The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review
Abstract
1. Introduction
2. Microecologics
2.1. Probiotics
2.2. Prebiotics
2.3. Synbiotics
2.4. Postbiotics
3. Microecologics Improve the Function of Locomotor System and Physical Performance
3.1. The Promotive Effects on Bone System
3.2. Improvement of Skeletal Muscle Content and Strength by Microecologics
3.3. The Promotive Effects of Microecologics on Muscular Endurance
4. Improvement of Mental Performance by Microecologics
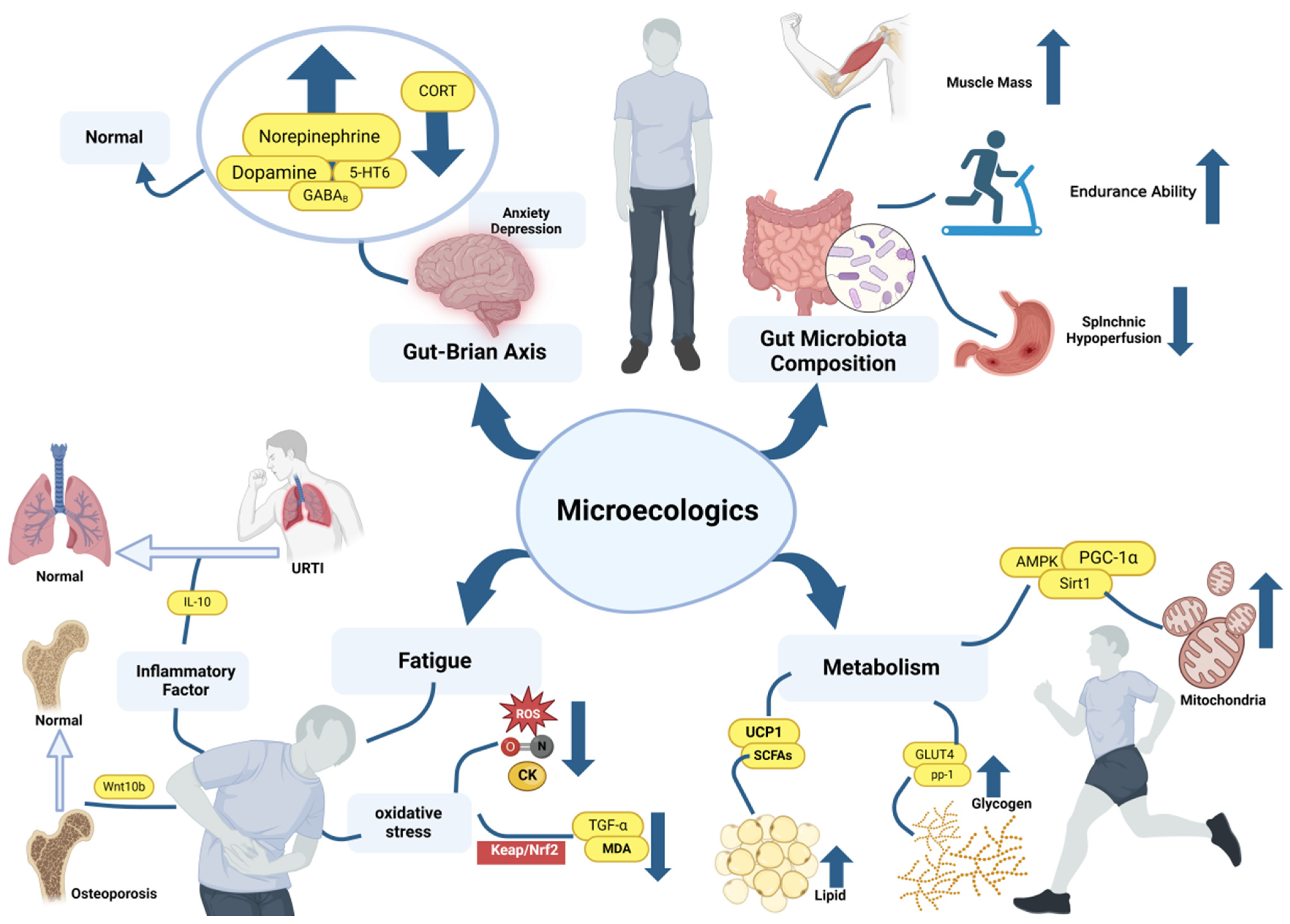
5. Promotion Mechanisms of Microecologics on Exercise Ability
5.1. Improvement of Gut Microbial Composition
5.2. Improving Mental State through Microbiota–Gut–Brain Axis
5.3. Enhancing the Activity of AMPK
5.4. Improving Glycogen and Lipid Metabolism
5.5. Reduce the Damage Caused by Oxidative Stress
5.6. Regulating on the Immune System
5.7. Relieving Bone Loss Caused by Excessive Glucocorticoid
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Zhu, H.; Ren, Z.; Zang, Y.; Hua, H.; Lu, J.; Xu, Q.; Zhu, S. Effects of Microecological Preparations on Obese Patients after Bariatric Surgery: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2020, 2020, 8724546. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Probiotics Market Size, Share & Trends Analysis Report by Product (Food & Beverages, Dietary Supplements), by Ingredient (Bacteria, Yeast), by Distribution Channel, by End-Use, by Region, and Segment Forecasts, 2023–2030. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/probiotics-market (accessed on 10 March 2024).
- Prebiotics Market Size, Share & Trends Analysis Report by Ingredients (FOS, Inulin, GOS, MOS), by Application (Food & Beverages, Dietary Supplements, Animal Feed), by Region, and Segment Forecasts, 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/prebiotics-market (accessed on 20 March 2024).
- Lee, C.C.; Liao, Y.C.; Lee, M.C.; Cheng, Y.C.; Chiou, S.Y.; Lin, J.S.; Huang, C.C.; Watanabe, K. Different Impacts of Heat-Killed and Viable Lactiplantibacillus plantarum TWK10 on Exercise Performance, Fatigue, Body Composition, and Gut Microbiota in Humans. Microorganisms 2022, 10, 2181. [Google Scholar] [CrossRef]
- Yeh, W.L.; Hsu, Y.J.; Ho, C.S.; Ho, H.H.; Kuo, Y.W.; Tsai, S.Y.; Huang, C.C.; Lee, M.C. Lactobacillus plantarum PL-02 Supplementation Combined with Resistance Training Improved Muscle Mass, Force, and Exercise Performance in Mice. Front. Nutr. 2022, 9, 896503. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of Human-origin Lactobacillus plantarum PL-02 in Improving Muscle Mass, Exercise Performance and Anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef]
- Meng, X.; Gao, Y.; Qi, H.; Ding, Y.; Sun, Y. Clinical Application Value of Lactobacillus Plantarum PS128 in Patients with Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2022, 20, 560–566. [Google Scholar] [CrossRef]
- Aykut, M.N.; Erdoğan, E.N.; Çelik, M.N.; Gürbüz, M. An Updated View of the Effect of Probiotic Supplement on Sports Performance: A Detailed Review. Curr. Nutr. Rep. 2024, Online ahead of print. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily Probiotic’s (Lactobacillus casei Shirota) Reduction of Infection Incidence in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef]
- Schepper, J.D.; Collins, F.L.; Rios-Arce, N.D.; Raehtz, S.; Schaefer, L.; Gardinier, J.D.; Britton, R.A.; Parameswaran, N.; McCabe, L.R. Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J. Bone Min. Res. 2019, 34, 681–698. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Liao, S.; Tang, W.; Peng, L.; Song, G. Bifidobacterium animalis subsp. lactis BB-12 Improves the State Anxiety and Sports Performance of Young Divers Under Stress Situations: A Single-Arm, Prospective Proof-of-Concept Study. Front. Psychol. 2020, 11, 570298. [Google Scholar]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides Improve Mineral Absorption and Bone Properties in Growing Rats through Gut Fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef]
- Zuhl, M.N.; Lanphere, K.R.; Kravitz, L.; Mermier, C.M.; Schneider, S.; Dokladny, K.; Moseley, P.L. Effects of Oral Glutamine Supplementation on Exercise-Induced Gastrointestinal Permeability and Tight Junction Protein Expression. J. Appl. Physiol. 2014, 116, 183–191. [Google Scholar] [CrossRef]
- Mao, Y.H.; Wang, M.; Yuan, Y.; Yan, J.K.; Peng, Y.; Xu, G.; Weng, X. Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model. Nutrients 2023, 15, 4206. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, A Synbiotic Supplement, Increases Fecal Lactobacillus Paracasei but has Little Effect on Immunity in Healthy Physically Active Individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bo, L.; Zhou, E.; Chen, Y.; Naranmandakh, S.; Xie, W.; Ru, Q.; Chen, L.; Zhu, Z.; et al. Progress of Linking Gut Microbiota and Musculoskeletal Health: Casualty, Mechanisms, and Translational Values. Gut Microbes 2023, 15, 2263207. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome Potentiates Endurance Exercise through Intestinal Acetate Production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of Heat-Killed Lactococcus lactis JCM 5805 on Immunity and Fatigue during Consecutive High Intensity Exercise in Male Athletes: A Randomized, Placebo-Controlled, Double-Blinded Trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, J.; Zhou, J.; Zhi, C.; Bai, Y.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Anti-Obesity Effect and Mechanism of Chitooligosaccharides Were Revealed Based on Lipidomics in Diet-Induced Obese Mice. Molecules 2023, 28, 5595. [Google Scholar] [CrossRef]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human Gut Microbiome Impacts Skeletal Muscle Mass via Gut Microbial Synthesis of the Short-chain Fatty Acid Butyrate among Healthy Menopausal Women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef]
- Ouwehand, A.C. Journal of Functional Foods special issue: Probiotics, prebiotics, microbiota and health. J. Funct. Foods 2024, 112, 105975. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on Immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or Synbiotic Alters the Gut Microbiota and Metabolism in a Randomised Controlled Trial of Weight Management in Overweight Adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.C.; Kwak, K.; Kim, J.S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, Prebiotics, Synbiotics and Insulin Sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Huang, W.C.; Lee, M.C.; Lee, C.C.; Ng, K.S.; Hsu, Y.J.; Tsai, T.Y.; Young, S.L.; Lin, J.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe that Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral Administration of the Probiotic Lactobacillus fermentum VRI-003 and Mucosal Immunity in Endurance Athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for Next-generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of Prebiotics by Human Colonic Microbiota In Vitro and Short-Chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as Functional Foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef]
- Li, D.D.; Ma, J.M.; Li, M.J.; Gao, L.L.; Fan, Y.N.; Zhang, Y.N.; Tao, X.J.; Yang, J.J. Supplementation of Lycium barbarum Polysaccharide Combined with Aerobic Exercise Ameliorates High-Fat-Induced Nonalcoholic Steatohepatitis via AMPK/PPARα/PGC-1α Pathway. Nutrients 2022, 14, 3247. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional Considerations for Single-Stage Ultra-Marathon Training and Racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- Morgan, P.T.; Breen, L. The Role of Protein Hydrolysates for Exercise-Induced Skeletal Muscle Recovery and Adaptation: A Current Perspective. Nutr. Metab. 2021, 18, 44. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary Polyphenol Impact on Gut Health and Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between Dietary Flavonoids and the Gut Microbiome: A Comprehensive Review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Chan, C.K.Y.; Tao, J.; Chan, O.S.; Li, H.B.; Pang, H. Preventing Respiratory Tract Infections by Synbiotic Interventions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for Improved Human Health: Recent Developments, Challenges, and Opportunities. Annu. Rev. Food Sci. Technol. 2018, 9, 451–479. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef]
- Ismaeel, A.; Valentino, T.R.; Burke, B.; Goh, J.; Saliu, T.P.; Albathi, F.; Owen, A.; McCarthy, J.J.; Wen, Y. Acetate and Succinate Benefit Host Muscle Energetics as Exercise-Associated Post-biotics. Physiol. Rep. 2023, 11, e15848. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Świetlicka, I.; Muszyński, S.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Żmuda, A.; Rycerz, K.; Blicharski, T.; et al. Effects of Maternal Treatment with β-hydroxy-β-metylbutyrate and 2-oxoglutaric Acid on Femur Development in Offspring of Minks of the Standard Dark Brown Type. J. Anim. Physiol. Anim. Nutr. 2018, 102, e299–e308. [Google Scholar] [CrossRef]
- Quach, D.; Britton, R.A. Gut Microbiota and Bone Health. Adv. Exp. Med. Biol. 2017, 1033, 47–58. [Google Scholar]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The Gut-Bone Axis: How Bacterial Metabolites Bridge the Distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Cheng, S.; Qi, X.; Ma, M.; Zhang, L.; Cheng, B.; Liang, C.; Liu, L.; Li, P.; Kafle, O.P.; Wen, Y.; et al. Assessing the Relationship between Gut Microbiota and Bone Mineral Density. Front. Genet. 2020, 11, 6. [Google Scholar] [CrossRef]
- Malmir, H.; Ejtahed, H.S.; Soroush, A.R.; Mortazavian, A.M.; Fahimfar, N.; Ostovar, A.; Esmaillzadeh, A.; Larijani, B.; Hasani-Ranjbar, S. Probiotics as a New Regulator for Bone Health: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2021, 2021, 3582989. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-Chain Fatty Acids Regulate Systemic Bone Mass and Protect from Pathological Bone Loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The Gut Microbiota Influences Skeletal Muscle Mass and Function in Mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Nucci, R.A.B.; Filho, V.A.N.; Jacob-Filho, W.; Otoch, J.P.; Pessoa, A.F.M. Role of Nutritional Supplements on Gut-Muscle Axis Across Age: A Mini-Review. Cell Physiol. Biochem. 2023, 57, 161–168. [Google Scholar]
- Chou, M.Y.; Wong, Y.C.; Wang, S.Y.; Chi, C.H.; Wang, T.H.; Huang, M.J.; Huang, P.H.; Li, P.H.; Wang, M.F. Potential Antidepressant Effects of a Dietary Supplement from Huáng Qí and its Complex in Aged Senescence-Accelerated Mouse Prone-8 mice. Front. Nutr. 2023, 10, 1235780. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Probiotic Influences on Motor Skills: A Review. Curr. Neuropharmacol. 2023, 21, 2481–2486. [Google Scholar] [CrossRef]
- Gupta, N.; El-Gawaad, N.S.A.; Mallasiy, L.O.; Gupta, H.; Yadav, V.K.; Alghamdi, S.; Qusty, N.F. Microbial Dysbiosis and the Aging Process: A Review on the Potential Age-Deceleration Role of Lactiplantibacillus plantarum. Front. Microbiol. 2024, 15, 1260793. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut Microbes and Muscle Function: Can Probiotics Make Our Muscles Stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The Histone Deacetylase Inhibitor Butyrate Improves Metabolism and Reduces Muscle Atrophy during Aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- de la Motte, S.J.; Gribbin, T.C.; Lisman, P.; Murphy, K.; Deuster, P.A. Systematic Review of the Association between Physical Fitness and Musculoskeletal Injury Risk: Part 2-Muscular Endurance and Muscular Strength. J. Strength. Cond. Res. 2017, 31, 3218–3234. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Jang, Y.-J.; Oh, I.; Chung, J.H.; Moon, J.S. Limosilactobacillus reuteri ID-D01 Improves Exercise Performance and Reduces Muscle Fatigue in C57BL/6 mice through Regulation of Oxidative Capacity. J. Funct. Foods 2024, 116, 106125. [Google Scholar] [CrossRef]
- Yu, C.H.; Lai, C.C.; Chen, J.H.; Chen, I.C.; Tai, H.L.; Fu, S.K. Effect of Lactobacillus plantarum PS128 on Neuromuscular Efficiency After a Half-Marathon. Front. Physiol. 2023, 14, 1254985. [Google Scholar] [CrossRef]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance Exercise and Gut Microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Lefevre, C.; Bindels, L.B. Role of the Gut Microbiome in Skeletal Muscle Physiology and Pathophysiology. Curr. Osteoporos. Rep. 2022, 20, 422–432. [Google Scholar] [CrossRef]
- Roberts, J.D.; Suckling, C.A.; Peedle, G.Y.; Murphy, J.A.; Dawkins, T.G.; Roberts, M.G. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients 2016, 8, 733. [Google Scholar] [CrossRef]
- Haywood, B.A.; Black, K.E.; Baker, D.; McGarvey, J.; Healey, P.; Brown, R.C. Probiotic Supplementation Reduces the Duration and Incidence of Infections but not Severity in Elite Rugby Union Players. J. Sci. Med. Sport 2014, 17, 356–360. [Google Scholar] [CrossRef]
- Coleman, J.L.; Hatch-McChesney, A.; Small, S.D.; Allen, J.T.; Sullo, E.; Agans, R.T.; Fagnant, H.S.; Bukhari, A.S.; Karl, J.P. Orally Ingested Probiotics, Prebiotics, and Synbiotics as Countermeasures for Respiratory Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 2277–2295. [Google Scholar] [CrossRef]
- Peluso, M.A.; Guerra de Andrade, L.H. Physical Activity and Mental Health: The Association between Exercise and Mood. Clinics 2005, 60, 61–70. [Google Scholar] [CrossRef]
- Mohr, A.E.; Pyne, D.B.; Leite, G.S.F.; Akins, D.; Pugh, J. A Systematic Scoping Review of Study Methodology for Randomized Controlled Trials Investigating Probiotics in Athletic and Physically Active Populations. J. Sport Health Sci. 2024, 13, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Williams, C.J.; Torquati, L.; Li, Z.; Lea, R.A.; Croci, I.; Keating, E.; Little, J.P.; Eynon, N.; Coombes, J.S. Oligofructose-Enriched Inulin Intake, Gut Microbiome Characteristics, and the VO2 Peak Response to High-Intensity Interval Training in Healthy Inactive Adults. J. Nutr. 2022, 152, 680–689. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, Z.; Luo, Y.; Yang, F.; Cao, F.; Luo, F.; Lin, Q. Dietary Polysaccharides Exert Anti-Fatigue Functions via the Gut-Muscle Axis: Advances and Prospectives. Foods. 2023, 12, 3083. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A Critical Review on the Impacts of β-glucans on Gut Microbiota and Human Health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, X.; Li, R.W.; Hou, E.; Wang, Y.; Xue, C.; Tang, Q. Neoagarotetraose Protects Mice against Intense Exercise-Induced Fatigue Damage by Modulating Gut Microbial Composition and Function. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef]
- Berg, D.; Clemente, J.C.; Colombel, J.F. Can Inflammatory Bowel Disease be Permanently Treated with Short-Term Interventions on the Microbiome? Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 781–795. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate Intake during Exercise and Performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournède, N.; Doré, J.; Melchior, J.C. Altered Host-Gut Microbes Symbiosis in Severely Malnourished Anorexia Nervosa (AN) Patients Undergoing Enteral Nutrition: An Explicative Factor of Functional Intestinal Disorders? Clin. Nutr. 2019, 38, 2304–2310. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A Combination of Quercetin and Resveratrol Reduces Obesity in High-Fat Diet-Fed Rats by Modulation of Gut Microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- van Wijck, K.; Wijnands, K.A.; Meesters, D.M.; Boonen, B.; van Loon, L.J.; Buurman, W.A.; Dejong, C.H.; Lenaerts, K.; Poeze, M. L-citrulline Improves Splanchnic Perfusion and Reduces Gut Injury during Exercise. Med. Sci. Sports Exerc. 2014, 46, 2039–2046. [Google Scholar] [CrossRef]
- Chunduri, A.; Reddy, S.D.M.; Jahanavi, M.; Reddy, C.N. Gut-Brain Axis, Neurodegeneration and Mental Health: A Personalized Medicine Perspective. Indian. J. Microbiol. 2022, 62, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.K.; Han, P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the Probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of Intestinal Microbiota on the Gut-Brain Axis. Iran. J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef]
- Gouttebarge, V.; Castaldelli-Maia, J.M.; Gorczynski, P.; Hainline, B.; Hitchcock, M.E.; Kerkhoffs, G.M.; Rice, S.M.; Reardon, C.L. Occurrence of Mental Health Symptoms and Disorders in Current and Former Elite Athletes: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2019, 53, 700–706. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Janzen, N.R.; Whitfield, J.; Hoffman, N.J. Interactive Roles for AMPK and Glycogen from Cellular Energy Sensing to Exercise Metabolism. Int. J. Mol. Sci. 2018, 19, 3344. [Google Scholar] [CrossRef]
- Tsuji, A.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. Tactics with Prebiotics for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease via the Improvement of Mitophagy. Int. J. Mol. Sci. 2023, 24, 5465. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Jaafar, M.H.; Lau, A.S.Y.; Ong, J.S.; Chuah, L.O.; Yap, K.P.; Azzam, G.; Azlan, A.; Liong, M.T. Lactobacilli modulated AMPK Activity and Prevented Telomere Shortening in Ageing Rats. Benef. Microbes 2019, 10, 883–892. [Google Scholar] [CrossRef]
- Jeong, H.W.; Cho, S.Y.; Kim, S.; Shin, E.S.; Kim, J.M.; Song, M.J.; Park, P.J.; Sohn, J.H.; Park, H.; Seo, D.B.; et al. Chitooligosaccharide Induces Mitochondrial Biogenesis and Increases Exercise Endurance through the Activation of Sirt1 and AMPK in rats. PLoS ONE 2012, 7, e40073. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Fact. 2020, 9, 168. [Google Scholar] [CrossRef]
- Carey, R.A.; Montag, D. Exploring the Relationship between Gut Microbiota and Exercise: Short-Chain Fatty Acids and their Role in Metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, C.S.; Choi, I.D.; Jeong, J.W.; Ku, H.K.; Ra, J.H.; Kim, T.Y.; Kim, G.B.; Sim, J.H.; Ahn, Y.T. The Anti-Diabetic Activity of Bifidobacterium lactis HY8101 In Vitro and In Vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]
- Nan, X.; Zhao, W.; Liu, W.H.; Li, Y.; Li, N.; Hong, Y.; Cui, J.; Shang, X.; Feng, H.; Hung, W.L.; et al. Bifidobacterium animalis subsp. lactis BL-99 Ameliorates Colitis-Related Lung Injury in Mice by Modulating Short-Chain Fatty Acid Production and Inflammatory Monocytes/Macrophages. Food Funct. 2023, 14, 1099–1112. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Hu, K.; Yang, Y.; Ma, J.; Zhai, Y.; Jiang, Y.; Zhang, D. Anti-Fatigue Effects of Lycium barbarum Polysaccharide and Effervescent Tablets by Regulating Oxidative Stress and Energy Metabolism in Rats. Int. J. Mol. Sci. 2022, 23, 10920. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Reardon, T.F.; Allen, D.G. Iron Injections in Mice Increase Skeletal Muscle Iron Content, Induce Oxidative Stress and Reduce Exercise Performance. Exp. Physiol. 2009, 94, 720–730. [Google Scholar] [CrossRef]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Huang, C.C. Live and Heat-Killed Probiotic Lactobacillus paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Chen, L.H.; Huang, S.Y.; Huang, K.C.; Hsu, C.C.; Yang, K.C.; Li, L.A.; Chan, C.H.; Huang, H.Y. Lactobacillus paracasei PS23 Decelerated Age-related Muscle Loss by Ensuring Mitochondrial Function in SAMP8 Mice. Aging 2019, 11, 756–770. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Zhang, L.; Chen, Q.; Tan, F.; Zhao, X. Effect of Lactobacillus fermentum HFY03 on the Antifatigue and Antioxidation Ability of Running Exhausted Mice. Oxid. Med. Cell Longev. 2021, 2021, 8013681. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef]
- Zhonghui, Z.; Xiaowei, Z.; Fang, F. Ganoderma lucidum Polysaccharides Supplementation Attenuates Exercise-Induced Oxidative Stress in Skeletal Muscle of Mice. Saudi J. Biol. Sci. 2014, 21, 119–123. [Google Scholar] [CrossRef]
- Hu, X.; Mu, L.; Zhu, L.; Chang, X.; Nie, L.; Wang, L.; Li, G. Lycium barbarum Polysaccharides Attenuate Cardiovascular Oxidative Stress Injury by Enhancing the Keap1/Nrf2 Signaling Pathway in Exhaustive Exercise Rats. Mol. Med. Rep. 2021, 24, 643. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Wang, X.; Zhang, C.L. Protective effects of Radix pseudostellariae polysaccharides against exercise-induced oxidative stress in male rats. Exp. Ther. Med. 2013, 5, 1089–1092. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Bruunsgaard, H.; Jensen, M.; Krzywkowski, K.; Ostrowski, K. Exercise and Immune Function: Effect of Ageing and Nutrition. Proc. Nutr. Soc. 1999, 58, 733–742. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can Exercise Affect Immune Function to Increase Susceptibility to Infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The Athlete Gut Microbiome and its Relevance to Health and Performance: A Review. Sports Med. 2022, 52 (Suppl. 1), 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gusmao-Silva, G.; Aguiar, S.L.F.; Miranda, M.C.G.; Guimarães, M.A.; Alves, J.L.; Vieira, A.T.; Cara, D.C.; Miyoshi, A.; Azevedo, V.A.; Oliveira, R.P.; et al. Hsp65-Producing Lactococcocus lactis Prevents Antigen-Induced Arthritis in Mice. Front. Immunol. 2020, 11, 562905. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A Central Player in Innate Immune Signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ikeda, T.; Homma, T.; Akimoto, T.; Suzuki, Y.; Kawahara, T. Effects of Acute Hypoxia on Metabolic and Hormonal Responses to Resistance Exercise. Med. Sci. Sports Exerc. 2010, 42, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.L.; Schlesinger, N.; Androulakis, I.P. Chronopharmacology of Glucocorticoids. Adv. Drug Deliv. Rev. 2019, 151–152, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr. Osteoporos. Rep. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Wend, P.; Wend, K.; Krum, S.A.; Miranda-Carboni, G.A. The Role of WNT10B in Physiology and Disease. Acta Physiol. 2012, 204, 34–51. [Google Scholar] [CrossRef]
- Schepper, J.D.; Collins, F.; Rios-Arce, N.D.; Kang, H.J.; Schaefer, L.; Gardinier, J.D.; Raghuvanshi, R.; Quinn, R.A.; Britton, R.; Parameswaran, N.; et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J. Bone Min. Res. 2020, 35, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Chargo, N.J.; Schepper, J.D.; Rios-Arce, N.; Kang, H.J.; Gardinier, J.D.; Parameswaran, N.; McCabe, L.R. Lactobacillus reuteri 6475 Prevents Bone Loss in a Clinically Relevant Oral Model of Glucocorticoid-Induced Osteoporosis in Male CD-1 Mice. JBMR Plus 2023, 7, e10805. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Irwin, R.; Bierhalter, H.; Schepper, J.; Britton, R.A.; Parameswaran, N.; McCabe, L.R. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS ONE 2016, 11, e0153180. [Google Scholar] [CrossRef]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef] [PubMed]
| Supplements | Reference | Doses | Duration | Subjects | Sample, Size | Pathways | Main Findings |
|---|---|---|---|---|---|---|---|
| Probiotic | |||||||
| L. plantarum TWK10 | [5] | 0, 2.05 × 10⁸, or 1.03 × 10⁹ CFU/kg/d | 6 weeks | Mice | n = 24 (all male) | Reduce inflammation through gut-muscle axis | Increased muscle mass, improved forelimb grip strength |
| L. plantarum PL-02 | [6] | 2.05 × 109 CFU/kg/d | 4 weeks | Mice | n = 32 (all male) | Generate SCFAs to improve the activity of AMPK and GLUT4 | |
| [7] | 0, 2.05 × 109, 4.10 × 109 and 1.03 × 1010 CFU/kg/d | 4 weeks | Mice | n = 40 (all male) | / | Reduce excess metabolic products | |
| L. Plantarum PS128 | [8] | Two capsules (3 × 1010 CFU/capsule) | 4 weeks | Human | n = 8 (male 4, female 4) | Improve microbial composition | Improve endurance, lower limb explosive strength, lower muscle damage indices et.al |
| Lactobacillus casei Shirota | [9] | 3 × 1010 (CFU) (80 mL/bottle) | 6 weeks | Human | n = 30 (not mentioned) | Gut–brain axis | Reduce competitive anxiety, perceived stress |
| [10] | 6.5 × 109 live LcS/pot | 16 weeks | Human | n = 84 (male 54, female 30) | Increase SIgA content | Reduce the incidence of URTI | |
| Lactobacillus reuteri | [11] | 3.3 × 108 CFU/mL | 4 weeks | Mice | n = 30 (all male) | Improve microbial composition | Increase the quantity and activity of osteoblasts |
| Bifidobacterium animalis subsp. lactis BB-12 | [12] | 1 × 109 CFU/100 g | 8 weeks | Human | n = 21 (all male) | Increase the abundance of Bifidobacteriaceae | Improve cognitive state anxiety, somatic state anxiety, and anxiety emotion |
| Prebiotic | |||||||
| GOSs | [13] | 0, 2, 4, 6, or 8% GOS by weight | 8 weeks | Mice | n = 75 (all male) | Improve microbial composition | Increase the quantity and activity of osteoblasts, increase the gut absorption of Ca2+ and Mg2+ |
| XOSs | [14] | 0, 1, 2, or 4% by concentration | 30 days | Mice | n = 96 (all male) | Upregulate the expression of (TRPV6) and Na+/Ca2+ transporters | Increase BMD |
| Glutamine | [15] | 0.9 g/kg of fat-free mass per day | 7 days | Human | n = 8 (male 5, female 3) | Activate HSF-1 | Reduce increased GI permeability and intestinal cellular damage |
| Konjac glucomannan | [16] | 1.25, 2.50, and 5.00 mg/mL | 42 days | Mice | n = 30 (all male) | Increase the abundance of bacteroidetes | Increase tolerance to excessive exercise |
| Synbiotic | |||||||
| BiosourceTM Gut Balance, Probiotech Pharma, Chr. Hansen A/S, Horsholm, Denmark | [17] | (Described in 2.3) | 21 days | Human | n = 22 (all male) | Improve gut microbial composition | Elicit a 14-fold increase in the recovery of fecal L. paracasei. |
| Postbiotic | |||||||
| Butyrate | [18] | high-fat diet at 5% wt/wt | 10 weeks | Mice | / | Increase Mitochondrial Biogenesis | Increase muscle mass |
| HKB-3 | [19] | 1 × 109 CFU/rat | 4 weeks | Rats | n = 52 (all male) | ||
| Acetate | [20] | 1 μL/h | 6 weeks | Mice | n = 12 (all male) | As an energy source for glycogen synthesis | Improve endurance ability |
| HKL.lactis JCM 5805 | [21] | 100 billion cells of HK LC-Plasma per day | 13 days | Human | n = 25 (all male) | Activate pDC activity | Alleviate the symptom of URTI and fatigue caused by high intensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Chen, Y.; Wang, M.; Zhang, Y.; Yuan, Y.; Hou, H.; Mao, Y.-H. The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review. Nutrients 2024, 16, 1602. https://doi.org/10.3390/nu16111602
Yang K, Chen Y, Wang M, Zhang Y, Yuan Y, Hou H, Mao Y-H. The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review. Nutrients. 2024; 16(11):1602. https://doi.org/10.3390/nu16111602
Chicago/Turabian StyleYang, Keer, Yonglin Chen, Minghan Wang, Yishuo Zhang, Yu Yuan, Haoyang Hou, and Yu-Heng Mao. 2024. "The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review" Nutrients 16, no. 11: 1602. https://doi.org/10.3390/nu16111602
APA StyleYang, K., Chen, Y., Wang, M., Zhang, Y., Yuan, Y., Hou, H., & Mao, Y.-H. (2024). The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review. Nutrients, 16(11), 1602. https://doi.org/10.3390/nu16111602






