Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Study Quality Assessment
2.5. Statistical Analysis
3. Results
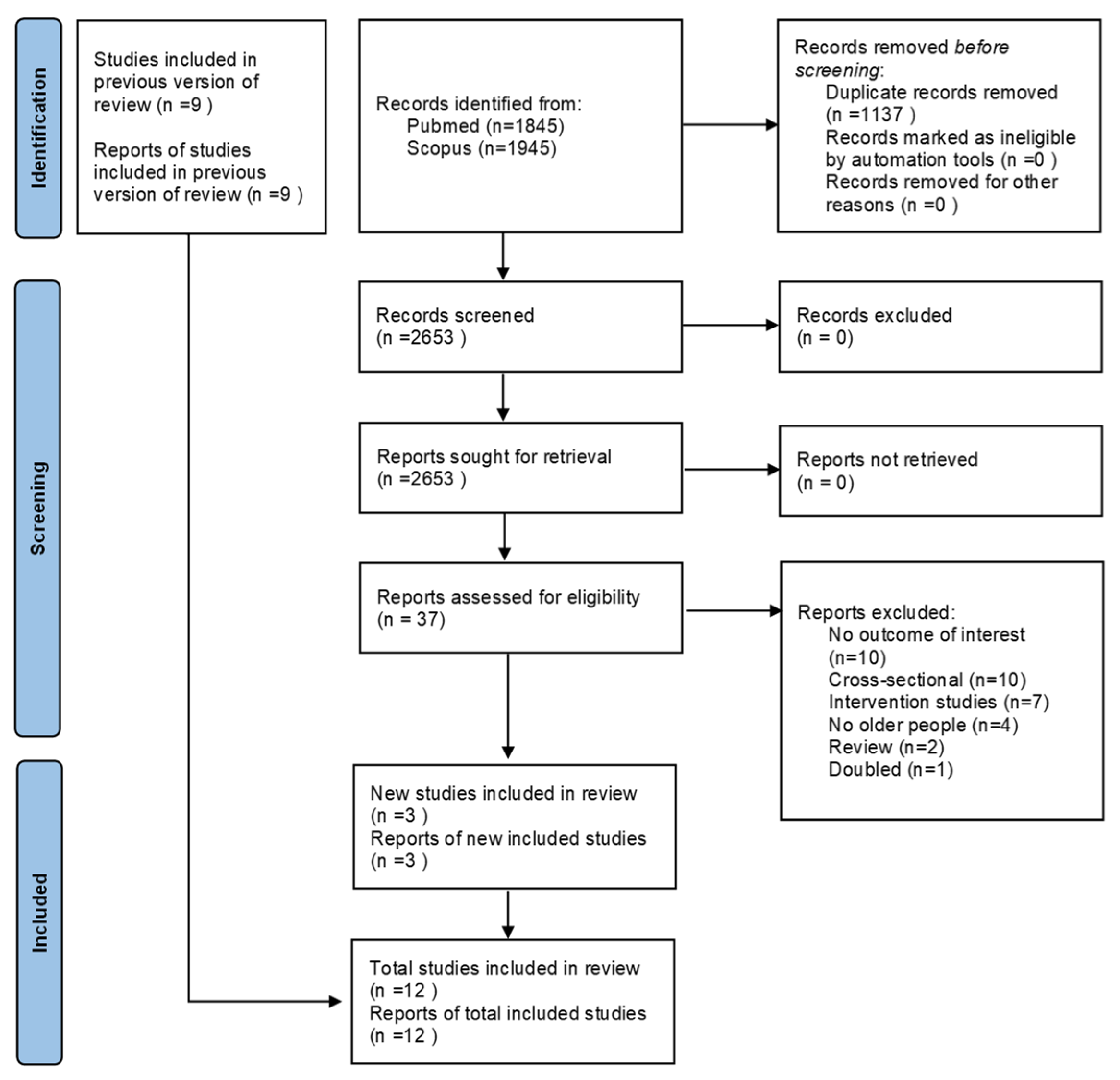
3.1. Literature Search
3.2. Study and Participants Characteristics
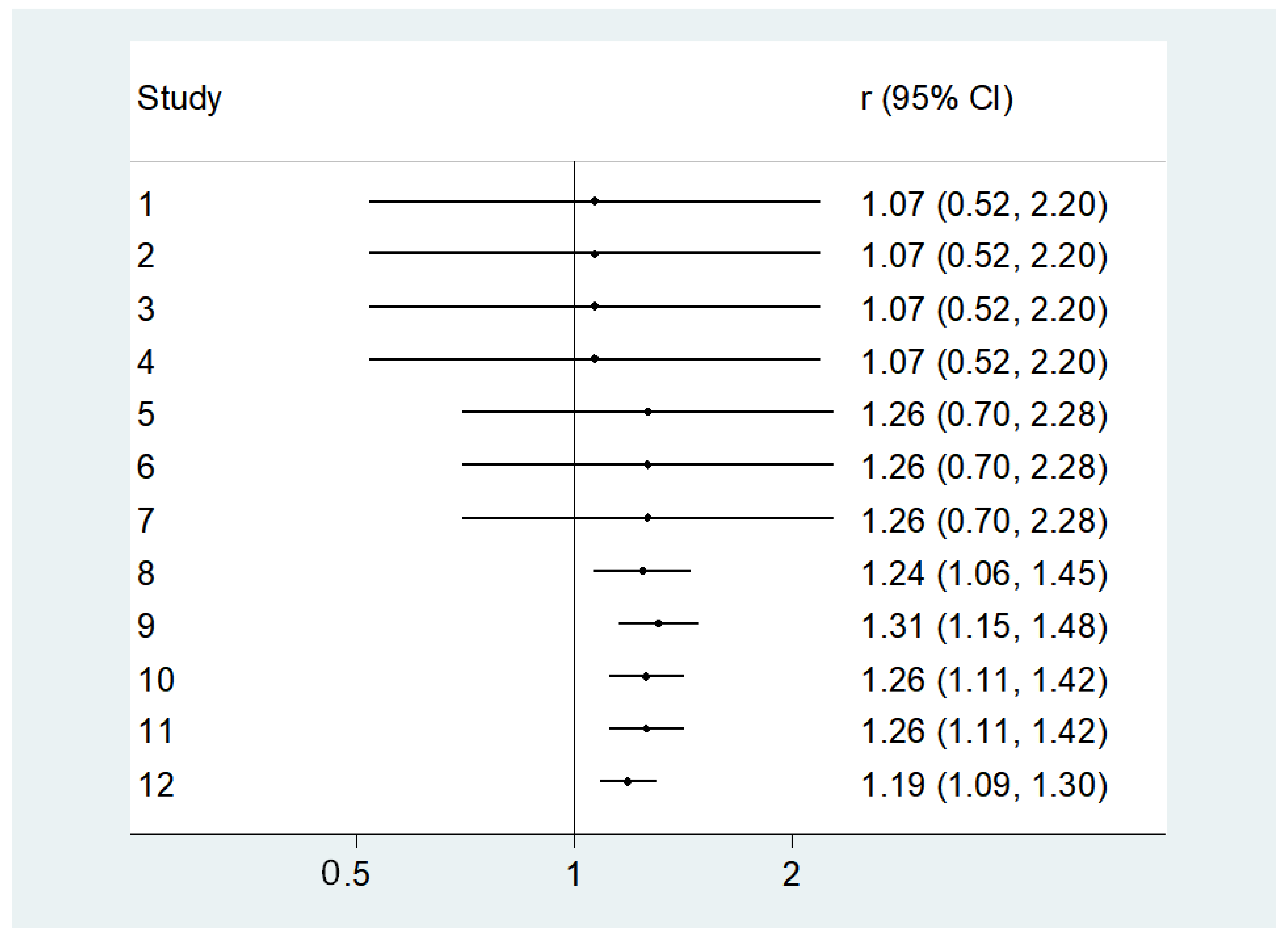
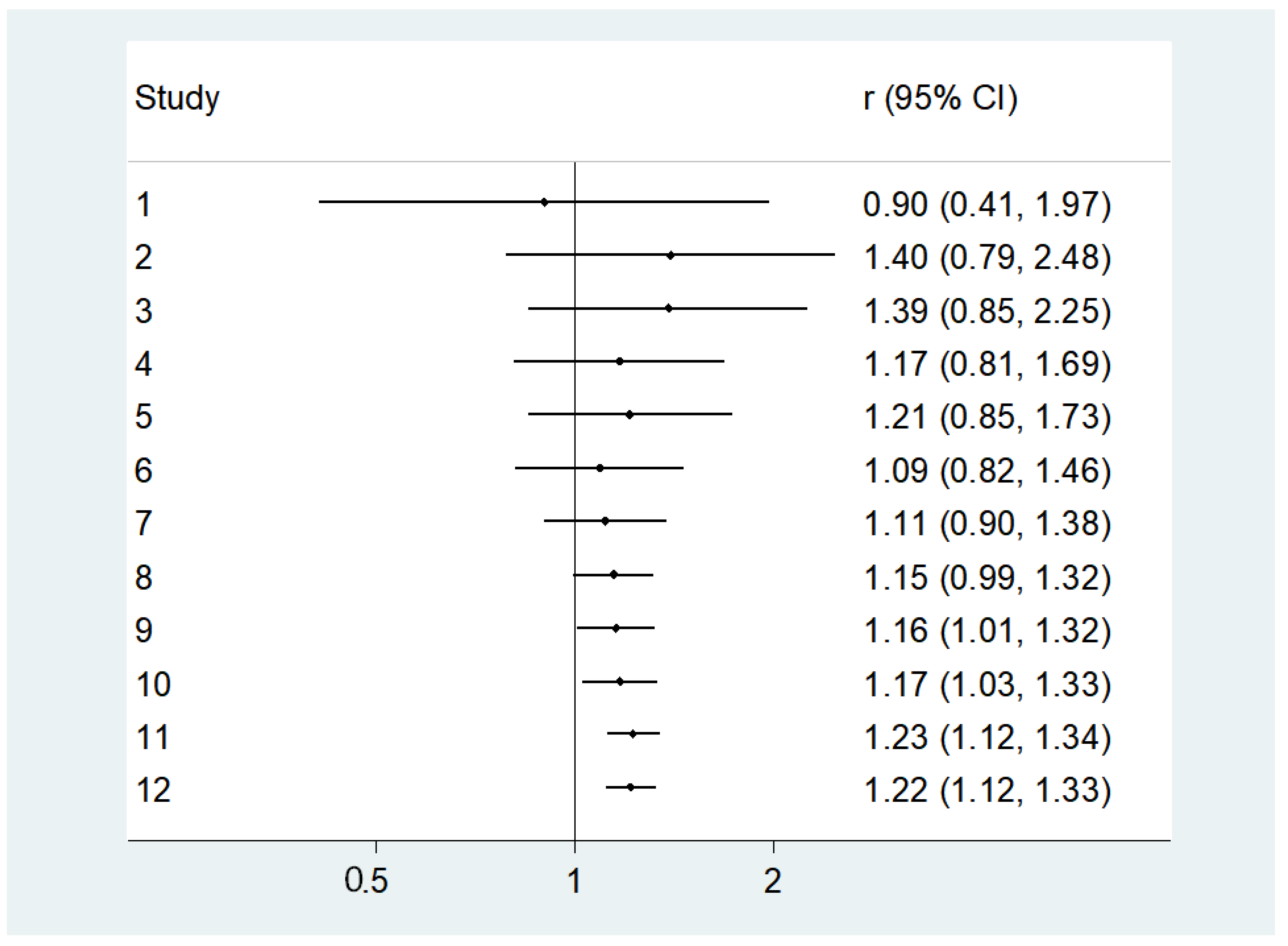
3.3. Unadjusted Findings Concerning Lower 25OHD Levels and Diabetes
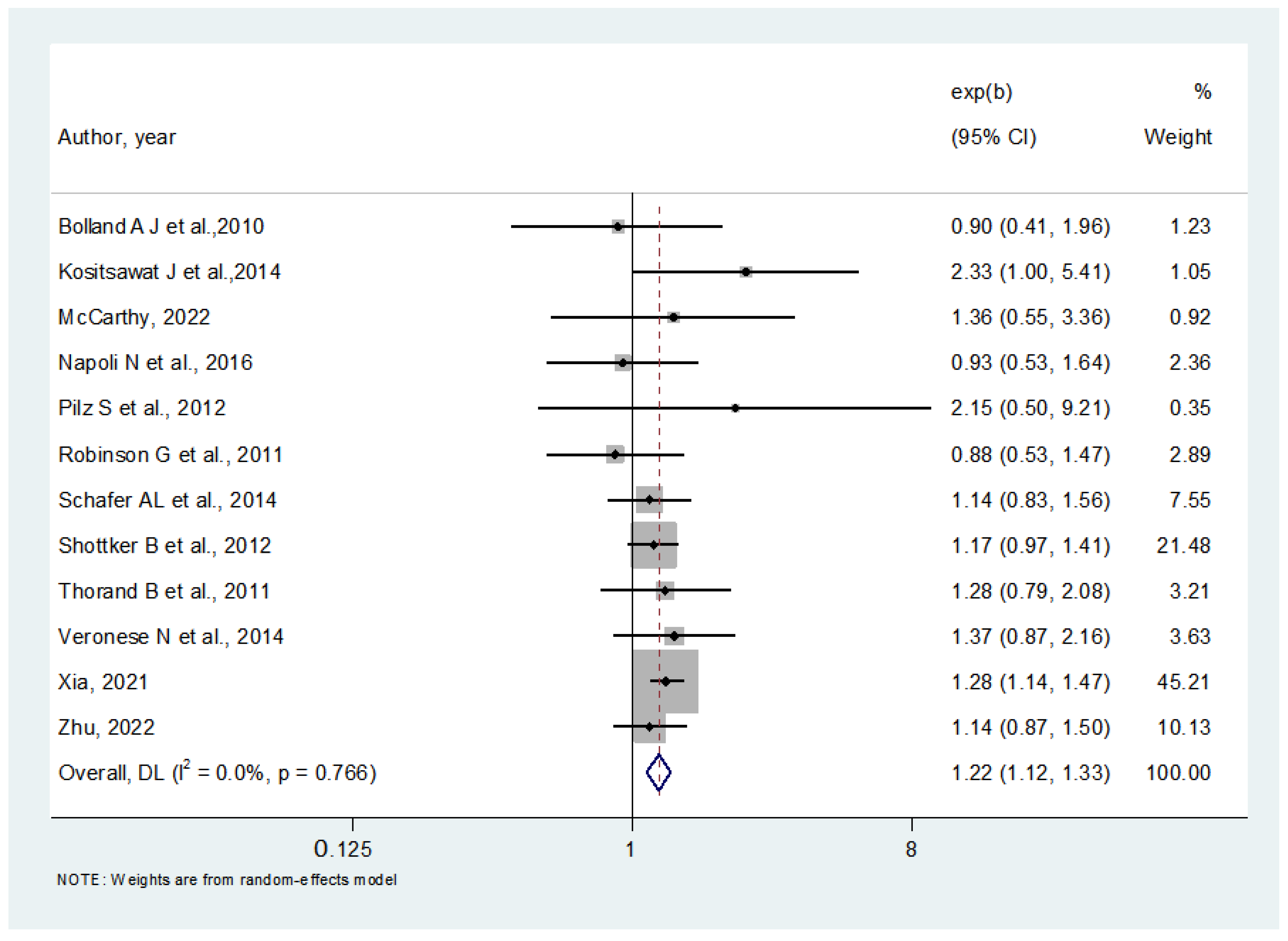
3.4. Adjusted Analyses Concerning Lower 25OHD Levels and Diabetes
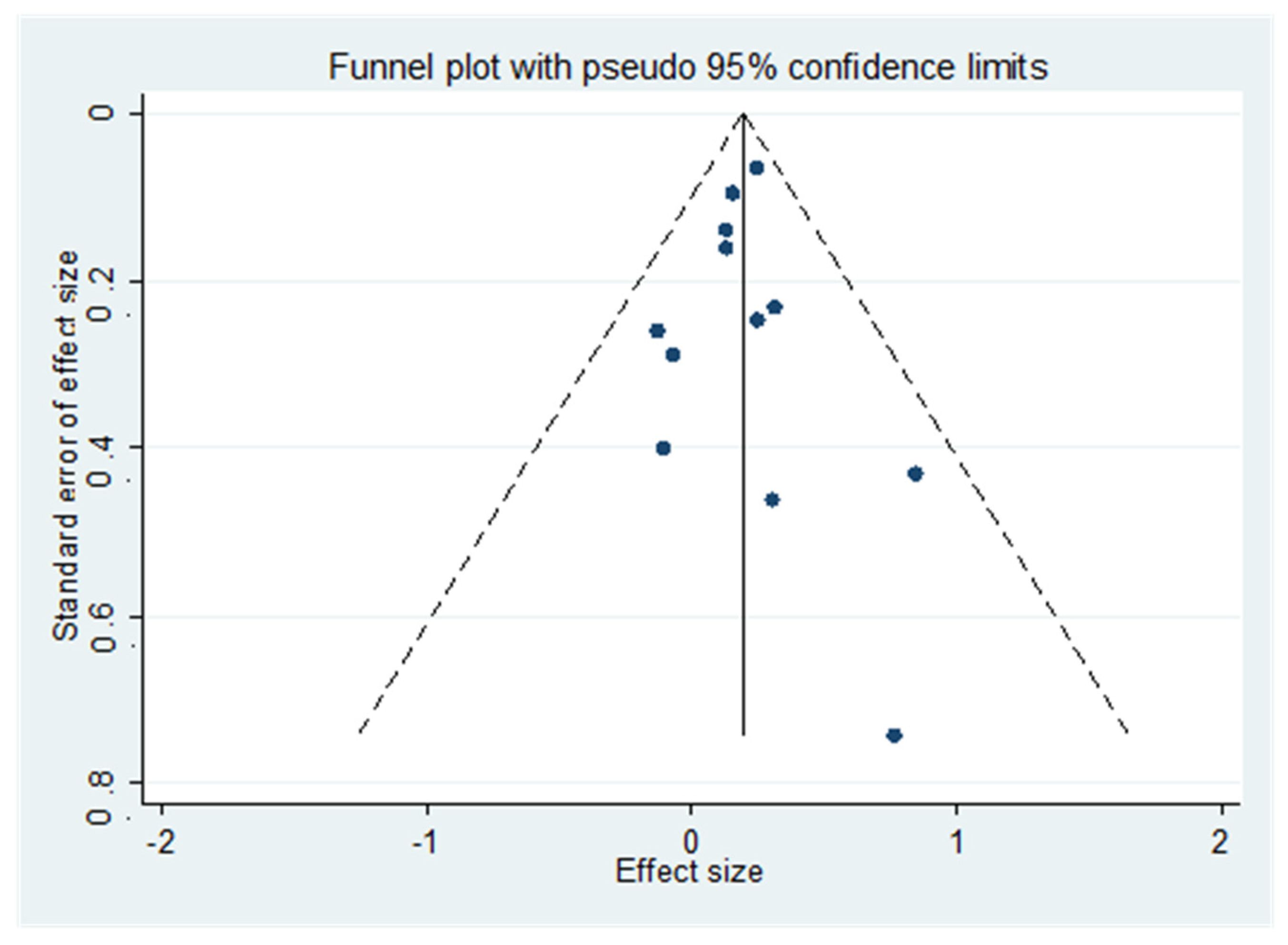
3.5. Metaregression and Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Jacob, L.; Rathmann, W.; Koyanagi, A.; Haro, J.M.; Kostev, K. Association between type 2 diabetes and chronic low back pain in general practices in Germany. BMJ Open Diabetes Res. Care 2021, 9, e002426. [Google Scholar] [CrossRef]
- Ying, A.F.; Tang, T.Y.; Jin, A.; Chong, T.T.; Hausenloy, D.J.; Koh, W.P. Diabetes and other vascular risk factors in association with the risk of lower extremity amputation in chronic limb-threatening ischemia: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 7. [Google Scholar] [CrossRef]
- Chaker, L.; Falla, A.; van der Lee, S.J.; Muka, T.; Imo, D.; Jaspers, L.; Colpani, V.; Mendis, S.; Chowdhury, R.; Bramer, W.M.; et al. The global impact of non-communicable diseases on macro-economic productivity: A systematic review. Eur. J. Epidemiol. 2015, 30, 357–395. [Google Scholar] [CrossRef]
- Folkerts, K.; Petruski-Ivleva, N.; Kelly, A.; Fried, L.; Blankenburg, M.; Gay, A.; Kovesdy, C.P. Annual health care resource utilization and cost among type 2 diabetes patients with newly recognized chronic kidney disease within a large U.S. administrative claims database. J. Manag. Care Spec. Pharm. 2020, 26, 1506–1516. [Google Scholar] [CrossRef]
- Park, J.; Zhang, P.; Wang, Y.; Zhou, X.; Look, K.A.; Bigman, E.T. High Out-of-pocket Health Care Cost Burden among Medicare Beneficiaries with Diabetes, 1999–2017. Diabetes Care 2021, 44, 1797–1804. [Google Scholar] [CrossRef]
- Freire, L.B.; Brasil-Neto, J.P.; da Silva, M.L.; Miranda, M.G.C.; de Mattos Cruz, L.; Martins, W.R.; da Silva Paz, L.P. Risk factors for falls in older adults with diabetes mellitus: Systematic review and meta-analysis. BMC Geriatr. 2024, 24, 201. [Google Scholar] [CrossRef]
- Grasset, L.; Frison, E.; Helmer, C.; Catheline, G.; Chene, G.; Dufouil, C. Understanding the relationship between type-2 diabetes, MRI markers of neurodegeneration and small vessel disease, and dementia risk: A mediation analysis. Eur. J. Epidemiol. 2024, 39, 409–417. [Google Scholar] [CrossRef]
- Titcomb, T.J.; Richey, P.; Casanova, R.; Phillips, L.S.; Liu, S.; Karanth, S.D.; Saquib, N.; Nuno, T.; Manson, J.E.; Shadyab, A.H.; et al. Association of type 2 diabetes mellitus with dementia-related and non-dementia-related mortality among postmenopausal women: A secondary competing risks analysis of the women’s health initiative. Alzheimer’s Dement. 2024, 20, 234–242. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Mann, J. Government inaction and the preventable diabetes pandemic. Nat. Med. 2023, 29, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Mazzei, L.; Garcia Menendez, S.; Martin Gimenez, V.M.; Al Anouti, F.; Manucha, W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 2023, 15, 767. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin d sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Taderegew, M.M.; Woldeamanuel, G.G.; Wondie, A.; Getawey, A.; Abegaz, A.N.; Adane, F. Vitamin D deficiency and its associated factors among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. BMJ Open 2023, 13, e075607. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Eekhoff, M.; van Schoor, N.; Oosterwerff, M.; de Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef]
- Kawahara, T.; Okada, Y.; Tanaka, Y. Vitamin D efficacy in type 1 and type 2 diabetes. J. Bone Miner. Metab. 2024. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef]
- Sollid, S.T.; Hutchinson, M.Y.; Fuskevag, O.M.; Figenschau, Y.; Joakimsen, R.M.; Schirmer, H.; Njolstad, I.; Svartberg, J.; Kamycheva, E.; Jorde, R. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014, 37, 2123–2131. [Google Scholar] [CrossRef]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann. Intern. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef]
- Barbarawi, M.; Zayed, Y.; Barbarawi, O.; Bala, A.; Alabdouh, A.; Gakhal, I.; Rizk, F.; Alkasasbeh, M.; Bachuwa, G.; Manson, J.E. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes: A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Bolzetta, F.; De Rui, M.; Zambon, S.; Corti, M.C.; Musacchio, E.; Baggio, G.; Crepaldi, G.; Perissinotto, E.; Manzato, E.; et al. Serum 25-hydroxyvitamin D and orthostatic hypotension in old people: The Pro.V.A. study. Hypertension 2014, 64, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Bellary, S.; Dashora, U.; Abdelhafiz, A.H.; Rowles, S.; Reedman, L.; Turner, B.; Green, M.; Forbes, A.; Middleton, A.; et al. Enhancing diabetes care for the most vulnerable in the 21st century: Interim findings of the National Advisory Panel on Care Home Diabetes (NAPCHD). Diabet. Med. 2023, 40, e15088. [Google Scholar] [CrossRef] [PubMed]
- Lucato, P.; Solmi, M.; Maggi, S.; Bertocco, A.; Bano, G.; Trevisan, C.; Manzato, E.; Sergi, G.; Schofield, P.; Kouidrat, Y.; et al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: A systematic review and meta-analysis. Maturitas 2017, 100, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Niedermaier, T.; Holleczek, B.; Schöttker, B.; Brenner, H. Consistent inverse associations of total,“bioavailable”, free, and “non-bioavailable” vitamin D with incidence of diabetes among older adults with lower baseline HbA1c (≤6%) levels. Nutrients 2022, 14, 3282. [Google Scholar] [CrossRef]
- McCarthy, K.; Laird, E.; O’Halloran, A.M.; Walsh, C.; Healy, M.; Fitzpatrick, A.L.; Walsh, J.B.; Hernández, B.; Fallon, P.; Molloy, A.M. Association between vitamin D deficiency and the risk of prevalent type 2 diabetes and incident prediabetes: A prospective cohort study using data from The Irish Longitudinal Study on Ageing (TILDA). EClinicalMedicine 2022, 53, 101654. [Google Scholar] [CrossRef]
- Xia, J.; Tu, W.; Manson, J.E.; Nan, H.; Shadyab, A.H.; Bea, J.W.; Gower, E.W.; Qi, L.; Cheng, T.-Y.D.; Song, Y. Combined associations of 25-hydroxivitamin D and parathyroid hormone with diabetes risk and associated comorbidities among US white and black women. Nutr. Diabetes 2021, 11, 29. [Google Scholar] [CrossRef]
- Bolland, M.J.; Bacon, C.J.; Horne, A.M.; Mason, B.H.; Ames, R.W.; Wang, T.K.; Grey, A.B.; Gamble, G.D.; Reid, I.R. Vitamin D insufficiency and health outcomes over 5 y in older women. Am. J. Clin. Nutr. 2010, 91, 82–89. [Google Scholar] [CrossRef]
- Kositsawat, J.; Kuchel, G.A.; Tooze, J.A.; Houston, D.K.; Cauley, J.A.; Kritchevsky, S.B.; Strotmeyer, E.S.; Kanaya, A.M.; Harris, T.B.; Johnson, K.C. Vitamin D insufficiency and abnormal hemoglobin A1c in black and white older persons. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 525–531. [Google Scholar] [CrossRef]
- Napoli, N.; Schafer, A.L.; Lui, L.-Y.; Cauley, J.A.; Strotmeyer, E.S.; Le Blanc, E.S.; Hoffman, A.R.; Lee, C.G.; Black, D.M.; Schwartz, A.V. Serum 25-hydroxyvitamin D level and incident type 2 diabetes in older men, the Osteoporotic Fractures in Men (MrOS) study. Bone 2016, 90, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Van Den Hurk, K.; Nijpels, G.; Stehouwer, C.; Van’t Riet, E.; Kienreich, K.; Tomaschitz, A.; Dekker, J. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 883–889. [Google Scholar] [CrossRef]
- Robinson, J.G.; Manson, J.E.; Larson, J.; Liu, S.; Song, Y.; Howard, B.V.; Phillips, L.; Shikany, J.M.; Allison, M.; Curb, J.D. Lack of association between 25 (OH) D levels and incident type 2 diabetes in older women. Diabetes Care 2011, 34, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Napoli, N.; Lui, L.; Schwartz, A.; Black, D.; Study of Osteoporotic Fractures. Serum 25-hydroxyvitamin D concentration does not independently predict incident diabetes in older women. Diabet. Med. 2014, 31, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Herder, C.; Rothenbacher, D.; Perna, L.; Müller, H.; Brenner, H. Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: A competing risk analysis in a large population-based cohort of older adults. Eur. J. Epidemiol. 2013, 28, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Thorand, B.; Zierer, A.; Huth, C.; Linseisen, J.; Meisinger, C.; Roden, M.; Peters, A.; Koenig, W.; Herder, C. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: Results from the MONICA/KORA Augsburg study. Diabetes Care 2011, 34, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Sergi, G.; De Rui, M.; Bolzetta, F.; Toffanello, E.D.; Zambon, S.; Corti, M.-C.; Sartori, L.; Musacchio, E.; Baggio, G. Serum 25-hydroxyvitamin D and incidence of diabetes in elderly people: The PRO. VA study. J. Clin. Endocrinol. Metab. 2014, 99, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sneve, M.; Hutchinson, M.; Emaus, N.; Figenschau, Y.; Grimnes, G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am. J. Epidemiol. 2010, 171, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, G.; Matsuo, L.H.; Ocker, G.; Grellert, M.; d’Orsi, E.; Venske, D.K.R.; Moreira, J.D. Adiposity and physical activity are among the main determinants of serum vitamin D concentrations in older adults: The EpiFloripa Aging Cohort Study. Nutr. Res. 2023, 111, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: A secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Park, S.K.; Garland, C.F.; Gorham, E.D.; BuDoff, L.; Barrett-Connor, E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS ONE 2018, 13, e0193070. [Google Scholar] [CrossRef]
- Schottker, B.; Ball, D.; Gellert, C.; Brenner, H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res. Rev. 2013, 12, 708–718. [Google Scholar] [CrossRef]
- Samefors, M.; Ostgren, C.J.; Molstad, S.; Lannering, C.; Midlov, P.; Tengblad, A. Vitamin D deficiency in elderly people in Swedish nursing homes is associated with increased mortality. Eur. J. Endocrinol. 2014, 170, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Rejnmark, L.; Bislev, L.S.; Cashman, K.D.; Eiriksdottir, G.; Gaksch, M.; Grubler, M.; Grimnes, G.; Gudnason, V.; Lips, P.; Pilz, S.; et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS ONE 2017, 12, e0180512. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Frankel, J.B.; Heldt, A.M.; Grodsky, G.M. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980, 209, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Cade, C.; Norman, A.W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986, 119, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.S.; Morisset, A.S.; Carreau, A.M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Kang, S.; Goyal, A.; Zhang, K.; You, J.Y.; Carey, M.; Jain, S.; Bhansali, S.; Kehlenbrink, S.; Guo, P.; et al. Insulin-sensitizing effects of vitamin D repletion mediated by adipocyte vitamin D receptor: Studies in humans and mice. Mol. Metab. 2020, 42, 101095. [Google Scholar] [CrossRef] [PubMed]
- Lalunio, H.; Parker, L.; Hanson, E.D.; Gregorevic, P.; Levinger, I.; Hayes, A.; Goodman, C.A. Detecting the vitamin D receptor (VDR) protein in mouse and human skeletal muscle: Strain-specific, species-specific and inter-individual variation. Mol. Cell Endocrinol. 2023, 578, 112050. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhong, C. Vitamin D Receptor Regulates Autophagy to Inhibit Apoptosis and Promote Proliferation in Hepatocyte Injury. J. Nippon. Med. Sch. 2023, 90, 89–95. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef]
- Wright, D.C.; Hucker, K.A.; Holloszy, J.O.; Han, D.H. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004, 53, 330–335. [Google Scholar] [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Rhoten, W.B. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology 1995, 136, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Egan, J.M. Pharmacological agents that directly modulate insulin secretion. Pharmacol. Rev. 2003, 55, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic beta-cells in health and in Type 2 diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and the Hallmarks of Aging. Nutrients 2024, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect beta Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, Y.; Su, Y.; Teng, L. The vitamin D receptor (VDR) protects pancreatic beta cells against Forkhead box class O1 (FOXO1)-induced mitochondrial dysfunction and cell apoptosis. Biomed. Pharmacother. 2019, 117, 109170. [Google Scholar] [CrossRef]
- Morro, M.; Vila, L.; Franckhauser, S.; Mallol, C.; Elias, G.; Ferre, T.; Molas, M.; Casana, E.; Rodo, J.; Pujol, A.; et al. Vitamin D Receptor Overexpression in beta-Cells Ameliorates Diabetes in Mice. Diabetes 2020, 69, 927–939. [Google Scholar] [CrossRef]
- Riek, A.E.; Oh, J.; Sprague, J.E.; Timpson, A.; de las Fuentes, L.; Bernal-Mizrachi, L.; Schechtman, K.B.; Bernal-Mizrachi, C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J. Biol. Chem. 2012, 287, 38482–38494. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Elkhwanky, M.S.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues. JBMR Plus 2020, 4, e10397. [Google Scholar] [CrossRef] [PubMed]
- Spyksma, E.E.; Alexandridou, A.; Mai, K.; Volmer, D.A.; Stokes, C.S. An Overview of Different Vitamin D Compounds in the Setting of Adiposity. Nutrients 2024, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar] [PubMed]
- Borissova, A.M.; Tankova, T.; Kirilov, G.; Dakovska, L.; Kovacheva, R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int. J. Clin. Pr. 2003, 57, 258–261. [Google Scholar] [CrossRef]
- Nyomba, B.L.; Auwerx, J.; Bormans, V.; Peeters, T.L.; Pelemans, W.; Reynaert, J.; Bouillon, R.; Vantrappen, G.; De Moor, P. Pancreatic secretion in man with subclinical vitamin D deficiency. Diabetologia 1986, 29, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Brodsky, I.G.; Chatterjee, R.; Kim, S.H.; Pratley, R.E.; Staten, M.A.; Pittas, A.G.; Group, D.d.R. Effects of Vitamin D Supplementation on Insulin Sensitivity and Secretion in Prediabetes. J. Clin. Endocrinol. Metab. 2022, 107, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Smogorzewski, M.; Massry, S.G. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology 1994, 135, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Rogers, S.D.; Sleeman, M.W.; Pasquini, G.M.; Bringhurst, F.R.; Ng, K.W.; Zajac, J.D.; Best, J.D. Modulation of glucose transport by parathyroid hormone and insulin in UMR 106-01, a clonal rat osteogenic sarcoma cell line. J. Mol. Endocrinol. 1995, 14, 263–275. [Google Scholar] [CrossRef]
- Cheng, Q.; Boucher, B.J.; Leung, P.S. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia 2013, 56, 553–562. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Chavez, A.O.; Gastaldelli, A.; Perego, L.; Tripathy, D.; Saad, M.J.; Velloso, L.; Folli, F. The crosstalk between insulin and renin-angiotensin-aldosterone signaling systems and its effect on glucose metabolism and diabetes prevention. Curr. Vasc. Pharmacol. 2008, 6, 301–312. [Google Scholar] [CrossRef]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E345–E351. [Google Scholar] [CrossRef] [PubMed]
- Kjalarsdottir, L.; Tersey, S.A.; Vishwanath, M.; Chuang, J.C.; Posner, B.A.; Mirmira, R.G.; Repa, J.J. 1,25-Dihydroxyvitamin D(3) enhances glucose-stimulated insulin secretion in mouse and human islets: A role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2019, 185, 17–26. [Google Scholar] [CrossRef]
- De Boland, A.R.; Boland, R.L. Non-genomic signal transduction pathway of vitamin D in muscle. Cell. Signal. 1994, 6, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, R.L.; Bucci, T.J.; Richard, B.; Empson, N.; Lufkin, E.G. Calcium-binding protein: Its cellular localization in jejunum, kidney and pancreas. Proc. Soc. Exp. Biol. Med. 1975, 149, 56–60. [Google Scholar] [CrossRef]
- Hardwick, L.L.; Jones, M.R.; Brautbar, N.; Lee, D.B. Magnesium absorption: Mechanisms and the influence of vitamin D, calcium and phosphate. J. Nutr. 1991, 121, 13–23. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Dominguez, L.J.; Pizzol, D.; Demurtas, J.; Smith, L.; Barbagallo, M. Oral magnesium supplementation for treating glucose metabolism parameters in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Nutrients 2021, 13, 4074. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]


| Author, Year | Country | Sample Size | N of Subjects with Diabetes at Follow-Up | Mean Age | Percentage of Females | Mean Serum 25OHD Levels | Follow-Up (Years) | Methods of Measurement of Serum 25OHD | Diagnostic Criteria for Diabetes | Type of Covariates | Number of Covariates | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bolland A J et al., 2010 [37] | New Zealand | 1472 | 29 | 74 | 100 | 50.9 | 5 | RIA (DiaSorin, Stillwater, MN, USA) | Self-reported with a final adjudication by a specialist | Treatment allocation, age, body weight, smoking status. | 4 | 5 |
| Kositsawat J et al., 2014 [38] | USA | 2193 | 477 | 74.6 | 52 | 65.8 | 2 | RIA (DiaSorin, Stillwater, MN, USA) | Abnormal HbA1c (≥6.5%) | Age, sex, recruitment site, BMI, physical activity, vitamin D supplementation, multivitaminic use, season, PTH, race, diabetes status at follow-up. | 11 | 5 |
| McCarthy, 2022 [35] | Ireland | 3373 | 110 | 62.9 | 53.5 | 57.1 | 4 | Liquid chromatography-mass spectrometry | Self-reported doctor diagnosis of diabetes, use of diabetes medications, or an HbA1c level ≥48 mmol/mol | age, sex, educational status attained, body mass index, smoking history, physical activity, use of statins, and the season | 8 | 7 |
| Napoli N et al., 2016 [39] | USA | 1939 | 139 | 73.3 | 0 | 27.0 | 6.4 | Liquid chromatography tandem mass spectrometry (Thermo Fisher Scientific, Franklin, MA, USA) | FPG ≥ 126 mg/dL, self-reported | Age, site, race, season, BMI, calcium intake | 6 | 7 |
| Pilz S et al., 2012 [40] | The Netherlands | 351 | 45 | 67.8 | 61 | 56.7 | 7.5 | RIA (DiaSorin, Stillwater, MN, USA) | FPG ≥ 126 mg/dL F | Fasting glucose, age, sex, BMI, HDL, TG, hypertension, physical activity level, season, PTH, eGFR | 11 | 7 |
| Robinson G et al., 2011 [41] | USA | 5140 | 317 | 66 | 100 | 7.3 | DiaSorin Liaison Chemiluminescence Method (DiaSorin, Stillwater, MN) | Self-reported, drugs | Age, ethnicity, latitude, season, WHI study indicators, BMI, hypertension, fiber intake, magnesium intake, physical activity level | 10 | 7 | |
| Schafer AL et al., 2014 [42] | USA | 5463 | 320 | 76.5 | 100 | 57.4 | 8.6 | Liquid chromatography–tandem mass spectrometry (Thermo Fisher Scientific, Franklin, MA, USA) | Self-reported | Age, clinic site, BMI, hypertension, self-reported health | 5 | 6 |
| Shottker B et al., 2012 [43] | Germany | 7791 | 829 | 62 | 58 | 46.1 | 8 | RIA (DiaSorin, Stillwater, MN, USA) | HbA1c ≥ 6.5% | Age, sex, season, multivitamin supplementation, frequent fish consumption, BMI, HbA1c, family history of diabetes, education, physical activity, smoking, hypertension, renal dysfunction, CRP, fasting TG | 15 | 8 |
| Thorand B et al., 2011 [44] | Germany | 1683 | 416 | >65 | 47 | 11 | Enzyme immunoassay IDA, Frankfurt, Germany | Self- reported | Age, sex, survey, season, BMI, smoking, alcohol use, physical activity, systolic BP, total cholesterol/HDL, family history of diabetes, CRP, IL6, ICAM-1, IFN-alpha Inducible protein, markers of inflammation, waist-to-hip ratio. | 17 | 6 | |
| Veronese N et al., 2014 [45] | Italy | 2227 | 291 | 76.1 | 59 | 80.1 | 4.4 | RIA (DiaSorin, Stillwater, MN, USA) | FPG ≥ 126 mg/dL or HbA1c ≥ 6.5% or use of medications | Age, gender, waist, hypertension, education, monthly income, smoking, eGFR, FPG, HbA1c, total cholesterol | 11 | 8 |
| Xia, 2021 [36] | USA | 4191 | 453 | 65 | 100 | 52.0 | 12 | Enzyme immunoassay with a competitive binding technique from Immunodiagnostic Systems Inc. (Fountain Hills, AZ, USA). | self-report of a new physician diagnosis of diabetes treated with oral drugs or insulin shots during study follow-up, | Age, clinical center, race/ethnicity, BMI, family history of diabetes, educational levels, alcohol intake, physical activity levels, cigarette smoking status, postmenopausal hormone therapy use, and season | 11 | 7 |
| Zhu, 2022 [34] | Germany | 4841 | 837 | 61.9 | 57.5 | 45.0 | 14 | RIA (DiaSorin, Stillwater, MN, USA) | General practitioner-confirmed patient self-reports | Age; sex; education; smoking and drinking status; vegetable, fruit, and fish consumption; regular intake of multivitamin supplements; body mass index; HbA1c; total cholesterol; high-density lipoprotein cholesterol; triglycerides; C-reactive protein; systolic blood pressure; estimated glomerular filtration rate; family history of diabetes; history of cardiovascular diseases and cancer; antihypertensive medication; lipid-lowering medication; and season of blood draw. | 23 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Marrone, E.; Di Palermo, C.; Iommi, C.; Ruggirello, R.; Caffarelli, C.; Gonnelli, S.; Barbagallo, M. Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1561. https://doi.org/10.3390/nu16111561
Dominguez LJ, Veronese N, Marrone E, Di Palermo C, Iommi C, Ruggirello R, Caffarelli C, Gonnelli S, Barbagallo M. Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis. Nutrients. 2024; 16(11):1561. https://doi.org/10.3390/nu16111561
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, Eliana Marrone, Carla Di Palermo, Candela Iommi, Rosaria Ruggirello, Carla Caffarelli, Stefano Gonnelli, and Mario Barbagallo. 2024. "Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis" Nutrients 16, no. 11: 1561. https://doi.org/10.3390/nu16111561
APA StyleDominguez, L. J., Veronese, N., Marrone, E., Di Palermo, C., Iommi, C., Ruggirello, R., Caffarelli, C., Gonnelli, S., & Barbagallo, M. (2024). Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis. Nutrients, 16(11), 1561. https://doi.org/10.3390/nu16111561









