Differential Associations of Erythrocyte Membrane Saturated Fatty Acids with Glycemic and Lipid Metabolic Markers in a Chinese Population: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
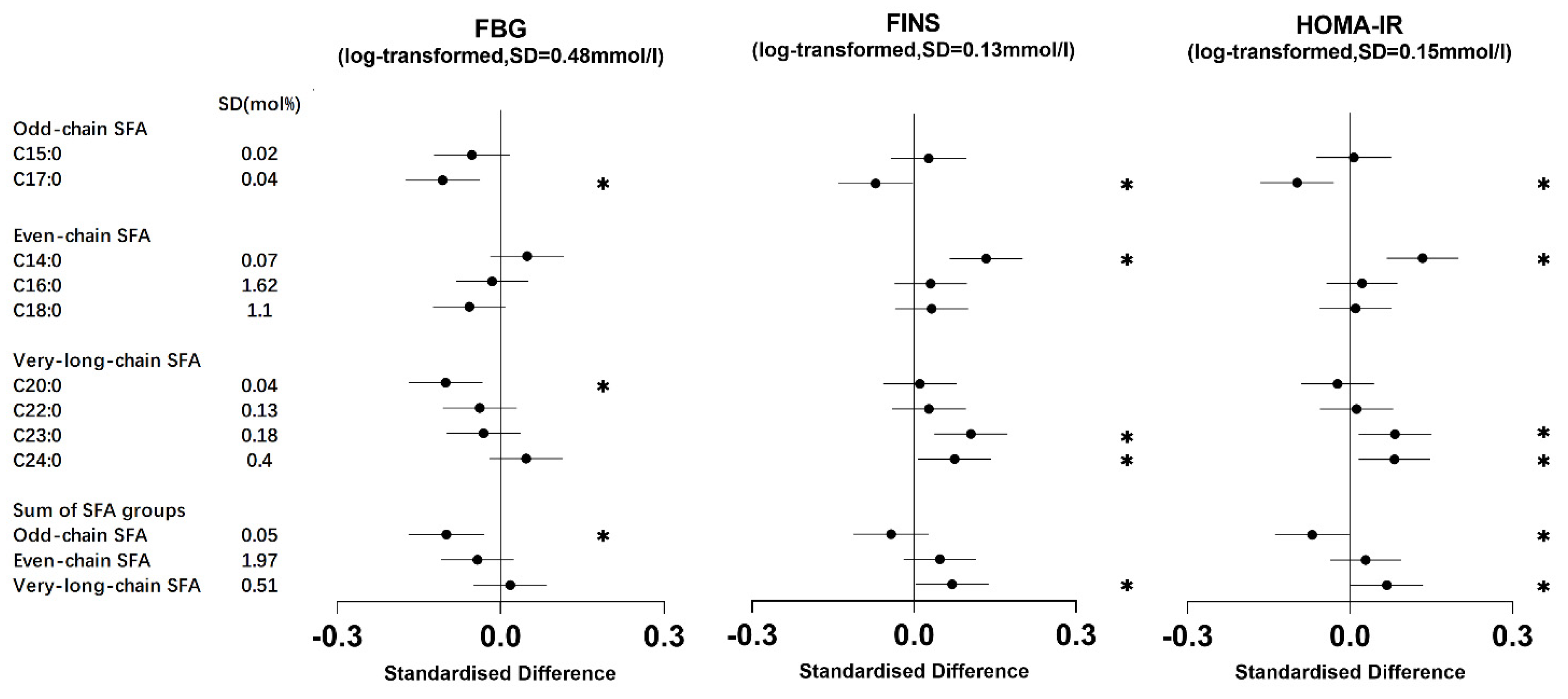
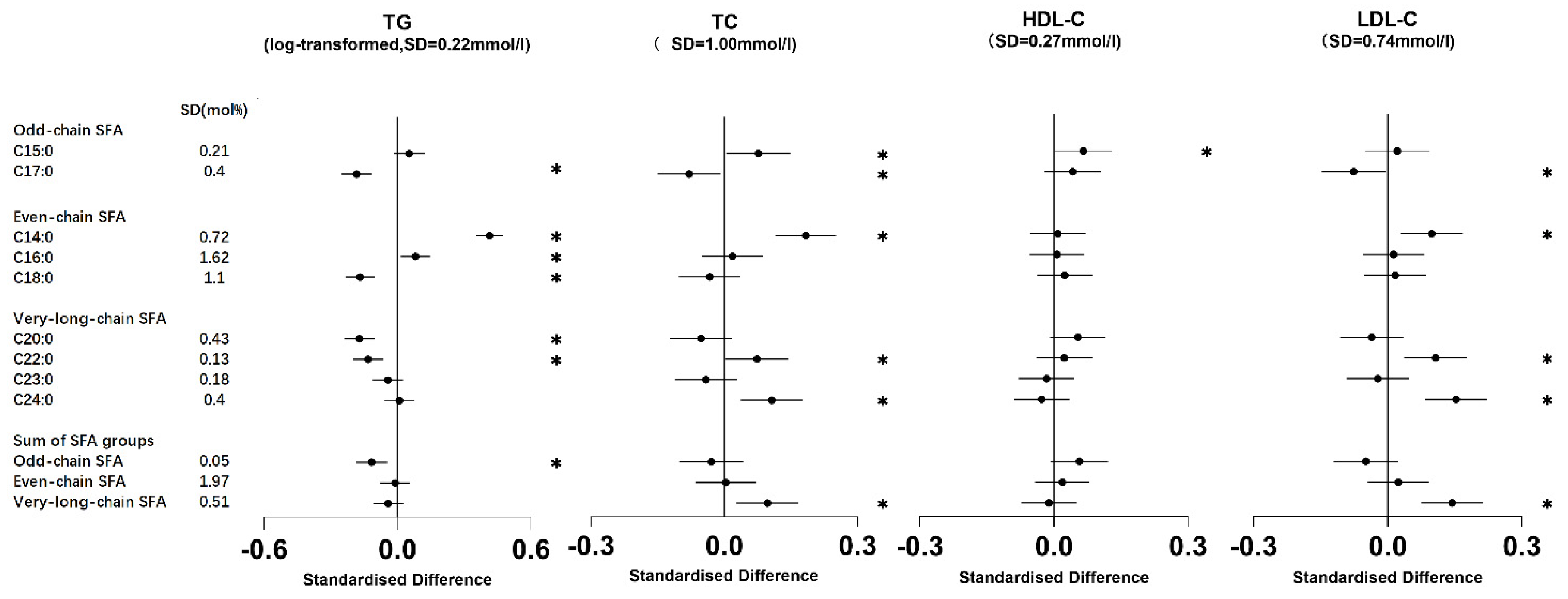
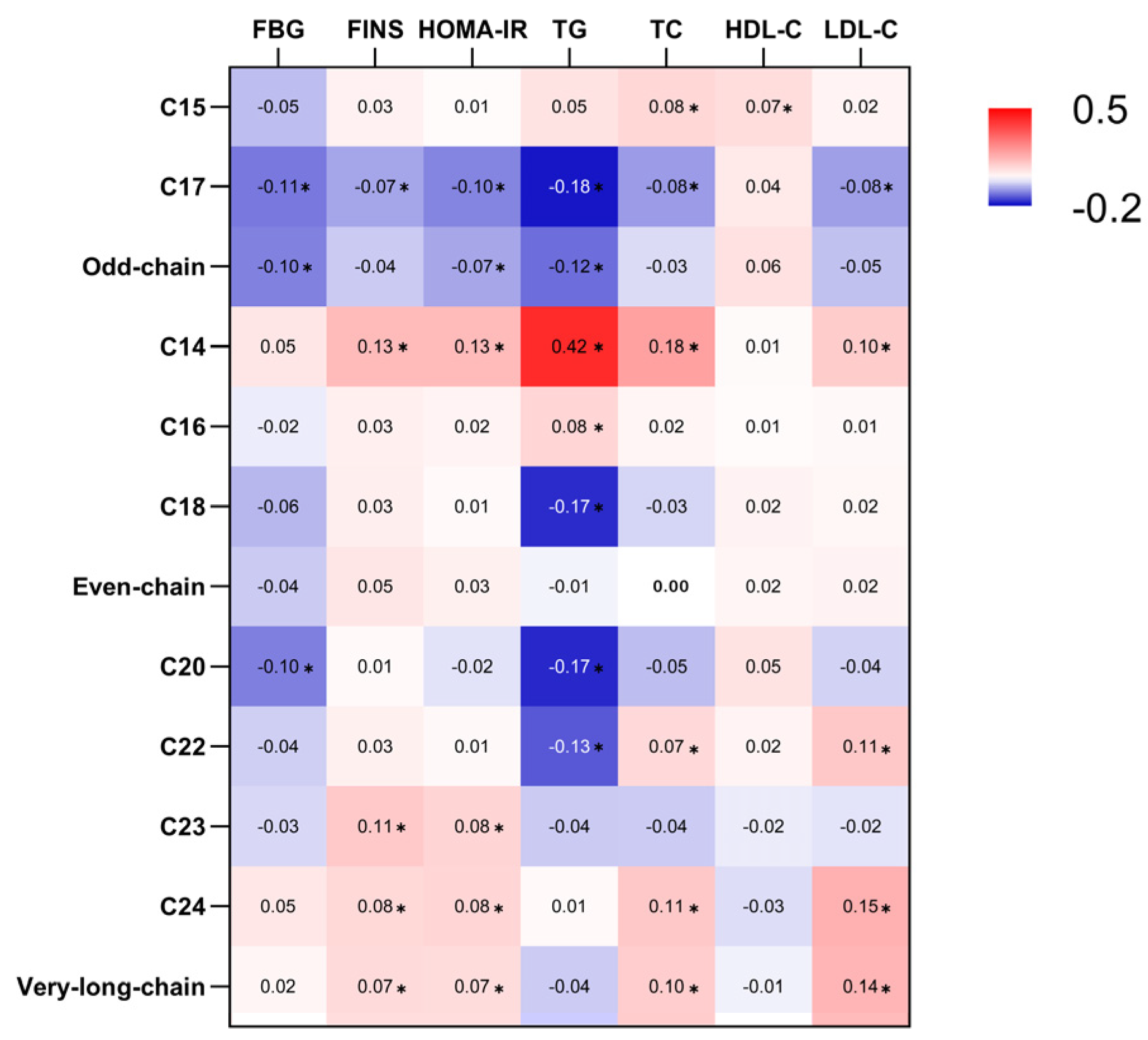
3.2. Association of Erythrocyte Membrane SFAs with Metabolic Markers
3.2.1. Odd-Chain SFAs
3.2.2. Even-Chain SFAs
3.2.3. Very-Long-Chain SFAs
3.3. Sensitivity and Interaction Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younus, A.; Aneni, E.C.; Spatz, E.S.; Osondu, C.U.; Roberson, L.; Ogunmoroti, O.; Malik, R.; Ali, S.S.; Aziz, M.; Feldman, T.; et al. A Systematic Review of the Prevalence and Outcomes of Ideal Cardiovascular Health in US and Non-US Populations. Mayo Clin. Proc. 2016, 91, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed. Environ. Sci. BES 2022, 35, 573–603. [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; King, I.B.; Rice, K.; McKnight, B.; Sotoodehnia, N.; Rea, T.D.; Johnson, C.O.; Raghunathan, T.E.; Cobb, L.A.; Mozaffarian, D.; et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 149–153. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; McKnight, B.; Sotoodehnia, N.; Fretts, A.M.; Qureshi, W.T.; Song, X.; King, I.B.; Sitlani, C.M.; Siscovick, D.S.; Psaty, B.M.; et al. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. J. Am. Heart Assoc. 2018, 7, e010019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Koulman, A.; Schulze, M.B.; Ye, Z.; Griffin, J.; Guevara, M.; Huerta, J.M.; Kröger, J.; et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: A cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017, 15, 203. [Google Scholar] [CrossRef]
- Li, Z.; Lei, H.; Jiang, H.; Fan, Y.; Shi, J.; Li, C.; Chen, F.; Mi, B.; Ma, M.; Lin, J.; et al. Saturated fatty acid biomarkers and risk of cardiometabolic diseases: A meta-analysis of prospective studies. Front. Nutr. 2022, 9, 963471. [Google Scholar] [CrossRef]
- Kirk, E.P.; Klein, S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J. Clin. Hypertens. 2009, 11, 761–765. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.; Chen, C.G.; He, Q.Q.; Zhuo, S.Y.; Chen, Y.M.; Su, Y.X. Validation of an FFQ to estimate the intake of fatty acids using erythrocyte membrane fatty acids and multiple 3d dietary records. Public Health Nutr. 2010, 13, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition; Peking University Medical Press: Being, China, 2009; Volume 42, pp. 795–799. [Google Scholar]
- Su, M.; Zhang, X.; Hu, W.; Yang, Z.; Chen, D.; Yang, Y.; Xie, K.; Chen, Y.; Zhang, Z. The associations of erythrocyte membrane polyunsaturated fatty acids with skeletal muscle loss: A prospective cohort study. Clin. Nutr. 2023, 42, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Lin, J.S.; Dong, H.L.; Zeng, F.F.; Li, D.; Song, Y.; Chen, Y.M. Association of erythrocyte n-3 polyunsaturated fatty acids with incident type 2 diabetes in a Chinese population. Clin. Nutr. 2019, 38, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Pikó, P.; Pál, L.; Szűcs, S.; Kósa, Z.; Sándor, J.; Ádány, R. Obesity-Related Changes in Human Plasma Lipidome Determined by the Lipidyzer Platform. Biomolecules 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- González-González, J.G.; Violante-Cumpa, J.R.; Zambrano-Lucio, M.; Burciaga-Jimenez, E.; Castillo-Morales, P.L.; Garcia-Campa, M.; Solis, R.C.; González-Colmenero, A.D.; Rodríguez-Gutiérrez, R. HOMA-IR as a predictor of Health Outcomes in Patients with Metabolic Risk Factors: A Systematic Review and Meta-analysis. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2022, 29, 547–564. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.S.; Aris, I.M.; Yang, G.; Chen, W.Q.; Li, L.J. Circulating Saturated Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Nettleton, J.A.; Lemaitre, R.N.; Steffen, L.M.; Kromhout, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R.; Mozaffarian, D. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000092. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Schiller, K.; Jansen, E.; Fritsche, A.; Weikert, C.; di Giuseppe, R.; Boeing, H.; Schulze, M.B.; Kröger, J. Association between erythrocyte membrane fatty acids and biomarkers of dyslipidemia in the EPIC-Potsdam study. Eur. J. Clin. Nutr. 2015, 69, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Dong, H.L.; Chen, G.D.; Chen, Z.Y.; Dong, X.W.; Zheng, J.S.; Chen, Y.M. Erythrocyte Saturated Fatty Acids and Incident Type 2 Diabetes in Chinese Men and Women: A Prospective Cohort Study. Nutrients 2018, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef]
- Sun, L.; Zong, G.; Li, H.; Lin, X. Fatty acids and cardiometabolic health: A review of studies in Chinese populations. Eur. J. Clin. Nutr. 2021, 75, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.S.; Enns, C.W.; Martin, C.L.; Goldman, J.D.; Moshfegh, A.J. Sweet Foods Consumption by Adults in the U.S. What We Eat in America, NHANES 2015–2018. In FSRG Dietary Data Briefs; United States Department of Agriculture (USDA): Beltsville, MD, USA, 2010. [Google Scholar]
- Leme, A.C.; Baranowski, T.; Thompson, D.; Philippi, S.; O’Neil, C.; Fulgoni, V.; Nicklas, T. Top food sources of percentage of energy, nutrients to limit and total gram amount consumed among US adolescents: National Health and Nutrition Examination Survey 2011–2014. Public Health Nutr. 2019, 22, 661–671. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Gao, X.; Yang, F.; Yang, Y.; Fang, H.; Ju, L.; Xu, X.; Guo, Q.; Li, S.; et al. Geographic Variations in Dietary Patterns and Their Associations with Overweight/Obesity and Hypertension in China: Findings from China Nutrition and Health Surveillance (2015–2017). Nutrients 2022, 14, 3949. [Google Scholar] [CrossRef]
- Patel, P.S.; Sharp, S.J.; Jansen, E.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Fretts, A.M.; Sitlani, C.M.; Biggs, M.L.; Mukamal, K.; King, I.B.; Song, X.; Djoussé, L.; Siscovick, D.S.; McKnight, B.; et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2015, 101, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Imamura, F.; Marklund, M.; Micha, R.; Wu, J.H.Y.; Murphy, R.A.; Chien, K.L.; McKnight, B.; Tintle, N.; Forouhi, N.G.; et al. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: A pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 2019, 109, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Liu, L.; Ma, P.; Hu, J.; Ming, Z.; Dang, K.; Zhang, Y.; Li, Y. Association of Circulating Very Long Chain Saturated Fatty Acids with Cardiovascular Mortality in NHANES 2015–2016. J. Clin. Endocrinol. Metab. 2023, 109, e633–e645. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, R.; Miyazaki, T.; Shimada, K.; Kume, A.; Kitamura, Y.; Oshida, K.; Yanagisawa, N.; Kiyanagi, T.; Hiki, M.; Fukao, K.; et al. High levels of very long-chain saturated fatty acid in erythrocytes correlates with atherogenic lipoprotein profiles in subjects with metabolic syndrome. Diabetes Res. Clin. Pract. 2013, 99, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zuo, L.S.; Sun, T.Y.; Wu, Y.Y.; Liu, Y.P.; Zeng, F.F.; Chen, Y.M. Circulating Very-Long-Chain Saturated Fatty Acids Were Inversely Associated with Cardiovascular Health: A Prospective Cohort Study and Meta-Analysis. Nutrients 2020, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Fattore, E.; Bosetti, C.; Brighenti, F.; Agostoni, C.; Fattore, G. Palm oil and blood lipid-related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014, 99, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Skeaff, C.M.; Green, T.J.; Gray, A.R.; Crowe, F.L. The serum fatty acids myristic acid and linoleic acid are better predictors of serum cholesterol concentrations when measured as molecular percentages rather than as absolute concentrations. Am. J. Clin. Nutr. 2010, 91, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Hellgren, L.I. Plasma phospholipid very-long-chain saturated fatty acids: A sensitive marker of metabolic dysfunction or an indicator of specific healthy dietary components? Am. J. Clin. Nutr. 2015, 101, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Chandra, J.; Zhivotovsky, B.; Zaitsev, S.; Juntti-Berggren, L.; Berggren, P.O.; Orrenius, S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes 2001, 50 (Suppl. 1), S44–S47. [Google Scholar] [CrossRef]
- Véret, J.; Coant, N.; Berdyshev, E.V.; Skobeleva, A.; Therville, N.; Bailbé, D.; Gorshkova, I.; Natarajan, V.; Portha, B.; Le Stunff, H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem. J. 2011, 438, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ardisson Korat, A.V.; Malik, V.S.; Furtado, J.D.; Sacks, F.; Rosner, B.; Rexrode, K.M.; Willett, W.C.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain SFA Concentrations Are Inversely Associated with Incident Type 2 Diabetes in US Men and Women. J. Nutr. 2020, 150, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; King, I.B. Very long-chain saturated fatty acids and diabetes and cardiovascular disease. Curr. Opin. Lipidol. 2022, 33, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hanzal-Bayer, M.F.; Hancock, J.F. Lipid rafts and membrane traffic. FEBS Lett. 2007, 581, 2098–2104. [Google Scholar] [CrossRef]
| Baseline Characteristics | /n (%) |
|---|---|
| Age (year) | 55.12 ± 7.75 |
| Sex | |
| Women | 547 (68.5) |
| Men | 251 (31.5) |
| Weight (kg) | 62.75 ± 10.43 |
| Height (cm) | 159.76 ± 8.12 |
| Body mass index, BMI (kg/m2) | |
| <24 | 356 (44.6) |
| 24–28 | 336 (42.1) |
| >28 | 106 (13.3) |
| Waist circumference (cm) | 85.57 ± 8.91 |
| Smoker | |
| No | 700 (87.7) |
| Yes | 98 (12.3) |
| Alcohol drinker | |
| No | 669 (83.8) |
| Yes | 129 (16.2) |
| Physical activity | |
| Light | 282 (35.3) |
| Moderate | 379 (47.5) |
| Vigorous | 137 (17.2) |
| Family history of diseases | |
| No | 652 (81.7) |
| Yes | 146 (18.3) |
| Total energy intake (kcal/day) | 1648 ± 468 |
| Dairy intake (g/day) | 90.18 ± 99.82 |
| Red and processed meat intake (g/day) | 78.90 ± 55.65 |
| Fruits and vegetables intake (g/day) | 412.09 ± 212.76 |
| Vegetable oil intake (g/day) | 32.60 ± 21.37 |
| Overall | Sex | Age (Years) | BMI (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 798 | Men (n = 251) | Women (n = 547) | p | ≤65 (n = 716) | >65 (n = 82) | p | <24 (n = 355) | 24–28 (n = 340) | >28 (n = 103) | p | |
| FBG (mmol/L) a | 5.50 (5.25, 5.89) | 5.48 (5.19, 5.86) | 5.47 (5.18, 5.83) | 0.337 | 5.46 (5.18, 5.84) | 5.58 (5.20, 5.85) | 0.353 | 5.36 (5.11, 5.67) | 5.57 (5.22, 5.98) | 5.62 (5.27, 6.03) | <0.001 |
| FINS (μU/mL) a | 6.92 (6.03, 8.32) | 6.91 (5.89, 8.20) | 6.85 (5.99, 8.41) | 0.793 | 6.85 (5.96, 8.31) | 6.86 (5.99, 8.49) | 0.815 | 6.48 (5.78, 7.67) | 7.31 (6.16, 8.70) | 7.39 (6.56, 9.91) | <0.001 |
| HOMA-IR a | 1.74 (1.41, 2.14) | 1.76 (1.42, 2.11) | 1.71 (1.41, 2.14) | 0.700 | 1.72 (1.41, 2.12) | 1.77 (1.44, 2.14) | 0.502 | 1.54 (1.35, 1.86) | 1.81 (1.48, 2.27) | 1.92 (1.63, 2.71) | <0.001 |
| TG (mmol/L) a | 1.26 (0.91, 1.78) | 1.48 (1.03, 2.11) | 1.17 (0.88, 1.67) | <0.001 | 1.25 (0.91, 1.77) | 1.35 (0.94, 1.72) | 0.757 | 1.13 (0.83, 1.52) | 1.36 (0.96, 2.03) | 1.48 (1.08, 1.94) | <0.001 |
| TC (mmol/L) b | 5.48 (1.00) | 5.36 (0.88) | 5.54 (1.04) | 0.047 | 5.49 (1.01) | 5.38 (0.93) | 0.403 | 5.55 (1.00) | 5.45 (0.98) | 5.35 (1.03) | 0.087 |
| HDL-C (mmol/L) b | 1.29 (0.27) | 1.15 (0.23) | 1.36 (0.27) | <0.001 | 1.29 (0.27) | 1.28 (0.28) | 0.812 | 1.39 (0.27) | 1.23 (0.24) | 1.16 (0.22) | <0.001 |
| LDL-C (mmol/L) b | 3.45 (0.74) | 3.45 (0.66) | 3.46 (0.78) | 0.934 | 3.46 (0.75) | 3.42 (0.72) | 0.675 | 3.45 (0.75) | 3.46 (0.74) | 3.44 (0.74) | 0.824 |
| Odd-chain SFAs (mol%) b | 0.36 (0.05) | 0.34 (0.05) | 0.36 (0.05) | <0.001 | 0.36 (0.06) | 0.36 (0.05) | 0.861 | 0.37 (0.05) | 0.35 (0.05) | 0.34 (0.05) | <0.001 |
| C15:0 (mol%) b | 0.08 (0.02) | 0.08 (0.02) | 0.09 (0.02) | <0.001 | 0.08 (0.02) | 0.08 (0.02) | 0.302 | 0.09 (0.02) | 0.08 (0.02) | 0.08 (0.02) | <0.001 |
| C17:0 (mol%) b | 0.27 (0.04) | 0.27 (0.04) | 0.28 (0.04) | <0.001 | 0.27 (0.04) | 0.27 (0.04) | 0.969 | 0.28 (0.04) | 0.27 (0.04) | 0.26 (0.04) | <0.001 |
| Even-chain SFAs (mol%) b | 44.34 (1.97) | 44.27 (2.04) | 44.37 (1.95) | 0.446 | 44.37 (1.96) | 44.04 (2.06) | 0.038 | 44.30 (1.99) | 44.37 (1.96) | 44.35 (1.96) | 0.993 |
| C14:0 (mol%) b | 0.25 (0.07) | 0.24 (0.07) | 0.26 (0.07) | 0.014 | 0.25 (0.07) | 0.26 (0.07) | 0.624 | 0.25 (0.07) | 0.25 (0.07) | 0.26 (0.07) | 0.086 |
| C16:0 (mol%) b | 31.40 (1.62) | 31.44 (1.70) | 31.40 (1.58) | 0.934 | 31.43 (1.62) | 31.26 (1.60) | 0.205 | 31.42 (1.67) | 31.40 (1.60) | 31.42 (1.46) | 0.973 |
| C18:0 (mol%) b | 12.67 (1.10) | 12.59 (1.03) | 12.71 (1.13) | 0.215 | 12.69 (1.11) | 12.52 (1.00) | 0.149 | 12.63 (1.12) | 12.72 (1.10) | 12.66 (1.07) | 0.674 |
| Very-long-chain SFAs (mol%) b | 3.85 (0.51) | 3.80 (0.50) | 3.88 (0.51) | 0.106 | 3.85 (0.51) | 3.88 (0.46) | 0.456 | 3.83 (0.51) | 3.87 (0.52) | 3.91 (0.44) | 0.205 |
| C20:0 (mol%) b | 0.24 (0.04) | 0.23 (0.03) | 0.25 (0.05) | <0.001 | 0.24 (0.04) | 0.24 (0.04) | 0.735 | 0.24 (0.04) | 0.24 (0.04) | 0.24 (0.04) | 0.574 |
| C22:0 (mol%) b | 0.85 (0.13) | 0.81 (0.12) | 0.86 (0.13) | <0.001 | 0.85 (0.14) | 0.85 (0.11) | 0.740 | 0.85 (0.13) | 0.84 (0.14) | 0.84 (0.11) | 0.571 |
| C23:0 (mol%) b | 0.09 (0.02) | 0.08 (0.02) | 0.09 (0.02) | <0.001 | 0.09 (0.02) | 0.08 (0.02) | 0.015 | 0.09 (0.02) | 0.08 (0.02) | 0.09 (0.02) | 0.298 |
| C24:0 (mol%) b | 2.68 (0.40) | 2.68 (0.40) | 2.68 (0.39) | 0.624 | 2.68 (0.40) | 2.71 (0.36) | 0.346 | 2.65 (0.40) | 2.70 (0.40) | 2.74 (0.36) | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Luo, H.; Zhong, J.; Su, M.; Lai, X.; Zhang, Z.; Zhou, Q. Differential Associations of Erythrocyte Membrane Saturated Fatty Acids with Glycemic and Lipid Metabolic Markers in a Chinese Population: A Cross-Sectional Study. Nutrients 2024, 16, 1507. https://doi.org/10.3390/nu16101507
Wu S, Luo H, Zhong J, Su M, Lai X, Zhang Z, Zhou Q. Differential Associations of Erythrocyte Membrane Saturated Fatty Acids with Glycemic and Lipid Metabolic Markers in a Chinese Population: A Cross-Sectional Study. Nutrients. 2024; 16(10):1507. https://doi.org/10.3390/nu16101507
Chicago/Turabian StyleWu, Shixin, Huiru Luo, Juncheng Zhong, Mengyang Su, Xiaoying Lai, Zheqing Zhang, and Quan Zhou. 2024. "Differential Associations of Erythrocyte Membrane Saturated Fatty Acids with Glycemic and Lipid Metabolic Markers in a Chinese Population: A Cross-Sectional Study" Nutrients 16, no. 10: 1507. https://doi.org/10.3390/nu16101507
APA StyleWu, S., Luo, H., Zhong, J., Su, M., Lai, X., Zhang, Z., & Zhou, Q. (2024). Differential Associations of Erythrocyte Membrane Saturated Fatty Acids with Glycemic and Lipid Metabolic Markers in a Chinese Population: A Cross-Sectional Study. Nutrients, 16(10), 1507. https://doi.org/10.3390/nu16101507







