Yerba Mate (Ilex paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Yerba Mate Preparation
2.3. Chemical Analysis of Yerba Mate
2.3.1. Chemicals
2.3.2. Equipment
2.3.3. Analysis of Extracts Compounds
2.4. Dextran Sodium Sulfate (DSS) Colitis Model
2.5. Colon Explants
2.6. Immunohistochemistry
2.7. Histological Analysis of the Colon
2.8. Cytokine Assays
2.9. Macrophage Polarization
2.10. Flow Cytometry
2.11. Microbiota Analysis
2.12. Statistical Analysis
3. Results
3.1. YM Supplementation Attenuates Colitis Symptoms and Improves Survival in the DSS-Induced Colitis Mouse Model

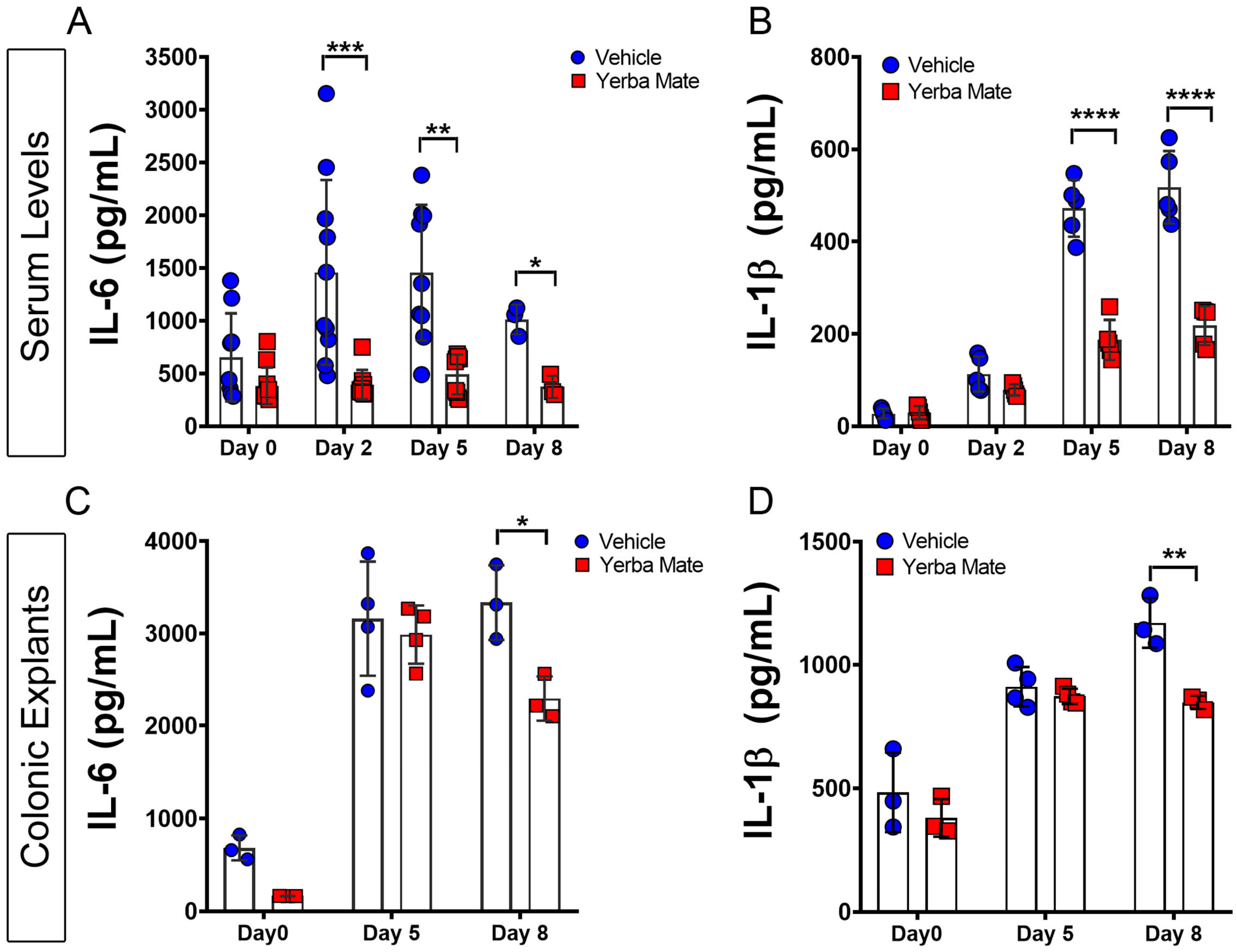
3.2. YM Reduces Local and Systemic Inflammation Triggered by DSS Administration
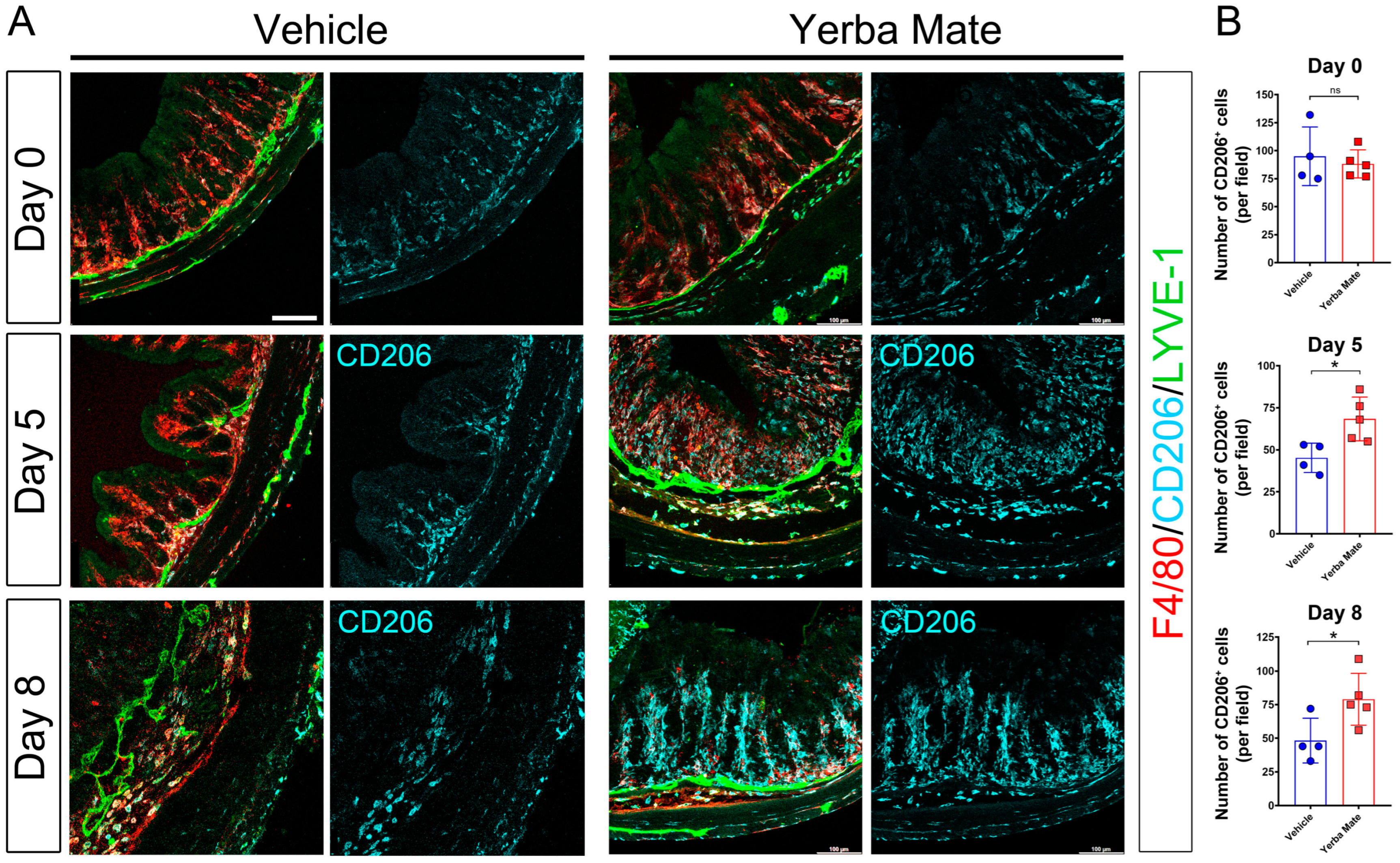
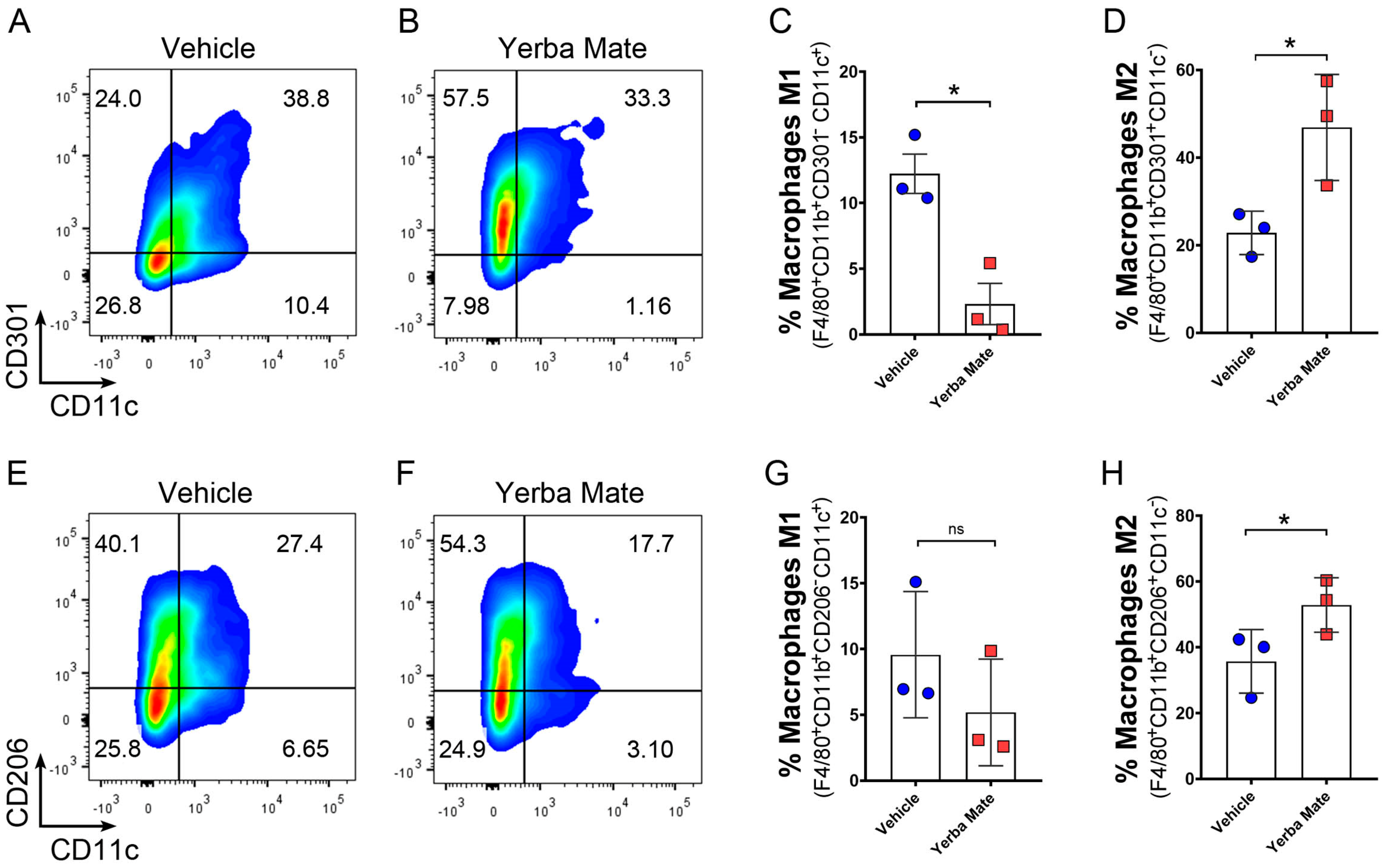
3.3. Increased M2 Macrophage Infiltration in Colon from YM-Treated Mice
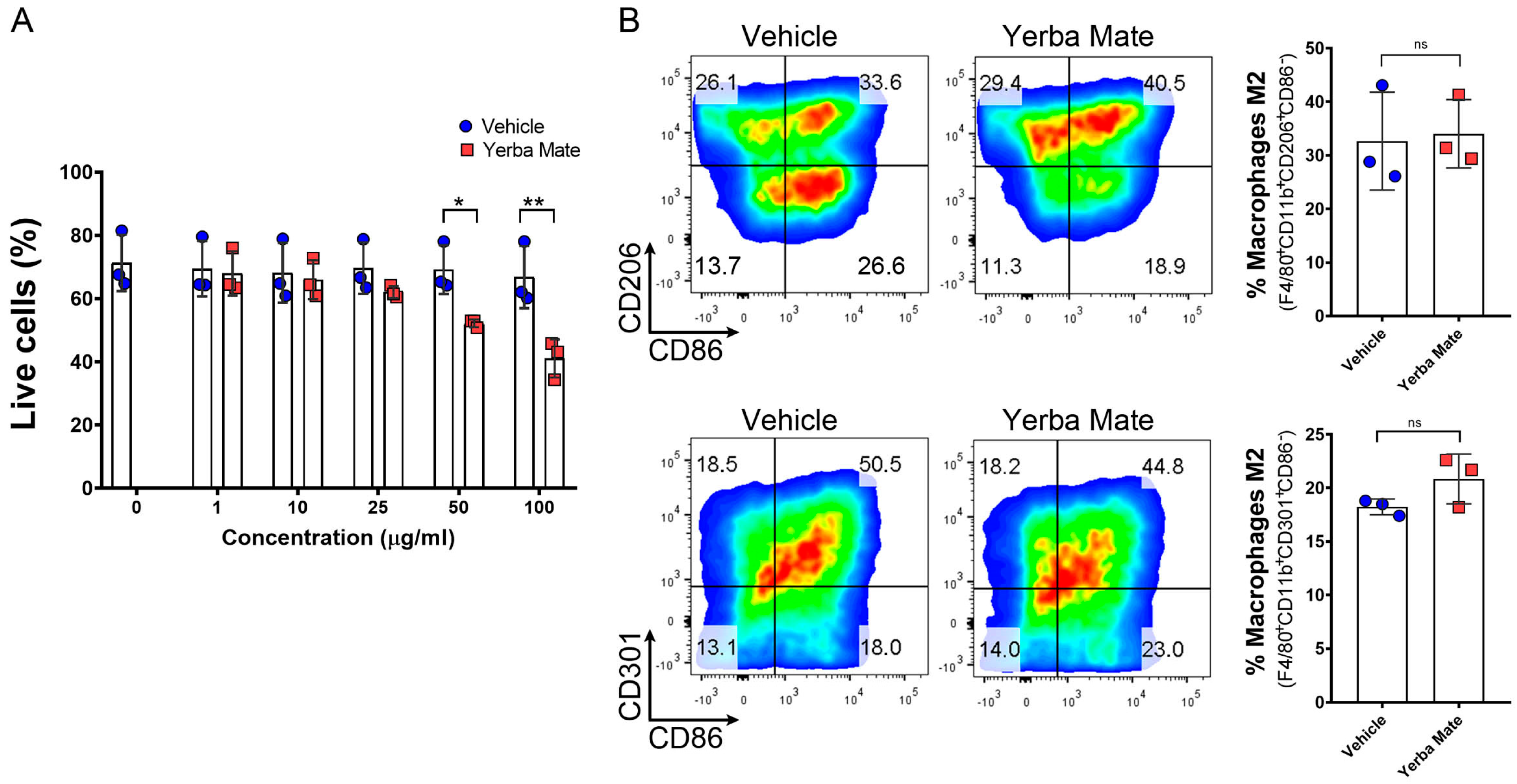
3.4. YM Administration Promotes M2 Macrophage Polarization via an Indirect Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gugliucci, A. Antioxidant Effects of Ilex paraguariensis: Induction of Decreased Oxidability of Human LDL in Vivo. Biochem. Biophys. Res. Commun. 1996, 224, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Berté, K.A.S.; Beux, M.R.; Spada, P.K.W.D.S.; Salvador, M.; Hoffmann-Ribani, R. Chemical Composition and Antioxidant Activity of Yerba-Mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) Extract as Obtained by Spray Drying. J. Agric. Food Chem. 2011, 59, 5523–5527. [Google Scholar] [CrossRef] [PubMed]
- Stein, F.L.P.; Schmidt, B.; Furlong, E.B.; Soares, L.A.S.; Soares, M.C.F.; Vaz, M.R.C.; Baisch, A.L.M. Vascular Responses to Extractable Fractions of Ilex paraguariensis in Rats Fed Standard and High-Cholesterol Diets. Biol. Res. Nurs. 2005, 7, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Arçari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; DeOliveira, C.C.; Gotardo, É.M.; Pedrazzoli, J.; Gambero, A.; Ferraz, L.F.C.; Carvalho, P.d.O.; et al. Anti-Inflammatory Effects of Yerba Maté Extract (Ilex paraguariensis) Ameliorate Insulin Resistance in Mice with High Fat Diet-Induced Obesity. Mol. Cell. Endocrinol. 2011, 335, 110–115. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, L.S.; Rogero, M.M.; Cortez, M.; Yamada, M.; Jacob, P.S.; Bastos, D.H.M.; Borelli, P.; Fock, R.A. The Effects of Yerba Maté (Ilex paraguariensis) Consumption on IL-1, IL-6, TNF-α and IL-10 Production by Bone Marrow Cells in Wistar Rats Fed a High-Fat Diet. Int. J. Vitam. Nutr. Res. 2013, 83, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Burris, K.P.; Harte, F.M.; Michael Davidson, P.; Stewart, C.N.; Zivanovic, S. Composición y Propiedades Bioactivas de La Yerba Mate (Ilex paraguariensis A. St.-Hil.): Una Revisión. Chil. J. Agric. Res. 2012, 72, 268–275. [Google Scholar] [CrossRef]
- Clifford, M.N.; Ramirez-Martinez, J.R. Chlorogenic Acids and Purine Alkaloids Contents of Maté (Ilex paraguariensis) Leaf and Beverage. Food Chem. 1990, 35, 13–21. [Google Scholar] [CrossRef]
- Isolabella, S.; Cogoi, L.; López, P.; Anesini, C.; Ferraro, G.; Filip, R. Study of the Bioactive Compounds Variation during Yerba Mate (Ilex paraguariensis) Processing. Food Chem. 2010, 122, 695–699. [Google Scholar] [CrossRef]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-Induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Yue, W.; Xu, X.; Li, B.; Zou, L.; He, R. Chemerin Aggravates DSS-Induced Colitis by Suppressing M2 Macrophage Polarization. Cell. Mol. Immunol. 2014, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, L.; Shi, Y.; Cao, H.; Liu, L.; Kay Washington, M.; Chaturvedi, R.; Israel, D.A.; Cao, H.; Wang, B.; et al. Berberine Promotes Recovery of Colitis and Inhibits Inflammatory Responses in Colonic Macrophages and Epithelial Cells in DSS-Treated Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G504–G514. [Google Scholar] [CrossRef] [PubMed]
- Ruder, B.; Becker, C. At the Forefront of the Mucosal Barrier: The Role of Macrophages in the Intestine. Cells 2020, 9, 2162. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Arranz, A.; Doxaki, C.; Vergadi, E.; de La Torre, Y.M.; Vaporidi, K.; Lagoudaki, E.D.; Ieronymaki, E.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; et al. Akt1 and Akt2 Protein Kinases Differentially Contribute to Macrophage Polarization. Proc. Natl. Acad. Sci. USA 2012, 109, 9517–9522. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, J.; Nie, Y.; Shi, X.; Liu, Y.; Li, F.; Zhang, X.L. Disequilibrium of M1 and M2 Macrophages Correlates with the Development of Experimental Inflammatory Bowel Diseases. Immunol. Investig. 2014, 43, 638–652. [Google Scholar] [CrossRef]
- Long, J.; Liu, X.K.; Kang, Z.P.; Wang, M.X.; Zhao, H.M.; Huang, J.Q.; Xiao, Q.P.; Liu, D.Y.; Zhong, Y.B. Ginsenoside Rg1 Ameliorated Experimental Colitis by Regulating the Balance of M1/M2 Macrophage Polarization and the Homeostasis of Intestinal Flora. Eur. J. Pharmacol. 2022, 917, 174742. [Google Scholar] [CrossRef]
- Horuluoglu, B.H.; Kayraklioglu, N.; Tross, D.; Klinman, D. PAM3 Protects against DSS-Induced Colitis by Altering the M2:M1 Ratio. Sci. Rep. 2020, 10, 6078. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokaria, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, Y.; Lin, L.; Lin, X.; Gong, W.; Xia, R.; He, J.; Sheng, J.; Cai, H.; et al. Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Ulcerative Colitis: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 14494. [Google Scholar] [CrossRef]
- Sahakian, L.; Robinson, A.M.; Sahakian, L.; Stavely, R.; Kelley, M.R.; Nurgali, K. APE1/Ref-1 as a Therapeutic Target for Inflammatory Bowel Disease. Biomolecules 2023, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.A.; Higgins, P.D.R. The Role of Upadacitinib in the Treatment of Ulcerative Colitis. Immunotherapy 2023, 15, 713–727. [Google Scholar] [CrossRef]
- Liu, J.; Di, B.; Xu, L.L. Recent Advances in the Treatment of IBD: Targets, Mechanisms and Related Therapies. Cytokine Growth Factor. Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Zografos, C.G.; Konstadoulakis, M.M.; Papalois, A.E. Combination Treatment of Inflammatory Bowel Disease: Present Status and Future Perspectives. World J. Gastroenterol. 2024, 30, 2068–2080. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef]
- Lewis, J.D.; Parlett, L.E.; Jonsson Funk, M.L.; Brensinger, C.; Pate, V.; Wu, Q.; Dawwas, G.K.; Weiss, A.; Constant, B.D.; McCauley, M.; et al. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology 2023, 165, 1197–1205.e2. [Google Scholar] [CrossRef] [PubMed]
- Kontola, K.; Oksanen, P.; Huhtala, H.; Jussila, A. Increasing Incidence of Inflammatory Bowel Disease, with Greatest Change among the Elderly: A Nationwide Study in Finland, 2000–2020. J. Crohn’s Colitis 2023, 17, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Yerba Mate—Statistics & Facts|Statista. Available online: https://www.statista.com/topics/7368/yerba-mate/#topicHeader__wrapper (accessed on 20 December 2022).
- Karp, N.A.; Mason, J.; Beaudet, A.L.; Benjamini, Y.; Bower, L.; Braun, R.E.; Brown, S.D.M.; Chesler, E.J.; Dickinson, M.E.; Flenniken, A.M.; et al. Prevalence of Sexual Dimorphism in Mammalian Phenotypic Traits. Nat. Commun. 2017, 8, 15475. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC Analysis of Diverse Grape and Wine Phenolics Using Direct Injection and Multidetection by DAD and Fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, G.; Yang, X.; Chen, Z.; Zhou, L. Behavioral Abnormalities in C57BL/6 Mice with Chronic Ulcerative Colitis Induced by DSS. BMC Gastroenterol. 2023, 23, 84. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Liu, Y.D.; Zhu, J.Q.; Zhou, Y.L.; Chen, H.T.; Xu, J.; Zhao, H.L.; Guo, X.; Shi, W.; et al. Selection Strategy of Dextran Sulfate Sodium-Induced Acute or Chronic Colitis Mouse Models Based on Gut Microbial Profile. BMC Microbiol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Li, D.; Ding, S.; Luo, M.; Chen, J.; Zhang, Q.; Liu, Y.; Li, A.; Zhong, S.; Ding, J. Differential Diagnosis of Acute and Chronic Colitis in Mice by Optical Coherence Tomography. Quant. Imaging Med. Surg. 2022, 12, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Herrada, A.A.; Olate-Briones, A.; Lazo-Amador, R.; Liu, C.; Hernández-Rojas, B.; Riadi, G.; Escobedo, N. Lymph Leakage Promotes Immunosuppression by Enhancing Anti-Inflammatory Macrophage Polarization. Front. Immunol. 2022, 13, 841641. [Google Scholar] [CrossRef]
- Wen, X.; Wang, H.G.; Zhang, M.N.; Zhang, M.H.; Wang, H.; Yang, X.Z. Fecal Microbiota Transplantation Ameliorates Experimental Colitis via Gut Microbiota and T-Cell Modulation. World J. Gastroenterol. 2021, 27, 2834–2849. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A Guide to Histomorphological Evaluation of Intestinal Inflammation in Mouse Models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar] [PubMed]
- Hong, J.Y.; Chung, Y.; Steenrod, J.; Chen, Q.; Lei, J.; Comstock, A.T.; Goldsmith, A.M.; Bentley, J.K.; Sajjan, U.S.; Hershenson, M.B. Macrophage Activation State Determines the Response to Rhinovirus Infection in a Mouse Model of Allergic Asthma. Respir. Res. 2014, 15, 63. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Lu, Q.; Gao, Y.; Cai, Y.; Sui, A.; Su, T.; Shen, X.; Xie, B. Identification of Different Macrophage Subpopulations with Distinct Activities in a Mouse Model of Oxygen-Induced Retinopathy. Int. J. Mol. Med. 2017, 40, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Unold, D.; Shifley, K.; Amir, E.; Hung, K.; Bos, N.; Salzman, N. Enteric Salmonellosis Disrupts the Microbial Ecology of the Murine Gastrointestinal Tract. Infect. Immun. 2008, 76, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.S.; Casagrande, L.; Model, J.F.A.; dos Santos, J.T.; Hoefel, A.L.; Kucharski, L.C. Effect of Yerba Mate (Ilex paraguariensis) Extract on the Metabolism of Diabetic Rats. Biomed. Pharmacother. 2018, 105, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Isidro, R.A.; Appleyard, C.B. Colonic Macrophage Polarization in Homeostasis, Inflammation, and Cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G59–G73. [Google Scholar] [CrossRef]
- Iyengar, P.; Godoy-Brewer, G.; Maniyar, I.; White, J.; Maas, L.; Parian, A.M.; Limketkai, B. Herbal Medicines for the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 934. [Google Scholar] [CrossRef]
- Lu, Q.; Xie, Y.; Luo, J.; Gong, Q.; Li, C. Natural Flavones from Edible and Medicinal Plants Exhibit Enormous Potential to Treat Ulcerative Colitis. Front. Pharmacol. 2023, 14, 1168990. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Wang, J.; Zheng, T.; Wu, C.; Cui, M.; Feng, Y.; Ye, H.; Dong, Z.; Dang, Y. Plant Polyphenols Attenuate DSS-Induced Ulcerative Colitis in Mice via Antioxidation, Anti-Inflammation and Microbiota Regulation. Int. J. Mol. Sci. 2023, 24, 10828. [Google Scholar] [CrossRef]
- Lee, Y.; Bae, C.S.; Ahn, T. Chlorogenic Acid Attenuates Pro-Inflammatory Response in the Blood of Streptozotocin-Induced Diabetic Rats. Lab. Anim. Res. 2022, 38, 37. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Li, Q.R.; Tan, S.R.; Yang, L.; He, W.; Chen, L.; Shen, F.X.; Wang, Z.; Wang, H.F. Mechanism of Chlorogenic Acid in Alveolar Macrophage Polarization in Klebsiella Pneumoniae-Induced Pneumonia. J. Leukoc. Biol. 2022, 112, 9–21. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Tian, D.; Hu, C.Y.; Meng, Y.H. Chlorogenic Acid Ameliorates High-Fat and High-Fructose Diet-Induced Cognitive Impairment via Mediating the Microbiota-Gut-Brain Axis. J. Agric. Food Chem. 2022, 70, 2600–2615. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhen, W.; Bai, D.; Zhong, J.; Zhang, R.; Zhang, H.; Zhang, Y.; Ito, K.; Zhang, B.; Ma, Y. Effects of Dietary Chlorogenic Acid on Cecal Microbiota and Metabolites in Broilers during Lipopolysaccharide-Induced Immune Stress. Front. Microbiol. 2024, 15, 1347053. [Google Scholar] [CrossRef]
- Håkansson, Å.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological Alteration and Changes of Gut Microbiota after Dextran Sulfate Sodium (DSS) Administration in Mice. Clin. Exp. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef]
- Tikunov, A.Y.; Fedorets, V.A.; Shrainer, E.V.; Morozov, V.V.; Bystrova, V.I.; Tikunova, N.V. Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. J. Clin. Med. 2023, 12, 7702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, X.; Wu, X.; Zhang, C.; Liu, J.; Hou, S. Eubacterium rectale Contributes to Colorectal Cancer Initiation via Promoting Colitis. Gut Pathog. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Asadzadeh Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Nadalian, B.; Nadalian, B.; Houri, H. Overrepresentation of Enterobacteriaceae and Escherichia coli Is the Major Gut Microbiome Signature in Crohn’s Disease and Ulcerative Colitis; a Comprehensive Metagenomic Analysis of IBDMDB Datasets. Front. Cell Infect. Microbiol. 2022, 12, 1015890. [Google Scholar] [CrossRef]
- Sovran, B.; Planchais, J.; Jegou, S.; Straube, M.; Lamas, B.; Natividad, J.M.; Agus, A.; Dupraz, L.; Glodt, J.; Da Costa, G.; et al. Enterobacteriaceae Are Essential for the Modulation of Colitis Severity by Fungi. Microbiome 2018, 6, 152. [Google Scholar] [CrossRef]
- Liu, Q.; Jian, W.; Wang, L.; Yang, S.; Niu, Y.; Xie, S.J.; Hayer, K.; Chen, K.; Zhang, Y.; Guo, Y.; et al. Alleviation of DSS-Induced Colitis in Mice by a New-Isolated Lactobacillus acidophilus C4. Front. Microbiol. 2023, 14, 1137701. [Google Scholar] [CrossRef]
- Yee, S.M.; Choi, H.; Seon, J.E.; Ban, Y.J.; Kim, M.J.; Seo, J.E.; Seo, J.H.; Kim, S.; Moon, S.H.; Yun, C.H.; et al. Axl Alleviates DSS-Induced Colitis by Preventing Dysbiosis of Gut Microbiota. Sci. Rep. 2023, 13, 5371. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- El-Sonbaty, S.M.; Araby, E. Microbial Regulation and Protective Effects of Yerba Mate (Ilex paraguariensis) in Gamma-Irradiated Mice Intestine. J. Radiat. Res. Appl. Sci. 2014, 7, 64–73. [Google Scholar] [CrossRef]
- Santos, D.; Frota, E.G.; Vargas, B.K.; Tonieto Gris, C.C.; dos Santos, L.F.; Bertolin, T.E. What Is the Role of Phenolic Compounds of Yerba Mate (Ilex paraguariensis) in Gut Microbiota? Phytochemistry 2022, 203, 113341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, H.; Wang, T.; Shi, F. Ginsenoside Rg1 Attenuates the Inflammatory Response in DSS-Induced Mice Colitis. Int. Immunopharmacol. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Modulation of Gut Microbiota by Ilex kudingcha Improves Dextran Sulfate Sodium-Induced Colitis. Food Res. Int. 2019, 126, 108595. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jeldres, T.; Pizarro, B.; Ascui, G.; Orellana, M.; Cerda-Villablanca, M.; Alvares, D.; De La Vega, A.; Cannistra, M.; Cornejo, B.; Baéz, P.; et al. Ethnicity Influences Phenotype and Clinical Outcomes: Comparing a South American with a North American Inflammatory Bowel Disease Cohort. Medicine 2022, 101, e30216. [Google Scholar] [CrossRef]
- Souza, R.F.; Caetano, M.A.F.; Magalhães, H.I.R.; Castelucci, P. Study of Tumor Necrosis Factor Receptor in the Inflammatory Bowel Disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef]
- da Silva, M.D.; Bobinski, F.; Sato, K.L.; Kolker, S.J.; Sluka, K.A.; Santos, A.R.S. IL-10 Cytokine Released from M2 Macrophages Is Crucial for Analgesic and Anti-Inflammatory Effects of Acupuncture in a Model of Inflammatory Muscle Pain. Mol. Neurobiol. 2015, 51, 19–31. [Google Scholar] [CrossRef]
- Makita, N.; Hizukuri, Y.; Yamashiro, K.; Murakawa, M.; Hayashi, Y. IL-10 Enhances the Phenotype of M2 Macrophages Induced by IL-4 and Confers the Ability to Increase Eosinophil Migration. Int. Immunol. 2015, 27, 131–141. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olate-Briones, A.; Albornoz-Muñoz, S.; Rodríguez-Arriaza, F.; Rodríguez-Vergara, V.; Aguirre, J.M.; Liu, C.; Peña-Farfal, C.; Escobedo, N.; Herrada, A.A. Yerba Mate (Ilex paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization. Nutrients 2024, 16, 1616. https://doi.org/10.3390/nu16111616
Olate-Briones A, Albornoz-Muñoz S, Rodríguez-Arriaza F, Rodríguez-Vergara V, Aguirre JM, Liu C, Peña-Farfal C, Escobedo N, Herrada AA. Yerba Mate (Ilex paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization. Nutrients. 2024; 16(11):1616. https://doi.org/10.3390/nu16111616
Chicago/Turabian StyleOlate-Briones, Alexandra, Sofía Albornoz-Muñoz, Francisca Rodríguez-Arriaza, Valentina Rodríguez-Vergara, Juan Machuca Aguirre, Chaohong Liu, Carlos Peña-Farfal, Noelia Escobedo, and Andrés A. Herrada. 2024. "Yerba Mate (Ilex paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization" Nutrients 16, no. 11: 1616. https://doi.org/10.3390/nu16111616
APA StyleOlate-Briones, A., Albornoz-Muñoz, S., Rodríguez-Arriaza, F., Rodríguez-Vergara, V., Aguirre, J. M., Liu, C., Peña-Farfal, C., Escobedo, N., & Herrada, A. A. (2024). Yerba Mate (Ilex paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization. Nutrients, 16(11), 1616. https://doi.org/10.3390/nu16111616





