Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Eligibility Criteria
2.3. Study Design
2.4. Measurements and Procedures
2.5. Statistics
3. Results
3.1. Baseline Characteristics
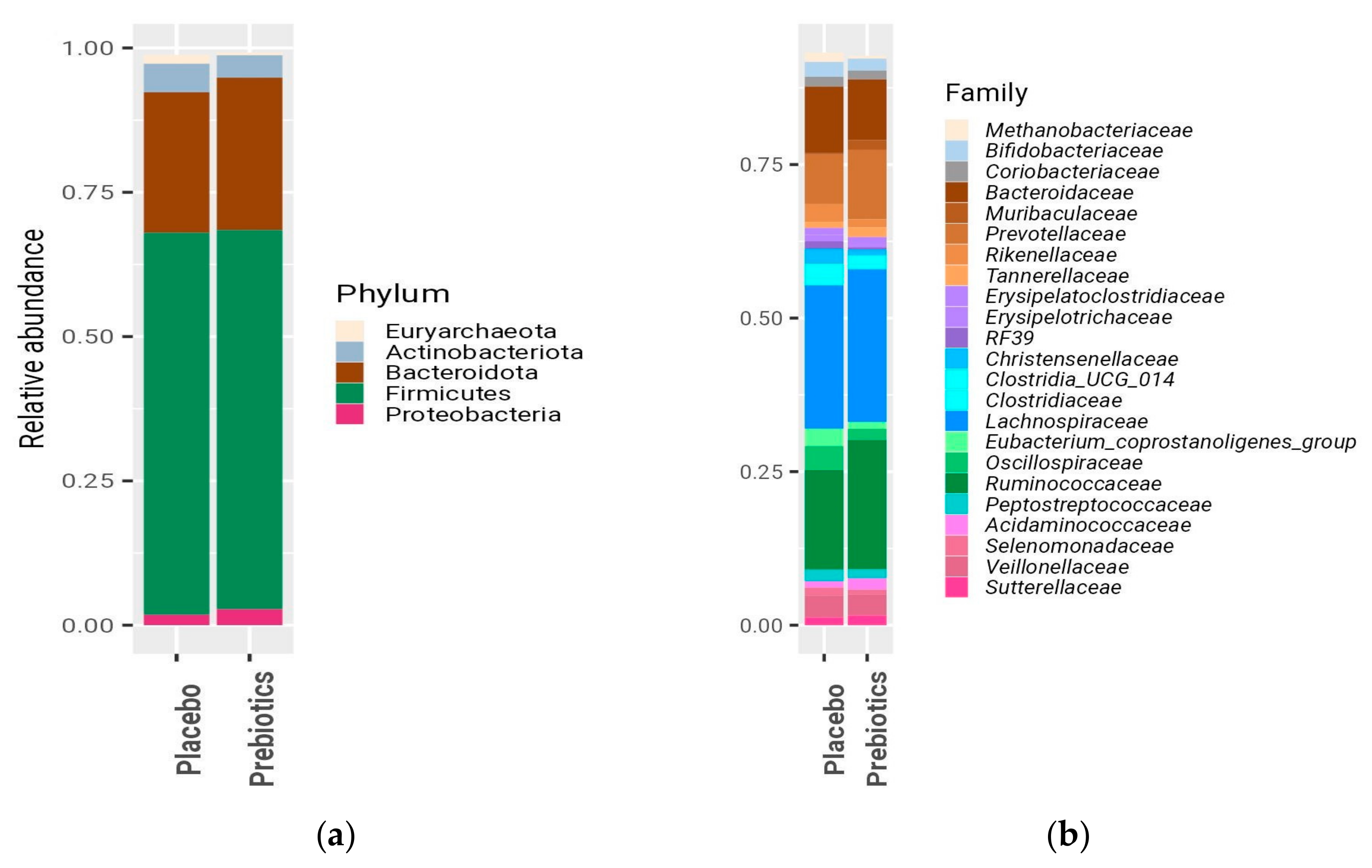
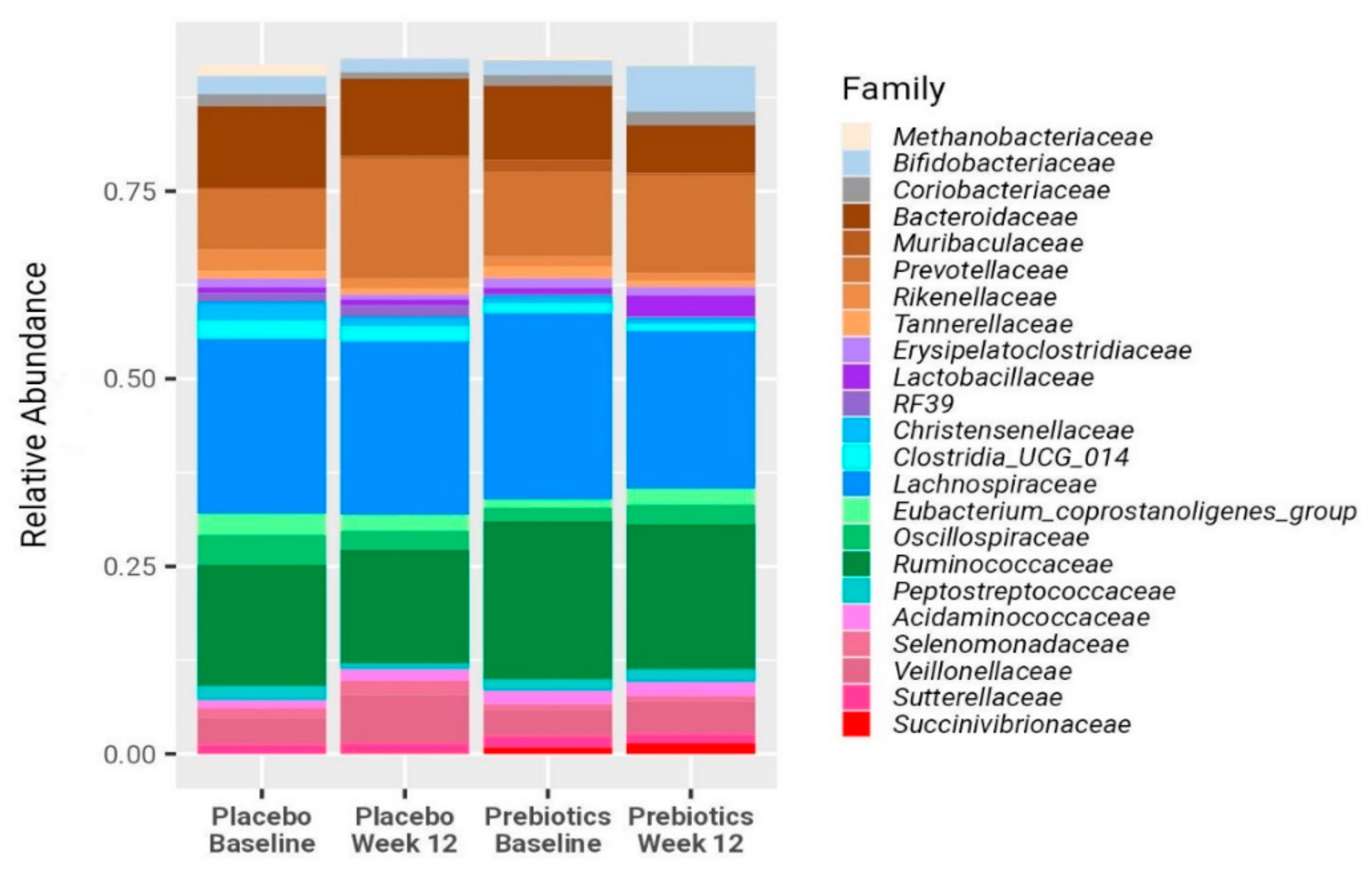
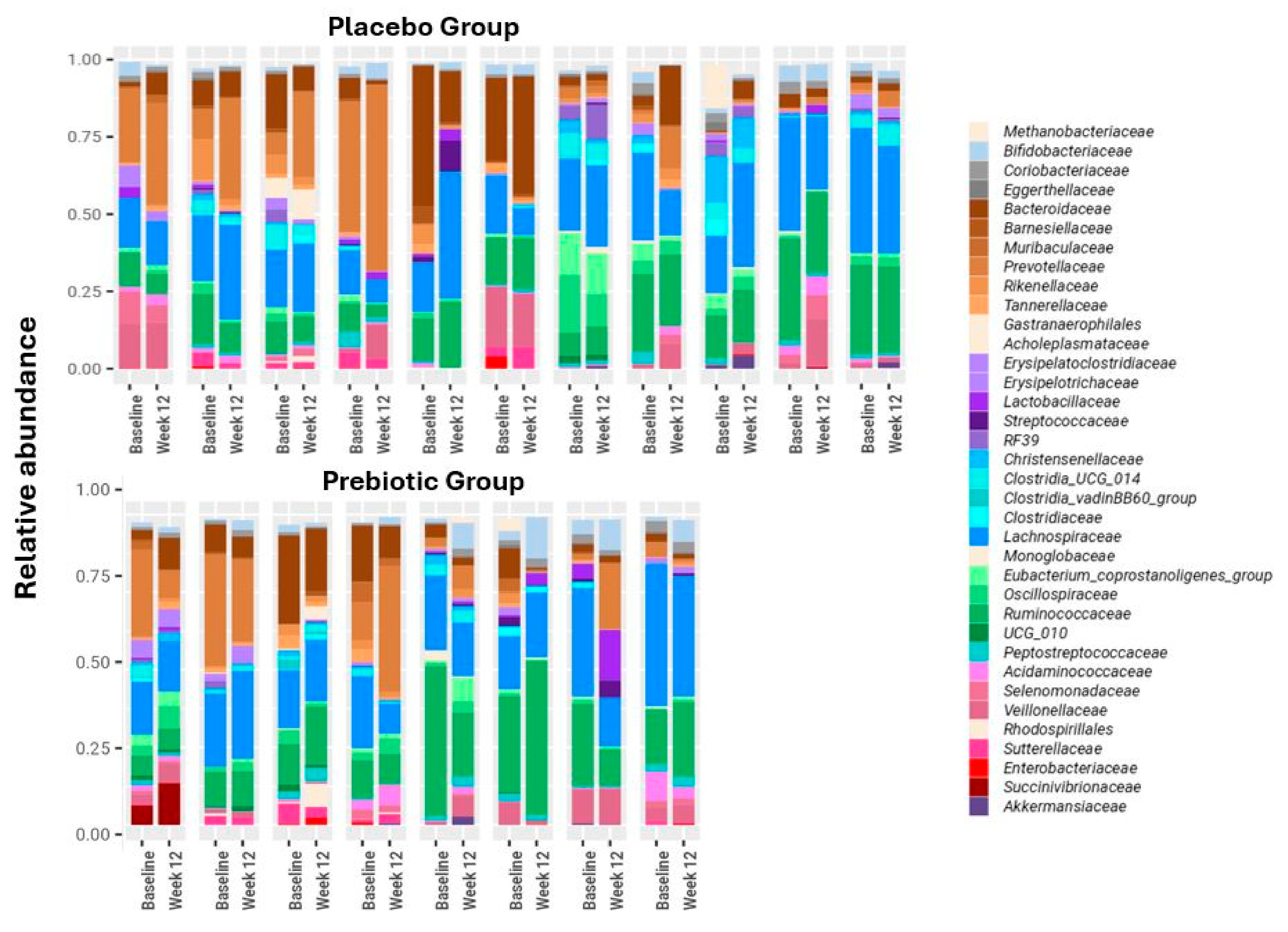
3.2. Anthropometrics Measurements, Bifidobacterium, LFC, and Biochemical Blood Test
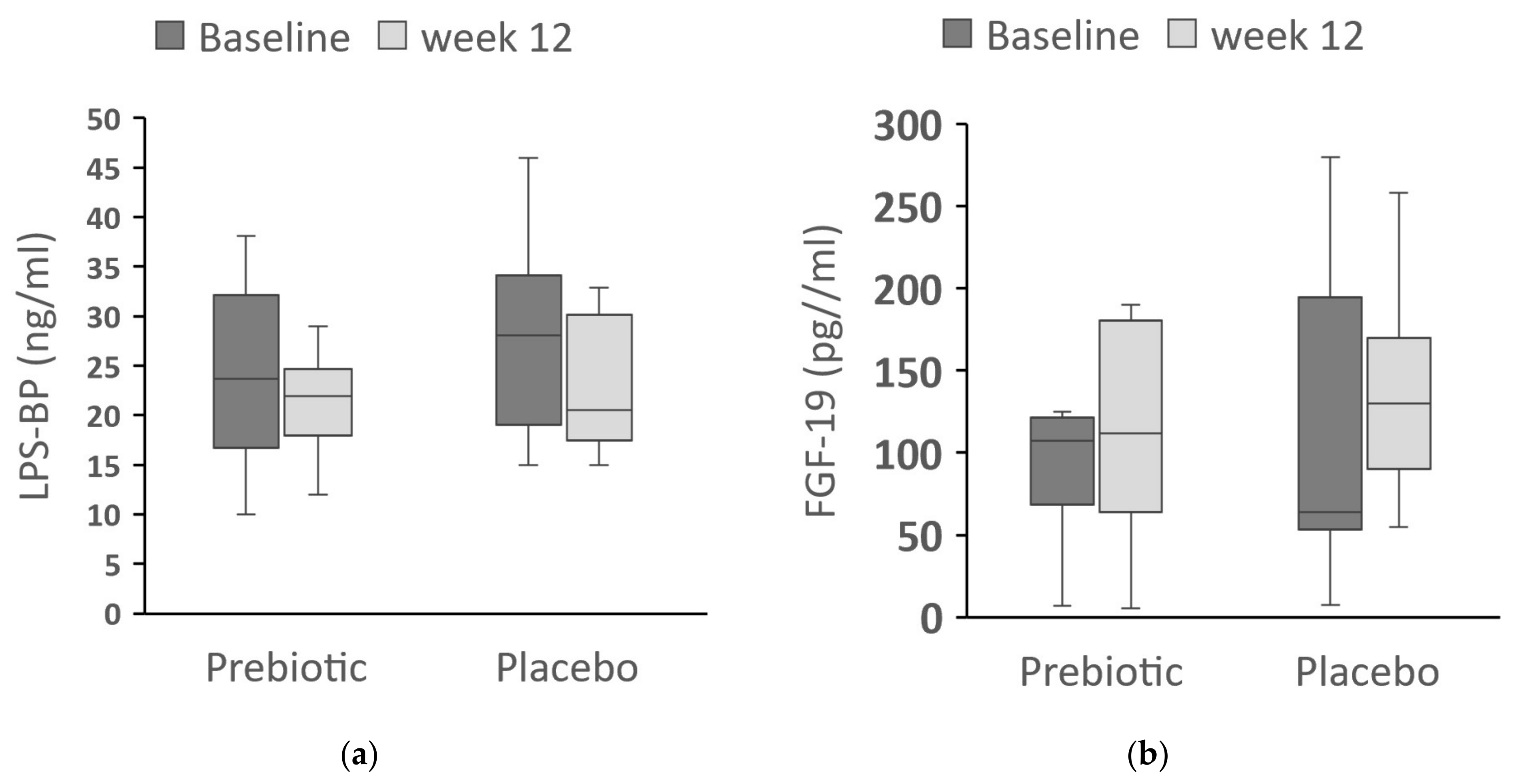
3.3. LPS-BP and FGF-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Beergabel, M.; Knobler, H. Non-alcoholic fatty liver: A common manifestation of a metabolic disorder. QJM 2003, 96, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mejía, M.M.; Qi, X.; Abenavoli, L.; Romero-Gómez, M.; Eslam, M.; Méndez-Sánchez, N. Metabolic dysfunction: The silenced connection with fatty liver disease. Ann. Hepatol. 2023, 28, 101138. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Min, B.-H.; Ganesan, R.; Gebru, Y.A.; Sharma, S.P.; Park, E.; Won, S.-M.; Jeong, J.-J.; Lee, S.-B.; Cha, M.-G.; et al. Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role. Biomedicines 2022, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Publisher Correction: Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1628. [Google Scholar] [CrossRef]
- Zhu, M.; Dagah, O.M.A.; Silaa, B.B.; Lu, J. Thioredoxin/glutaredoxin systems and gut microbiota in NAFLD: Interplay, mechanism, and therapeutical potential. Antioxidants 2023, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Qu, S. Non-alcoholic fatty liver disease and gut microbial dysbiosis- underlying mechanisms and gut microbiota mediated treatment strategies. Rev. Endocr. Metab. Disord. 2023, 24, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.-E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef] [PubMed]
- Stofan, M.; Guo, G.L. Bile acids and FXR: Novel targets for liver diseases. Front. Med. 2020, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Struik, D.; Dommerholt, M.B.; Jonker, J.W. Fibroblast growth factors in control of lipid metabolism: From biological function to clinical application. Curr. Opin. Lipidol. 2019, 30, 235–243. [Google Scholar] [CrossRef]
- Henriksson, E.; Andersen, B. FGF19 and FGF21 for the Treatment of NASH-Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Front. Endocrinol. 2020, 11, 601349. [Google Scholar] [CrossRef] [PubMed]
- Vespasiani-Gentilucci, U.; Carotti, S.; Perrone, G.; Mazzarelli, C.; Galati, G.; Onetti-Muda, A.; Picardi, A.; Morini, S. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015, 35, 569–581. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Chen, L.; Zhao, Z.; Du, S.; Dong, Q.; Xin, Y.; Xuan, S. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Exp. Ther. Med. 2019, 18, 1935–1944. [Google Scholar] [CrossRef]
- Loman, B.R.; Hernández-Saavedra, D.; An, R.; Rector, R.S. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics—A review: Part 1. Altern. Med. Rev. 2008, 13, 315–329. [Google Scholar]
- Kelly, G. Inulin-type prebiotics: A review. (Part 2). Altern. Med. Rev. 2009, 14, 36–55. [Google Scholar] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Salazar, N.; Dewulf, E.M.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Mahillon, J.; de Vos, W.M.; Thissen, J.-P.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015, 34, 501–507. [Google Scholar] [CrossRef]
- Daubioul, C.A.; Horsmans, Y.; Lambert, P.; Danse, E.; Delzenne, N.M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur. J. Clin. Nutr. 2005, 59, 723–726. [Google Scholar] [CrossRef]
- Valenti, L.; Pelusi, S.; Bianco, C.; Ceriotti, F.; Berzuini, A.; Iogna Prat, L.; Trotti, R.; Malvestiti, F.; D’Ambrosio, R.; Lampertico, P.; et al. Definition of healthy ranges for alanine aminotransferase levels: A 2021 update. Hepatol. Commun. 2021, 5, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Biagi, E.; Rampelli, S.; Fiori, J.; Soverini, M.; Audu, H.J.; Cristino, S.; Caporali, L.; Schnorr, S.L.; Carelli, V.; et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep. 2018, 23, 3056–3067. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-type Fructans: A Systematic Review. Adv. Nutr. 2021, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Virchenko, O.; Mykhalchyshyn, G.; Bodnar, P.; Spivak, M.; Yankovsky, D.; Beregova, T.; Ostapchenko, L. Comparative experimental investigation on the efficacy of mono- and multiprobiotic strains in non-alcoholic fatty liver disease prevention. BMC Gastroenterol. 2016, 16, 34. [Google Scholar] [CrossRef]
- Yu, J.S.; Youn, G.S.; Choi, J.; Kim, C.-H.; Kim, B.Y.; Yang, S.-J.; Lee, J.H.; Park, T.-S.; Kim, B.K.; Kim, Y.B.; et al. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin. Transl. Med. 2021, 11, e634. [Google Scholar] [CrossRef]
- Luo, M.; Yan, J.; Wu, L.; Wu, J.; Chen, Z.; Jiang, J.; Chen, Z.; He, B. Probiotics Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats via Gut Microbiota/FXR/FGF15 Signaling Pathway. J. Immunol. Res. 2021, 2021, 2264737. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Abadia-Molina, F.; Saez-Lara, M.J.; Campaña-Martin, L.; Muñoz-Quezada, S.; Romero, F.; Gil, A.; Fontana, L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS ONE 2014, 9, e98401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Li, F.; Gu, Z.; Liu, M.; Shao, T.; Zhang, L.; Zhou, G.; Pan, C.; He, L.; et al. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J. Nutr. Biochem. 2020, 75, 108256. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ren, Y.; Bao, T.; Wang, T.; Li, Y.; Liu, Y.; Zhang, X.; Yang, S.; Wang, H. Inulin activates FXR-FGF15 signaling and further increases bile acids excretion in non-alcoholic fatty liver disease mice. Biochem. Biophys. Res. Commun. 2022, 600, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef]
- Long, X.; Liu, D.; Gao, Q.; Ni, J.; Qian, L.; Ni, Y.; Fang, Q.; Jia, W.; Li, H. Bifidobacterium adolescentis Alleviates Liver Steatosis and Steatohepatitis by Increasing Fibroblast Growth Factor 21 Sensitivity. Front. Endocrinol. 2021, 12, 773340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, H.; Lv, Y.; Tao, H.; Jiang, Y.; Ni, Z.; Peng, L.; Chen, X. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res. Int. 2023, 163, 112309. [Google Scholar] [CrossRef] [PubMed]
- Mijangos-Trejo, A.; Nuño-Lambarri, N.; Barbero-Becerra, V.; Uribe-Esquivel, M.; Vidal-Cevallos, P.; Chávez-Tapia, N. Prebiotics and probiotics: Therapeutic tools for nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2023, 24, 14918. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cassard, A.-M.; Ciocan, D. Pectin in metabolic liver disease. Nutrients 2022, 15, 157. [Google Scholar] [CrossRef]
- Castillo, V.; Figueroa, F.; González-Pizarro, K.; Jopia, P.; Ibacache-Quiroga, C. Probiotics and Prebiotics as a Strategy for Non-Alcoholic Fatty Liver Disease, a Narrative Review. Foods 2021, 10, 1719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bomhof, M.R.; Parnell, J.A.; Ramay, H.R.; Crotty, P.; Rioux, K.P.; Probert, C.S.; Jayakumar, S.; Raman, M.; Reimer, R.A. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2019, 58, 1735–1745. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Z.; Duan, F.; Jia, Z.; Chen, X.; Li, S. The promising role of probiotics/prebiotics/synbiotics in energy metabolism biomarkers in patients with NAFLD: A systematic review and meta-analysis. Front. Public Health 2022, 10, 862266. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions with Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escouto, G.S.; Port, G.Z.; Tovo, C.V.; Fernandes, S.A.; Peres, A.; Dorneles, G.P.; Houde, V.P.; Varin, T.V.; Pilon, G.; Marette, A.; et al. Probiotic Supplementation, Hepatic Fibrosis, and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J. Nutr. 2023, 153, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mψ-Nuclear Factor-κB-Nod-Like Receptor Protein 3 Pathway via gut-Liver Axis in Mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Kang, B.-K.; Lim, J.H.; Lim, S.; Chung, M.-J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef] [PubMed]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Shalimar; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lawitz, E.J.; Frias, J.P.; Ortiz-Lasanta, G.; Johansson, L.; Franey, B.B.; Morrow, L.; Rosenstock, M.; Hartsfield, C.L.; Chen, C.-Y.; et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol. Hepatol. 2023, 8, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Haring, H.-U.; Claussen, C.D.; Fritsche, A.; Schick, F. Hepatic lipid accumulation in healthy subjects: A comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn. Reson. Med. 2006, 55, 913–917. [Google Scholar] [CrossRef] [PubMed]

| Placebo (n = 11) | Prebiotic (n = 8) | p | |
|---|---|---|---|
| Age (years) | 50.0 (40.9–60.5) | 47.8 (44.4–58.4) | 1.0 |
| Gender, male/female | 9/2 | 6/2 | 0.7 |
| Hypertension | |||
| Yes | 4 | 3 | 0.9 |
| No | 7 | 5 | |
| Prediabetes | |||
| Yes | 5 | 4 | 0.8 |
| No | 6 | 4 | |
| Diabetes | |||
| Yes | 2 | 0 | 0.2 |
| No | 9 | 8 | |
| Anthropometrics | |||
| Weight (kg) | 94.4 (85.9–98.4) | 94.1 (85.7–103.0) | 0.7 |
| BMI (kg/m2) | 32.5 (30.2–35.1) | 32.6 (29.5–33.2) | 0.7 |
| Waist circumference (cm) | 107 (99–112) | 106 (95–110) | 0.6 |
| Body fat (%) | 34 (29–35) | 33 (30–41) | 0.9 |
| Blood Pressure | |||
| SBP (mmHg) | 123 (120–128) | 125 (117–132) | 0.8 |
| DBP (mmHg) | 81 (76–83) | 79 (77–82) | 1 |
| Blood chemistries | |||
| Fasting glucose (mg/dL) | 100 (90–110) | 100 (93–105) | 0.8 |
| Insulin (IU/dL) | 11.6 (9.0–20.8) | 13.7 (7.6–21.4) | 0.9 |
| HbA1c (%) | 5.8 (5.5–5.9) | 5.6 (5.4–5.8) | 0.1 |
| HOMA-IR | 3.7 (2.1–5.1) | 3.5 (1.9–5.5) | 0.9 |
| Total cholesterol (mg/dL) | 189 (145–228) | 197 (181–210) | 0.5 |
| HDL-C (mg/dL) | 41 (32–42) | 38 (34–44) | 0.6 |
| LDL-C (mg/dL) | 113 (94–150) | 113 (87–141) | 0.7 |
| Triglycerides (mg/dL) | 197 (125–296) | 168 (138–259) | 0.8 |
| ALT (IU/L) | 50 (30–69) | 36 (31–54) | 0.6 |
| AST (IU/L) | 25 (20–39) | 27 (25–29) | 0.5 |
| GGT (IU/L) | 40 (28–102) | 34 (27–48) | 0.6 |
| CRP (mg/dL) | 2.5 (1.8–5.7) | 3.0 (1.3–5.0) | 1 |
| LFC (%) | 18 (8–21) | 24 (12–30) | 0.2 |
| Placebo (n = 11) | Prebiotic (n = 8) | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 12 | p-Value | Baseline | Week 12 | p-Value | |
| Weight (kg) | 94.4 (85.9–98.4) | 91.5 (83.5–98.9) | 0.5 | 94.1 (85.7–103.0) | 93.9 (85.0–100.9) | 0.1 |
| BMI (kg/m2) | 32.5 (30.2–35.1) | 31.4 (29.7–36.0) | 0.5 | 32.6 (29.5–33.2) | 32.4 (29.7–32.9) | 0.3 |
| Waist circumference (cm) | 107 (99–112) | 106 (95–112) | 0.1 | 106 (95–110) | 106 (93–110) | 0.3 |
| Body fat (%) | 34 (29–35) | 32 (29–41) | 0.3 | 33 (30–41) | 34 (28–40) | 0.2 |
| Fasting glucose (mg/dL) | 100 (90–110) | 96 (88–110) | 0.7 | 100 (93–105) | 101 (95–106) | 0.6 |
| Insulin (mU/L) | 11.6 (9.0–20.8) | 10.4 (7.4–14.7) | 0.3 | 13.7 (7.6–21.4) | 14.7 (9.8–19.9) | 0.6 |
| HbA1c (%) | 5.8 (5.5–5.9) | 5.7 (5.5–6.0) | 0.9 | 5.6 (5.4–5.8) | 5.6 (5.4–5.8) | 0.2 |
| HOMA-IR | 3.7 (2.1–5.1) | 2.82 (1.6–3.7) | 0.2 | 3.5 (1.9–5.5) | 3.7 (2.3–5.1) | 0.7 |
| Total Cholesterol (mg/dL) | 189 (145–228) | 199 (148–239) | 0.7 | 197 (181–210) | 199 (178–236) | 0.1 |
| HDL-C (mg/dL) | 41 (32–42) | 35 (30–42) | 0.5 | 38 (34–44) | 38 (35–41) | 0.7 |
| LDL-C (mg/dL) | 113 (94–150) | 127 (90–154) | 0.7 | 113 (87–141) | 103 (91–134) | 0.6 |
| Triglycerides (mg/dL) | 197 (125–296) | 206 (152–252) | 0.6 | 168 (138–259) | 176 (150–272) | 0.7 |
| ALT (U/L) | 50 (30–69) | 44 (30–52) | 0.2 | 36 (31–54) | 42 (28–50) | 0.5 |
| AST (U/L) | 25 (20–39) | 22 (21–30) | 0.27 | 27 (25–29) | 25 (22–41) | 0.5 |
| GGT (U/L) | 40 (28–102) | 32 (29–83) | 0.3 | 34 (27–48) | 40 (26–50) | 0.9 |
| CRP (mg/dL) | 2.5 (1.8–5.7) | 1.7 (1.1–4.5) | 0.1 | 3.0 (1.3–5.0) | 3.0 (1.1–4.4) | 0.3 |
| LFC (%) | 18 (8–21) | 11 (4–20) | 0.4 | 24 (12–30) | 19 (13–27) | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshef, N.; Gophna, U.; Reshef, L.; Konikoff, F.; Gabay, G.; Zornitzki, T.; Knobler, H.; Maor, Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial. Nutrients 2024, 16, 1571. https://doi.org/10.3390/nu16111571
Reshef N, Gophna U, Reshef L, Konikoff F, Gabay G, Zornitzki T, Knobler H, Maor Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial. Nutrients. 2024; 16(11):1571. https://doi.org/10.3390/nu16111571
Chicago/Turabian StyleReshef, Naama, Uri Gophna, Leah Reshef, Fred Konikoff, Gila Gabay, Taiba Zornitzki, Hilla Knobler, and Yaakov Maor. 2024. "Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial" Nutrients 16, no. 11: 1571. https://doi.org/10.3390/nu16111571
APA StyleReshef, N., Gophna, U., Reshef, L., Konikoff, F., Gabay, G., Zornitzki, T., Knobler, H., & Maor, Y. (2024). Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial. Nutrients, 16(11), 1571. https://doi.org/10.3390/nu16111571







