scholar] [CrossRef]
- Higueras, C.; Rey, A.I.; Escudero, R.; Díaz-Regañón, D.; Rodríguez-Franco, F.; García-Sancho, M.; Agulla, B.; Sainz, A. Short-Chain and Total Fatty Acid Profile of Faeces or Plasma as Predictors of Food-Responsive Enteropathy in Dogs: A Preliminary Study. Animals 2022, 12, 89. [Google Scholar]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids With Gut Microbiota and Clinical Severity in Patients With Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Diet-Related Changes of Short-Chain Fatty Acids in Blood and Feces in Obesity and Metabolic Syndrome. Biology 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.A.; Tucker, M.; Marfori, Z.; Shishani, R.; Bustamante, J.M.; Moreno, R.; Goodson, M.L.; Ehrlich, A.; Taha, A.Y.; Lein, P.J.; et al. Dietary resistant starch supplementation increases gut luminal deoxycholic acid abundance in mice. Gut Microbes 2024, 16, 2315632. [Google Scholar] [CrossRef]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Canibe, N.; Montagne, L.; Freire, J.; Bosi, P.; Prates, J.A.M.; Tanghe, S.; Trevisi, P. Resistant starch reduces large intestinal pH and promotes fecal lactobacilli and bifidobacteria in pigs. Animal 2019, 13, 64–73. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ho, C.L. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, S.; Murakami, S.; Nishimoto, Y.; Higashi, K.; Watarai, N.; Umetsu, J.; Ishii, C.; Ito, Y.; Mori, Y.; et al. Integrated gut microbiome and metabolome analyses identified fecal biomarkers for bowel movement regulation by Bifidobacterium longum BB536 supplementation: A RCT. Comput. Struct. Biotechnol. J. 2022, 20, 5847–5858. [Google Scholar] [CrossRef]
- Sela, D.A.; Price, N.; Mills, D.A. Metabolism of bifidobacteria. Bifidobact. Genom. Mol. Asp. 2010, 45–70. [Google Scholar]
- Bello-Medina, P.C.; Hernández-Quiroz, F.; Pérez-Morales, M.; González-Franco, D.A.; Cruz-Pauseno, G.; García-Mena, J.; Díaz-Cintra, S.; Pacheco-López, G. Spatial Memory and Gut Microbiota Alterations Are Already Present in Early Adulthood in a Pre-clinical Transgenic Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 595583. [Google Scholar] [CrossRef]
- Sánchez, B.; Champomier-Vergès, M.C.; Collado Mdel, C.; Anglade, P.; Baraige, F.; Sanz, Y.; de los Reyes-Gavilán, C.G.; Margolles, A.; Zagorec, M. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microbiol. 2007, 73, 6450–6459. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium animalis subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Schmitz-Esser, S.; Mann, E.; Grüll, D.; Molnar, T.; Zebeli, Q. Adaptation of the cecal bacterial microbiome of growing pigs in response to resistant starch type 4. Appl. Environ. Microbiol. 2015, 81, 8489–8499. [Google Scholar]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- Ahmed, S.; Busetti, A.; Fotiadou, P.; Vincy Jose, N.; Reid, S.; Georgieva, M.; Brown, S.; Dunbar, H.; Beurket-Ascencio, G.; Delday, M.I.; et al. In vitro Characterization of Gut Microbiota-Derived Bacterial Strains with Neuroprotective Properties. Front. Cell. Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar]
- Usta-Gorgun, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electron. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar]
- Du, X.; Xiang, Y.; Lou, F.; Tu, P.; Zhang, X.; Hu, X.; Lyu, W.; Xiao, Y. Microbial Community and Short-Chain Fatty Acid Mapping in the Intestinal Tract of Quail. Animal 2020, 10, 1006. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar]
- Osborne, G.; Wu, F.; Yang, L.; Kelly, D.; Hu, J.; Li, H.; Jasmine, F.; Kibriya, M.G.; Parvez, F.; Shaheen, I. The association between gut microbiome and anthropometric measurements in Bangladesh. Gut Microbes 2020, 11, 63–76. [Google Scholar]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Luo, S.; He, L.; Zhang, H.; Li, Z.; Liu, C.; Chen, T. Arabinoxylan from rice bran protects mice against high-fat diet-induced obesity and metabolic inflammation by modulating gut microbiota and short-chain fatty acids. Food Funct. 2022, 13, 7707–7719. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. mBio 2020, 11, e02179-19. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Bulut, N.; Chambry, E.; Ruthes, A.; Iacomini, M.; Keshavarzian, A.; Johnson, T.A.; Hamaker, B.R. Dietary Fiber Hierarchical Specificity: The Missing Link for Predictable and Strong Shifts in Gut Bacterial Communities. mBio 2021, 12, e0102821. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. Tuning Expectations to Reality: Don’t Expect Increased Gut Microbiota Diversity with Dietary Fiber. J. Nutr. 2023, 153, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Diop, A.; Bittar, F.; Maraninchi, M.; Valero, R.; Armstrong, N.; Dubourg, G.; Labas, N.; Richez, M.; Delerce, J.; et al. Description of Mediterraneibacter massiliensis, gen. nov., sp. nov., a new genus isolated from the gut microbiota of an obese patient and reclassification of Ruminococcus faecis, Ruminococcus lactaris, Ruminococcus torques, Ruminococcus gnavus and Clostridium glycyrrhizinilyticum as Mediterraneibacter faecis comb. nov., Mediterraneibacter lactaris comb. nov., Mediterraneibacter torques comb. nov., Mediterraneibacter gnavus comb. nov. and Mediterraneibacter glycyrrhizinilyticus comb. nov. Antonie Van Leeuwenhoek 2018, 111, 2107–2128. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar]
- Torres-Maravilla, E.; Holowacz, S.; Delannoy, J.; Lenoir, L.; Jacouton, E.; Gervason, S.; Meynier, M.; Boucard, A.-S.; Carvalho, F.A.; Barbut, F.; et al. Serpin-positive Bifidobacterium breve CNCM I-5644 improves intestinal permeability in two models of irritable bowel syndrome. Sci. Rep. 2022, 12, 19776. [Google Scholar] [CrossRef]
- Pratt, C.; Campbell, M.D. The Effect of Bifidobacterium on Reducing Symptomatic Abdominal Pain in Patients with Irritable Bowel Syndrome: A Systematic Review. Probiot. Antimicrob. Proteins 2020, 12, 834–839. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Palsson, O.S.; Maier, D.; Carroll, I.; Galanko, J.A.; Leyer, G.; Ringel, Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011, 45, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Rajilić–Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [PubMed]
- Gong, H.; Gao, H.; Ren, Q.; He, J. The abundance of bifidobacterium in relation to visceral obesity and serum uric acid. Sci. Rep. 2022, 12, 13073. [Google Scholar] [CrossRef]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.Z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zou, Y.; Gong, J.; Ge, Z.; Lin, X.; Zhang, W.; Huang, H.; Zhao, J.; Saw, P.E.; et al. A high-fat diet promotes cancer progression by inducing gut microbiota–mediated leucine production and PMN-MDSC differentiation. Proc. Natl. Acad. Sci. USA 2024, 121, e2306776121. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, H.-F.; Wu, C.-C.; Chen, C.-L.; Ni, Y.-H. Pathogenic effects of Desulfovibrio in the gut on fatty liver in diet-induced obese mice and children with obesity. J. Gastroenterol. 2022, 57, 913–925. [Google Scholar] [CrossRef]
- Xie, R.; Gu, Y.; Li, M.; Li, L.; Yang, Y.; Sun, Y.; Zhou, B.; Liu, T.; Wang, S.; Liu, W.; et al. Desulfovibrio vulgaris interacts with novel gut epithelial immune receptor LRRC19 and exacerbates colitis. Microbiome 2024, 12, 4. [Google Scholar] [CrossRef]
- Kushkevych, I.; Castro Sangrador, J.; Dordević, D.; Rozehnalová, M.; Černý, M.; Fafula, R.; Vítězová, M.; Rittmann, S.K.R. Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People. J. Clin. Med. 2020, 9, 1920. [Google Scholar] [CrossRef]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Schipper, L.; Harvey, L.; van der Beek, E.M.; van Dijk, G. Home alone: A systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obes. Rev. 2018, 19, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.; van Heijningen, S.; Karapetsas, G.; van der Beek, E.M.; van Dijk, G. Individual housing of male C57BL/6J mice after weaning impairs growth and predisposes for obesity. PLoS ONE 2020, 15, e0225488. [Google Scholar] [CrossRef]
- Chourbaji, S.; Zacher, C.; Sanchis-Segura, C.; Spanagel, R.; Gass, P. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned helplessness paradigm in male mice. Behav. Brain Res. 2005, 164, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, O.; Teilmann, A.C.; Jacobsen, K.R.; Abelson, K.S.; Hau, J. The lonely mouse—Single housing affects serotonergic signaling integrity measured by 8-OH-DPAT-induced hypothermia in male mice. PLoS ONE 2014, 9, e111065. [Google Scholar] [CrossRef]
- Macedo, G.C.; Morita, G.M.; Domingues, L.P.; Favoretto, C.A.; Suchecki, D.; Quadros, I.M.H. Consequences of continuous social defeat stress on anxiety- and depressive-like behaviors and ethanol reward in mice. Horm. Behav. 2018, 97, 154–161. [Google Scholar] [CrossRef]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar]
- Ndongo, S.; Lagier, J.C.; Fournier, P.E.; Raoult, D.; Khelaifia, S. “Prevotellamassilia timonensis,” a new bacterial species isolated from the human gut. New Microbes New Infect. 2016, 13, 102–103. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong-Nguyen, K.; O’Hely, M.; Kowalski, G.M.; McGee, S.L.; Aston-Mourney, K.; Connor, T.; Mahmood, M.Q.; Rivera, L.R. The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice. Nutrients 2024, 16, 3138. https://doi.org/10.3390/nu16183138
Phuong-Nguyen K, O’Hely M, Kowalski GM, McGee SL, Aston-Mourney K, Connor T, Mahmood MQ, Rivera LR. The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice. Nutrients. 2024; 16(18):3138. https://doi.org/10.3390/nu16183138
Chicago/Turabian StylePhuong-Nguyen, Kate, Martin O’Hely, Greg M. Kowalski, Sean L. McGee, Kathryn Aston-Mourney, Timothy Connor, Malik Q. Mahmood, and Leni R. Rivera. 2024. "The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice" Nutrients 16, no. 18: 3138. https://doi.org/10.3390/nu16183138
APA StylePhuong-Nguyen, K., O’Hely, M., Kowalski, G. M., McGee, S. L., Aston-Mourney, K., Connor, T., Mahmood, M. Q., & Rivera, L. R. (2024). The Impact of Yoyo Dieting and Resistant Starch on Weight Loss and Gut Microbiome in C57Bl/6 Mice. Nutrients, 16(18), 3138. https://doi.org/10.3390/nu16183138






