Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development
Abstract
1. Introduction
2. Materials and Methods
- -
- Epidemiological parameter evaluations included age, gender, and medical comorbidities such as diabetes, hypertension, dyslipidemia).
- -
- The anthropometric data included weight, height, and body mass index.
- -
- Bioelectrical impedance analysis (Akern® BIA 101 New Edition-sinusoidal 50 kHz waveform current, intensity 0.8 A) was used to analyze body composition, particularly appendicular skeletal muscle mass. Data were analyzed through the software Bodygram® Dashboard by Akern Srl V. 1.0.
- -
- Muscle strength was measured through the Handgrip test (Jamar Hydraulic Hand Dynamometer—5030J1 by Petterson Medical; dual-scale readout displays isometric grip force from 0 to 200 lbs [90 kg]).
- -
- Physical performance was evaluated through the Gait Speed test (measurement of walking speed by timing the time it takes to cover a distance of 4 m).
2.1. Dietary Intervention
- Energy: 25–30 kcal/kg/day;
- Proteins: 0.3–0.7 g/kg/day. A very low-protein diet (0.3–0.4 g/kg/day) was supported by essential amino acids or keto-analogues, according to patient weight;
- Carbohydrates: At least 60% of total energy intake;
- Lipids: At least 30% of total energy intake;
- Sodium: 2.3 g/day (corresponding to 6 g of NaCl);
- Phosphorus and potassium: adequate to maintain normal blood levels;
- Supplementations: carbonate calcium, vitamins, iron.
2.2. Sarcopenia Definition
2.3. Statistical Analysis
2.4. Sample Size
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- ISN—Global Kidney Health Atlas: A Report by the International Society of Nephrology: An Assessment of Global Kidney Health Care Status Focussing on Capacity, Availability, Accessibility, Affordability and Outcomes of Kidney Disease. International Society of Nephrology, Brussels, Belgium. Available online: www.theisn.org/global-atlas (accessed on 1 March 2024).
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Martino, F.K.; Fanton, G.; Zanetti, F.; Carta, M.; Nalesso, F.; Novara, G. Stage 5 Chronic Kidney Disease: Epidemiological Analysis in a NorthEastern District of Italy Focusing on Access to Nephrological Care. J. Clin. Med. 2024, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus On Definition And Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, G.J.; Calado, C.K.M.; Souza de Oliveira, B.C.; Araujo, R.P.O.; Filgueira, T.O.; de Sousa Fernandes, M.S.; Castoldi, A.; Vajgel, G.; Valente, L.M.; de Lima-Filho, J.L.; et al. Sarcopenia in Non-Dialysis Chronic Kidney Disease Patients: Prevalence and Associated Factors. Front. Med. 2022, 9, 854410. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Zaitseva, A.P.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetrou, V.; Bégin, M.; Hars, M.; Trombetti, A. Sarcopenia in Chronic Kidney Disease: A Scoping Review of Prevalence, Risk Factors, Association with Outcomes, and Treatment. Calcif. Tissue Int. 2022, 110, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef]
- Martino, F.K.; Novara, G.; Nalesso, F.; Calò, L.A. Conservative Management in End-Stage Kidney Disease between the Dialysis Myth and Neglected Evidence-Based Medicine. J. Clin. Med. 2023, 13, 41. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Marzocco, S.; Bellasi, A.; De Simone, E.; Dal Piaz, F.; Rocchetti, M.T.; Cosola, C.; Di Micco, L.; Gesualdo, L. Nutritional therapy reduces protein carbamylation through urea lowering in chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 804–813. [Google Scholar] [CrossRef]
- AlimentiNUTrizione—Tabelle Composizione Alimenti. Available online: https://www.alimentinutrizione.it/sezioni/tabelle-nutrizionali (accessed on 1 March 2024).
- Barril, G.; Nogueira, A.; Ruperto López, M.; Castro, Y.; Sánchez-Tomero, J.A. Influence of dietary protein intake on body composition in chronic kidney disease patients in stages 3-5: A cross-sectional study. Nefrologia 2018, 38, 647–654. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2018, 10, CD001892. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, I.; De Simone, I.; Biazzi, C.; Buzzi, L.; Ferrario, F.; Luise, M.C.; Santagostino, G.; Furiani, S.; Alberghini, E.; Capitanio, C.; et al. The low-protein diet for chronic kidney disease: 8 years of clinical experience in a nephrology ward. Clin. Kidney J. 2020, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Garofalo, C.; Ferrara, C.; Calella, P. Ketoanalogue Supplementation in Patients with Non-Dialysis Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, G.; Sofia, A.; Parodi, E.L.; Ansaldo, F.; Bonanni, A.; Picciotto, D.; Signori, A.; Vettore, M.; Tessari, P.; Verzola, D. Effects of Low-Protein, and Supplemented Very Low-Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018, 3, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Obi, Y.; Tortorici, A.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-Protein Diets for Chronic Kidney Disease Patients: The Italian Experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef]
- Ellison, K.M.; Ehrlicher, S.E.; El Zein, A.; Sayer, R.D. Fat and fat-free mass measurement agreement by dual-energy X-ray absorptiometry versus bioelectrical impedance analysis: Effects of posture and waist circumference. Obes. Sci. Pract. 2024, 10, e744. [Google Scholar] [CrossRef] [PubMed]
| n.(%) | |
|---|---|
| Male | 33 (73.3%) |
| Age (years) | 78 (±6.2) |
| Comorbidities | |
| Diabetes | 18 (40%) |
| Hypertension | 23 (51.1%) |
| Dyslipidemia | 6 (13.3%) |
| n. | T0 | T1 | p-Value | |
|---|---|---|---|---|
| Weight (kg) (1) | 45 | 74.5 (±15.9) | 72.9 (±15.5) | 0.002 |
| - Weight with BMI < 25 kg/m2 (kg) | 15 | 58.4 (±9.5) | 57.9 (±8.9) | 0.37 |
| - Weight with BMI > 25 kg/m2 (kg) | 30 | 82.6 (±11.8) | 80.4 (±12.4) | 0.003 |
| BMI (kg/m2) (1) | 45 | 28 (±5.7) | 27.4 (±5.6) | 0.003 |
| - BMI < 25 (kg/m2) | 15 | 22 (±2.3) | 21.8 (±2.1) | 0.47 |
| - BMI > 25 (kg/m2) | 30 | 31 (±4.3) | 30.2 (±4.5) | 0.004 |
| BIA | ||||
| - Reactance (Ohm) (2) | 45 | 454 [414–519] | 452 [416–502.2] | 0.12 |
| - Resistance (Ohm) (2) | 45 | 35.1 [29.2–39.8] | 34.2 [29.2–38.4] | 0.16 |
| - Phase Angle (°) (2) | 45 | 4.2 [3.7–4.8] | 4.2 [3.7–4.7] | 0.46 |
| Lean Mass (kg) (2) | 45 | 54 [46.1–59.8] | 54.1 [47.2–58.7] | 0.66 |
| Fat mass (kg) (2) | 45 | 19.5 [13.9–29.6] | 18 [10.9–27.7] | <0.001 |
| Total Body Water (L) (2) | 45 | 41 [37.2–47.5] | 40.9 [38–46.5] | 0.9 |
| Extracellular Water (L) (2) | 45 | 22.5 [20.6–26.7] | 22.7 [20.4–25] | 0.97 |
| n. | T0 | T1 | p-Value | |
|---|---|---|---|---|
| Sarcopenia diagnosis (1) | 45 | |||
| - Sarcopenic | 6 (13.3%) | 6 (13.3%) | 1 | |
| - Not sarcopenic | 39 (86.7%) | 39 (86.7%) | ||
| Handgrip (kg) (2) | 45 | 25.2 (±8.8) | 25.2 (±8.7) | 0.95 |
| ASM/h2 (kg/m2) (3) | 45 | 7.44 [6.46–8.23] | 7.39 [6.47–8.15] | 0.53 |
| Gait Speed (m/s) (3) | 45 | 0.95 (±0.34) | 0.96 (±0.34) | 0.64 |
| n. | T0 | T1 | p-Value | |
|---|---|---|---|---|
| GFR (mL/min/1.73 m2) (1) | 45 | 16.8 (±4.9) | 17 (±6.2) | 0.66 |
| Creatinine (umol/L) (2) | 45 | 291 [234–356] | 296 [215–417] | 0.57 |
| Plasma urea (mmol/l) (1) | 45 | 22.5 (±7) | 17.9 (±5.9) | <0.001 |
| Uric acid (mmol/l) (2) | 44 | 0.34 [0.3–0.46] | 0.38 [0.31–0.47] | 0.27 |
| Sodium (mmol/L) (1) | 45 | 139.3 (±2.7) | 138.6 (±4.8) | 0.33 |
| Potassium (mmol/L) (2) | 45 | 4.4 (±0.5) | 4.3 (±0.5) | 0.14 |
| Phosphorus (mmol/L) (2) | 44 | 1.3 [1.15–1.43] | 1.27 [1.04–1.41] | 0.09 |
| Calcium (mmol/L) (2) | 44 | 2.35 [2.25–2.4] | 2.33 [2.26–2.4] | 0.12 |
| PTH (ng/L) | 35 | 119 (64–219) | 99 (65–178) | 0.64 |
| Albumin (g/L) (1) | 45 | 38.9 (±3.9) | 38.6 (±2.9) | 0.73 |
| Hemoglobin (g/L) (1) | 44 | 118.4 (±13.9) | 116 (±11) | 0.64 |
| Blood glucose (mg/dl) (2) | 45 | 99 [92–117] | 92 [86.5–118.3] | 0.86 |
| Proteinuria 24 h (g/24 h) (2) | 42 | 1.02 [0.53–2.8] | 0.57 [0.29–2.14] | 0.01 |
| n. | T0 | T1 | p-Value | |
|---|---|---|---|---|
| Energy (kcal) (3) | 45 | 1700 [1523–1836] | 1656 [1567–1815] | 0.73 |
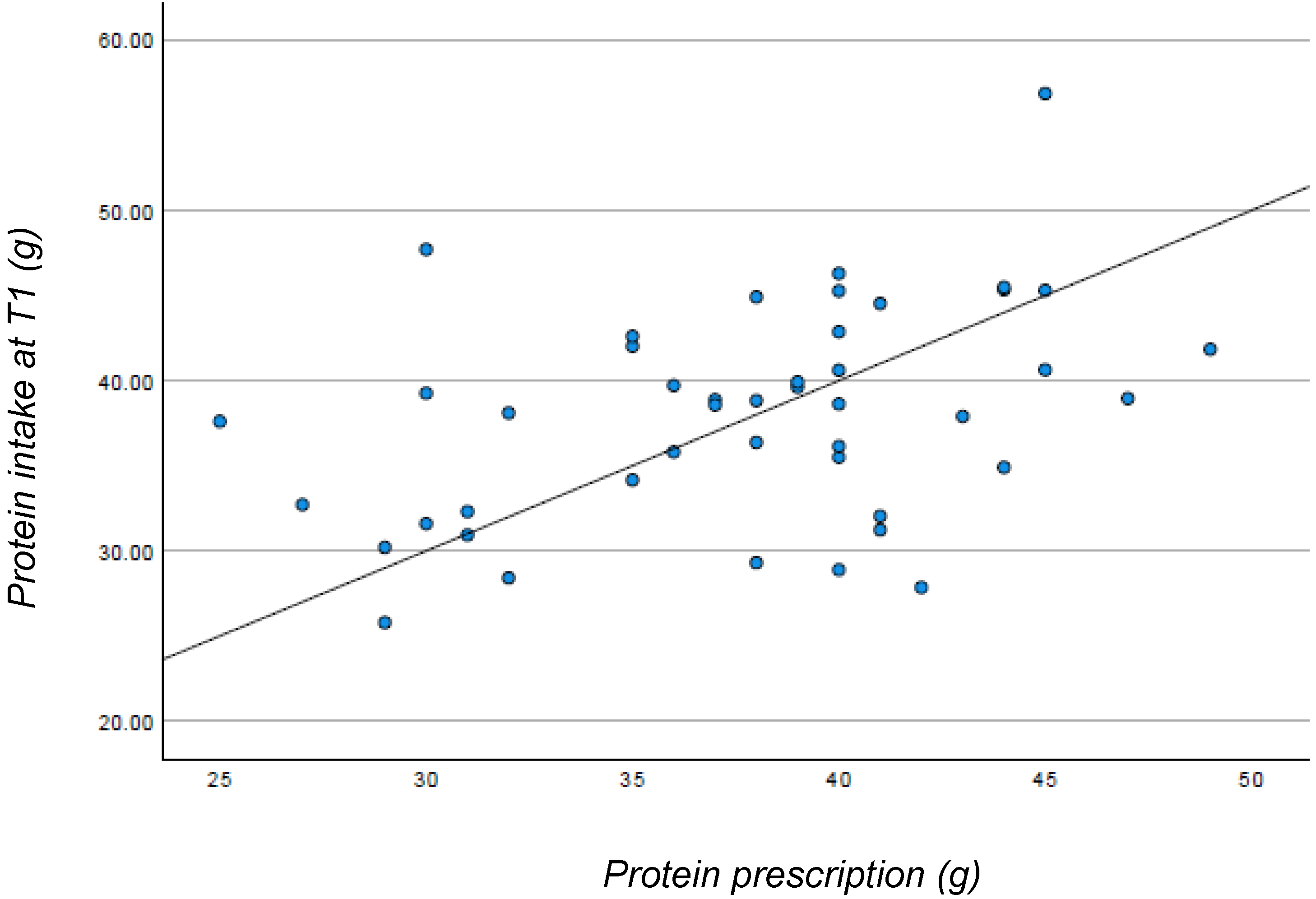
| Proteins (g) (3) | 45 | 61.1 [56.5–70.1] | 38.6 [32.5–42.3] | <0.001 |
| Lipids (g) (3) | 45 | 64.1 [56.6–67.7] | 66 [59.7–72.6] | 0.01 |
| Carbohydrates (g) (3) | 45 | 206.8 [180–228.5] | 228.6 [202.3–250] | <0.001 |
| Fiber (g) (3) | 45 | 17.4 [15.2–19.5] | 18.6 [16.3–22.3] | <0.001 |
| Sodium (mg) (3) | 45 | 965 [800–1223] | 681 [575–822] | <0.001 |
| Potassium (mg) (3) | 45 | 2409 [2061–2760] | 2105 [1782–2412] | <0.001 |
| Phosphorus (mg) (3) | 45 | 986 [855–1117] | 666 [550–771] | <0.001 |
| Use of free-protein food (1) | 45 | 42 (±91.1%) | ||
| Prescribed diet | 45 | |||
| - Energy (kcal) (2) | 45 | 1846 (±167.5) | ||
| - Proteins (g) (2) | 45 | 38 (±5.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, F.K.; Zattarin, A.; Cinquini, C.; Toniazzo, S.; Francini Pesenti, F.; Stefanelli, L.F.; Cacciapuoti, M.; Bettin, E.; Calò, L.A.; Spinella, P. Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development. Nutrients 2024, 16, 1498. https://doi.org/10.3390/nu16101498
Martino FK, Zattarin A, Cinquini C, Toniazzo S, Francini Pesenti F, Stefanelli LF, Cacciapuoti M, Bettin E, Calò LA, Spinella P. Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development. Nutrients. 2024; 16(10):1498. https://doi.org/10.3390/nu16101498
Chicago/Turabian StyleMartino, Francesca K., Alessandra Zattarin, Chiara Cinquini, Silvia Toniazzo, Francesco Francini Pesenti, Lucia Federica Stefanelli, Martina Cacciapuoti, Elisabetta Bettin, Lorenzo A. Calò, and Paolo Spinella. 2024. "Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development" Nutrients 16, no. 10: 1498. https://doi.org/10.3390/nu16101498
APA StyleMartino, F. K., Zattarin, A., Cinquini, C., Toniazzo, S., Francini Pesenti, F., Stefanelli, L. F., Cacciapuoti, M., Bettin, E., Calò, L. A., & Spinella, P. (2024). Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development. Nutrients, 16(10), 1498. https://doi.org/10.3390/nu16101498








