Relationship between Circulating 25-Hydroxyvitamin D and Metabolic Syndrome in Chinese Adults: A Large Nationwide Longitudinal Study
Abstract
1. Introduction
2. Study Participants and Methods
2.1. Study Participants
2.2. Research Method
2.2.1. Data Collection
2.2.2. Physical Examination
2.2.3. Laboratory Examination
2.3. Relevant Definitions
2.4. Statistical Methods
3. Results
3.1. Participant Characteristics
3.2. Relationships between Serum 25(OH)D Levels and MetS Risk—Hybrid Mixed-Effect Model
3.3. Mean 25 Hydroxyvitamin D Values and the Incidence of MetS—Cox Regression Model
4. Discussion
4.1. Prevalence of VD Deficiency and Insufficiency and VD Supplement Users in Chinese Adults
4.2. Serum 25(OH)D and Metabolic Syndrome
4.3. Serum 25(OH)D and Abdominal Obesity and Dyslipidemia
4.4. Serum 25(OH)D and Hyperglycemia
4.5. Serum 25(OH)D and Hypertension
4.6. Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42 (Suppl. S1), S10–S15. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Mosekilde, L. Vitamin D and the elderly. Clin. Endocrinol. 2005, 62, 265–281. [Google Scholar] [CrossRef]
- Pinelli, N.R.; Jaber, L.A.; Brown, M.B.; Herman, W.H. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care 2010, 33, 1373–1375. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Mader, J.K.; Hoeller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. High-dose cholecalciferol supplementation significantly increases peripheral CD4⁺ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur. J. Nutr. 2014, 53, 751–759. [Google Scholar] [CrossRef]
- Tarcin, O.; Yavuz, D.G.; Ozben, B.; Telli, A.; Ogunc, A.V.; Yuksel, M.; Toprak, A.; Yazici, D.; Sancak, S.; Deyneli, O.; et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J. Clin. Endocrinol. Metab. 2009, 94, 4023–4030. [Google Scholar] [CrossRef]
- Tran, V.; De Silva, T.M.; Sobey, C.G.; Lim, K.; Drummond, G.R.; Vinh, A.; Jelinic, M. The Vascular Consequences of Metabolic Syndrome: Rodent Models, Endothelial Dysfunction, and Current Therapies. Front. Pharmacol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Krisnamurti, D.G.B.; Louisa, M.; Poerwaningsih, E.H.; Tarigan, T.J.E.; Soetikno, V.; Wibowo, H.; Nugroho, C.M.H. Vitamin D supplementation alleviates insulin resistance in prediabetic rats by modifying IRS-1 and PPARγ/NF-κB expressions. Front. Endocrinol. 2023, 14, 1089298. [Google Scholar] [CrossRef]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.-M.; Negru, A.-G.; Căinap, S.-S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and Its Role in the Lipid Metabolism and the Development of Atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Faraji, S.; Alizadeh, M. Mechanistic Effects of Vitamin D Supplementation on Metabolic Syndrome Components in Patients with or without Vitamin D Deficiency. J. Obes. Metab. Syndr. 2020, 29, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sexton, R.C.; Rudney, H. Effect of vitamin D3 derivatives on cholesterol synthesis and HMG-CoA reductase activity in cultured cells. J. Lipid Res. 1989, 30, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yu, Y.; Zhao, Y.; Deng, Z.; Zhang, L.; Liang, G. Insight into the Interaction Mechanism of Vitamin D against Metabolic Syndrome: A Meta-Analysis and In Silico Study. Foods 2023, 12, 3973. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, W.; Zhang, W.; Tong, R.; Yuan, A.; Li, Z.; Jiang, H.; Hu, L.; Huang, L.; Xu, Y.; et al. Plasma metabolic fingerprints for large-scale screening and personalized risk stratification of metabolic syndrome. Cell Rep. Med. 2023, 4, 101109. [Google Scholar] [CrossRef]
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Reynolds, R.F.; Whelton, P.K.; He, J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005, 365, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, L.; Li, M.; Xu, Y.; Jiang, Y.; Wang, W.; Li, J.; Mi, S.; Zhang, M.; Li, Y.; et al. Metabolic Syndrome Among Adults in China: The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2016, 102, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, E.Y.; Lee, J.H.; Kim, J.E.; Kim, K.J.; Rhee, Y.; Kim, H.C.; Youm, Y.; Kim, C.O. Associations of serum 25-hydroxyvitamin D with metabolic syndrome and its components in elderly men and women: The Korean Urban Rural Elderly cohort study. BMC Geriatr. 2019, 19, 102. [Google Scholar] [CrossRef]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Petri Nahás, E.A. Vitamin D deficiency is associated with metabolic syndrome in postmenopausal women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, H.H.; Lu, C.W.; Tseng, F.Y.; Lee, L.T.; Huang, K.C. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clin. Nutr. 2015, 34, 484–489. [Google Scholar] [CrossRef]
- Zhu, W.; Heil, D.P. Associations of vitamin D status with markers of metabolic health: A community-based study in Shanghai, China. Diabetes Metab. Syndr. 2018, 12, 727–732. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Nascimento, C.M.C.; Costa-Guarisco, L.P.; Gomes, G.A.O.; Gramani-Say, K.; Orlandi, F.S.; Gratão, A.C.M.; Orlandi, A.; Pavarini, S.C.I.; Vasilceac, F.A.; et al. Vitamin D Deficient Older Adults Are More Prone to Have Metabolic Syndrome, but Not to a Greater Number of Metabolic Syndrome Parameters. Nutrients 2020, 12, 748. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Lu, F.; Liu, Y.; Lv, Y.; Qu, Y.; Gu, H.; Li, C.; Cai, J.; Ji, S.; et al. Vitamin D deficiency and metabolic syndrome in elderly Chinese individuals: Evidence from CLHLS. Nutr. Metab. 2020, 17, 58. [Google Scholar] [CrossRef]
- Lu, L.; Yu, Z.; Pan, A.; Hu, F.B.; Franco, O.H.; Li, H.; Li, X.; Yang, X.; Chen, Y.; Lin, X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 2009, 32, 1278–1283. [Google Scholar] [CrossRef]
- Pham, T.M.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J. The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program. Nutrients 2015, 7, 7271–7284. [Google Scholar] [CrossRef]
- Qi, K.J.; Zhao, Z.T.; Zhang, W.; Yang, F. The impacts of vitamin D supplementation in adults with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Hajhashemy, Z.; Saneei, P. Circulating serum vitamin D levels in relation to metabolic syndrome in children: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13314. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Han, L.; Zhao, Y.; Li, G.; Zhu, Y.; Li, Y.; Li, M.; Gao, S.; Willi, S.M. Vitamin D levels are associated with metabolic syndrome in adolescents and young adults: The BCAMS study. Clin. Nutr. 2019, 38, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Abuhijleh, H.; Alkhatib, D.; Ganji, V. Hypovitaminosis D and Metabolic Syndrome in Postmenopausal Women. Healthcare 2022, 10, 2026. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Song, Y.; Manson, J.E.; Van Horn, L.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S.; et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am. J. Clin. Nutr. 2011, 94, 209–217. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Røislien, J.; Hofsø, D.; Bollerslev, J. Plasma 25-hydroxyvitamin d concentration and metabolic syndrome among middle-aged and elderly chinese individuals: Response to Lu et al. Diabetes Care 2010, 33, e13. [Google Scholar] [CrossRef][Green Version]
- Reis, J.P.; von Mühlen, D.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care 2007, 30, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- ISO 15189:2022; Medical Laboratories—Requirements for Quality and Competence. ISO: Geneva, Switzerland, 2022.
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Method for Vitamin D Deficiency Screening. Biomed. Environ. Sci. 2023, 36, 785.
- Twisk, J.W.R.; de Vente, W. Hybrid models were found to be very elegant to disentangle longitudinal within- and between-subject relationships. J. Clin. Epidemiol. 2019, 107, 66–70. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Q.; Zhang, X.; Lu, Y.; Sun, C.; Cui, Y.; Wang, S. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutr. J. 2012, 11, 68. [Google Scholar] [CrossRef]
- Ke, N.; Jin-Zi, W.; Huan, W. Investigation on the influencing factors of calcium and vitamin D supplementations in children from 9 areas of China. Matern. Child Health Care China 2014, 29, 5289–5293. [Google Scholar]
- Yin, W.J.; Tao, R.X.; Hu, H.L.; Zhang, Y.; Jiang, X.M.; Zhang, M.X.; Jin, D.; Yao, M.N.; Tao, F.B.; Zhu, P. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: A Chinese prospective birth cohort study. Am. J. Clin. Nutr. 2020, 111, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Mao, D.; Chen, J.; Li, M.; Li, W.; Yang, Y.; Zhao, L.; Zhang, J.; Piao, J.; et al. Vitamin D Nutritional Status of Chinese Pregnant Women, Comparing the Chinese National Nutrition Surveillance (CNHS) 2015-2017 with CNHS 2010-2012. Nutrients 2021, 13, 2237. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.B.; Clarke, A.; Franco, O.H. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; von Mühlen, D.; Miller, E.R., 3rd. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur. J. Endocrinol. 2008, 159, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; McGuire, L.C.; Liu, S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005, 28, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Tangpricha, V.; Zhang, X. Serum Vitamin D Concentration ≥75 nmol/L Is Related to Decreased Cardiometabolic and Inflammatory Biomarkers, Metabolic Syndrome, and Diabetes; and Increased Cardiorespiratory Fitness in US Adults. Nutrients 2020, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Boucher, B.J.; Berry, D.J.; Power, C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008, 57, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Chang, C.S.; Chang, Y.F.; Wu, S.J.; Chiu, C.J.; Hou, M.T.; Chen, C.Y.; Liu, P.Y.; Wu, C.H. Inverse Relationship between Metabolic Syndrome and 25-Hydroxyvitamin D Concentration in Elderly People without Vitamin D deficiency. Sci. Rep. 2018, 8, 17052. [Google Scholar] [CrossRef]
- Mansouri, M.; Abasi, R.; Nasiri, M.; Sharifi, F.; Vesaly, S.; Sadeghi, O.; Rahimi, N.; Sharif, N.A. Association of vitamin D status with metabolic syndrome and its components: A cross-sectional study in a population of high educated Iranian adults. Diabetes Metab. Syndr. 2018, 12, 393–398. [Google Scholar] [CrossRef]
- Mehri, Z.; Salehi-Abargouei, A.; Shahvazi, S.; Samadi, M.; Zare, F.; Nadjarzadeh, A. The association between vitamin D status and metabolic syndrome and its components among female teachers residing in Yazd city. Endocrinol. Diabetes Nutr. 2019, 66, 628–638. [Google Scholar] [CrossRef]
- Vitezova, A.; Zillikens, M.C.; van Herpt, T.T.; Sijbrands, E.J.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Kiefte-de Jong, J.C. Vitamin D status and metabolic syndrome in the elderly: The Rotterdam Study. Eur. J. Endocrinol. 2015, 172, 327–335. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Harris, S.B.; Retnakaran, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J. Clin. Endocrinol. Metab. 2011, 96, 168–175. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Hou, F.F.; Guo, Z.J.; Liang, M.; Wang, G.B.; Zhang, X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab. Res. Rev. 2008, 24, 459–464. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y. Research progress of mechanism of vitamin D in regulating obesity and insulin resistance on children. Chin. J. Appl. Clin. Pediatr. 2015, 30, 1598–1600. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Ebeling, P.R.; Daly, R.M. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: Results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Sciacqua, A.; Perticone, M.; Grillo, N.; Falbo, T.; Bencardino, G.; Angotti, E.; Arturi, F.; Parlato, G.; Sesti, G.; Perticone, F. Vitamin D and 1-hour post-load plasma glucose in hypertensive patients. Cardiovasc. Diabetol. 2014, 13, 48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kabadi, S.M.; Lee, B.K.; Liu, L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: Results from the NHANES 2001-2006. Diabetes Care 2012, 35, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ford, E.S.; Li, C.; Kris-Etherton, P.M.; Etherton, T.D.; Balluz, L.S. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J. Hypertens. 2010, 28, 1821–1828. [Google Scholar] [CrossRef]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Luo, X.; Zhang, P.; Luan, Y.; Cai, X.; He, X. Effect of vitamin D supplementation on markers of cardiometabolic risk in children and adolescents: A meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2800–2814. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
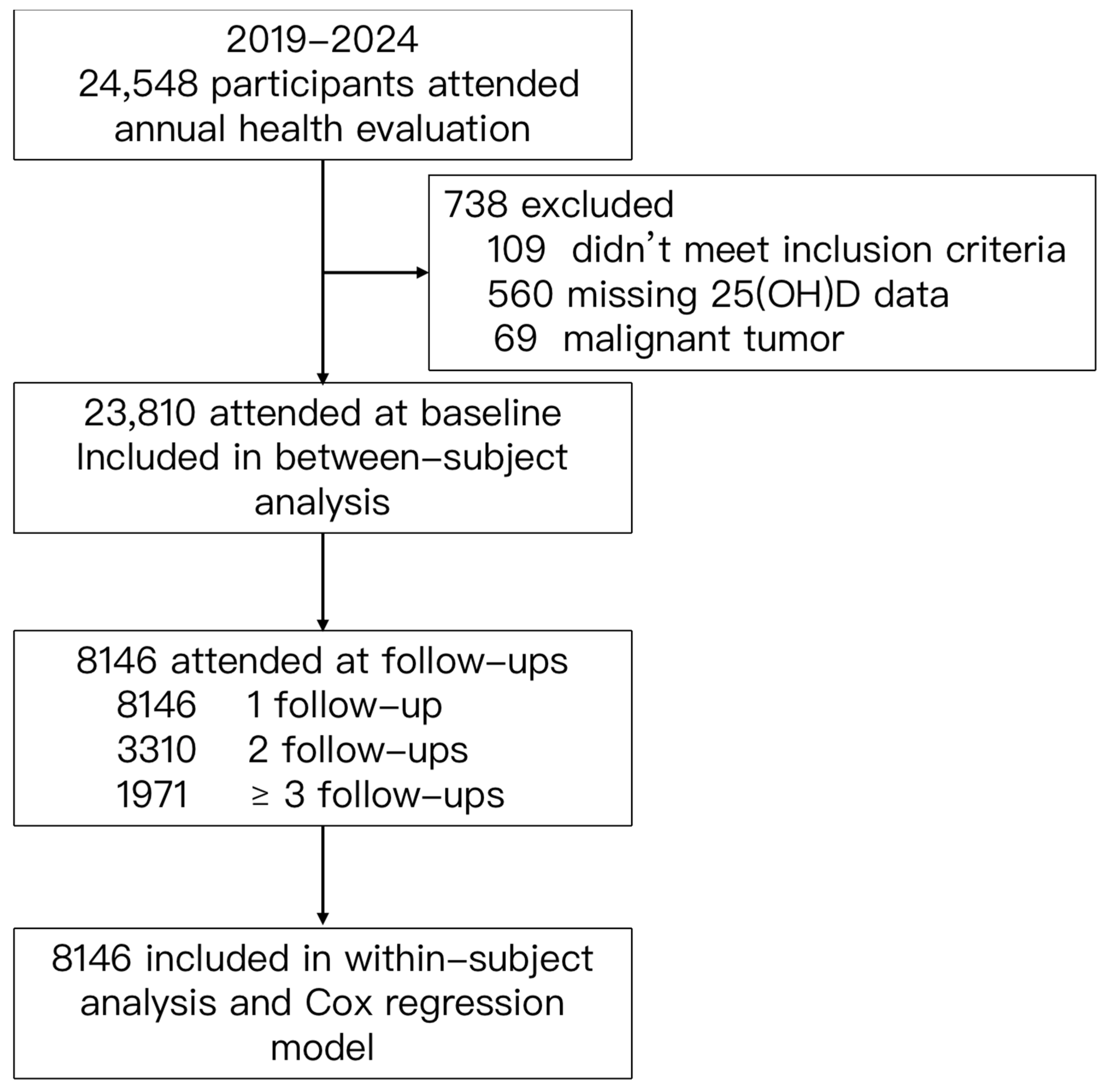
| Variables | Overall | Q1 | Q2 | Q3 | Q4 | p-Value |
|---|---|---|---|---|---|---|
| n | 23,810 | 6663 | 6172 | 5865 | 5110 | |
| Sex, n (%) | 0.490 | |||||
| Male | 12,596 (52.9) | 3539 (53.1) | 3246 (52.6) | 3068 (52.3) | 2743 (53.7) | |
| Female | 11,214 (47.1) | 3124 (46.9) | 2926 (47.4) | 2797 (47.7) | 2367 (46.3) | |
| Age, y | 43.6 (13.3) | 43.9 (13.3) | 43.7 (13.4) | 43.4 (13.3) | 43.3 (13.2) | 0.028 |
| Age, n (%) | 0.278 | |||||
| 18–44 | 12,366 (51.9) | 3384 (50.8) | 3199 (51.8) | 3093 (52.7) | 2690 (52.6) | |
| 45–59 | 8544 (35.9) | 2442 (36.7) | 2205 (35.7) | 2069 (35.3) | 1828 (35.8) | |
| ≥60 | 2900 (12.2) | 837 (12.6) | 768 (12.4) | 703 (12.0) | 592 (11.6) | |
| Season, n (%) | <0.001 | |||||
| Spring | 3879 (16.3) | 1348 (20.2) | 1019 (16.5) | 862 (14.7) | 650 (12.7) | |
| Summer | 6131 (25.7) | 1127 (16.9) | 1510 (24.5) | 1786 (30.5) | 1708 (33.4) | |
| Fall | 6139 (25.8) | 1308 (19.6) | 1526 (24.7) | 1629 (27.8) | 1676 (32.8) | |
| Winter | 7661 (32.2) | 2880 (43.2) | 2117 (34.3) | 1588 (27.1) | 1076 (21.1) | |
| Smoking, n (%) | 4852 (20.9) | 1366 (21.2) | 1195 (19.9) | 1164 (20.3) | 1127 (22.5) | 0.005 |
| Drinking, n (%) | 6969 (30.1) | 1948 (30.3) | 1788 (29.8) | 1675 (29.2) | 1558 (31.1) | 0.186 |
| Systolic blood pressure, mmHg | 122.3 (18.4) | 122.7 (18.6) | 122.3 (18.4) | 122.2 (18.4) | 122.1 (18.0) | 0.335 |
| Diastolic blood pressure, mmHg | 71.8 (12.0) | 72.2 (12.1) | 71.8 (12.0) | 71.7 (12.0) | 71.5 (11.8) | 0.008 |
| Body mass index, kg/m2 | 23.8 (3.4) | 23.9 (3.6) | 23.9 (3.4) | 23.8 (3.3) | 23.6 (3.2) | <0.001 |
| Waist circumference, cm | 83.0 (10.2) | 83.4 (10.8) | 83.2 (10.3) | 82.9 (10.0) | 82.5 (9.5) | <0.001 |
| 25(OH)D, ng/mL | 18.8 (7.3) | 11.9 (3.1) | 16.8 (3.6) | 20.8 (4.2) | 28.0 (6.6) | <0.001 |
| Fasting glucose, mmol/L | 5.6 (1.1) | 5.7 (1.2) | 5.6 (1.1) | 5.6 (1.2) | 5.6 (1.0) | <0.001 |
| FBI, μU/mL | 9.0 (14.3) | 9.8 (21.7) | 9.0 (13.1) | 8.7 (9.6) | 8.2 (5.3) | 0.001 |
| HOMA-IR | 2.4 (5.0) | 2.7 (7.4) | 2.5 (5.1) | 2.3 (3.0) | 2.2 (1.7) | 0.002 |
| HbA1c, % | 5.7 (0.9) | 5.7 (0.9) | 5.7 (0.9) | 5.7 (0.9) | 5.6 (0.8) | <0.001 |
| Total cholesterol, mmol/L | 5.0 (1.0) | 5.0 (1.0) | 5.0 (1.0) | 5.0 (0.9) | 5.0 (1.0) | 0.006 |
| LDL-C, mmol/L | 3.1 (0.9) | 3.0 (0.9) | 3.1 (0.9) | 3.1 (0.8) | 3.1 (0.9) | 0.028 |
| HDL-C, mmol/L | 1.4 (0.3) | 1.3 (0.3) | 1.4 (0.3) | 1.4 (0.3) | 1.4 (0.3) | <0.001 |
| Triglycerides, mmol/L | 1.5 (1.4) | 1.6 (1.8) | 1.5 (1.3) | 1.5 (1.2) | 1.4 (1.0) | <0.001 |
| Metabolic syndrome, n (%) | 6231 (27.5) | 1907 (30.1) | 1680 (28.7) | 1485 (26.6) | 1159 (23.8) | <0.001 |
| Abdominal obesity, n (%) | 9307 (40.3) | 2651 (41.4) | 2501 (41.9) | 2248 (39.2) | 1907 (38.1) | <0.001 |
| High Triglycerides, n (%) | 6260 (26.7) | 1853 (28.6) | 1717 (28.3) | 1506 (26.0) | 1184 (23.3) | <0.001 |
| Low HDL-C, n (%) | 4550 (21.9) | 1383 (23.5) | 1220 (22.6) | 1101 (21.7) | 846 (19.4) | <0.001 |
| Hypertension, n (%) | 8055 (34.4) | 2284 (35.2) | 2079 (34.3) | 1988 (34.3) | 1704 (33.7) | 0.340 |
| Hyperglycemia, n (%) | 8829 (37.7) | 2588 (39.9) | 2284 (37.6) | 2158 (37.2) | 1799 (35.4) | <0.001 |
| Variables | Between-Subject | Within-Subject | ||
|---|---|---|---|---|
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | |
| Metabolic syndrome | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.77 (0.63, 0.94) | 0.78 (0.65, 0.95) | 0.21 (0.17, 0.25) | 0.76 (0.63, 0.90) |
| Q3 | 0.58 (0.48, 0.72) | 0.61 (0.51, 0.75) | 0.30 (0.24, 0.36) | 0.63 (0.52, 0.75) |
| Q4 | 0.38 (0.31, 0.47) | 0.43 (0.35, 0.52) | 0.28 (0.23, 0.34) | 0.60 (0.50, 0.73) |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| Abdominal obesity | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.84 (0.63, 1.11) | 0.85 (0.64, 1.13) | 0.13 (0.10, 0.17) | 0.34 (0.26, 0.46) |
| Q3 | 0.54 (0.40, 0.71) | 0.54 (0.40, 0.72) | 0.16 (0.12, 0.22) | 0.29 (0.21, 0.39) |
| Q4 | 0.37 (0.28, 0.49) | 0.40 (0.29, 0.54) | 0.15 (0.11, 0.20) | 0.26 (0.19, 0.36) |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| HTG | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.99 (0.84, 1.18) | 0.99 (0.84, 1.16) | 0.46 (0.40, 0.54) | 0.90 (0.77, 1.05) |
| Q3 | 0.70 (0.59, 0.84) | 0.71 (0.60, 0.84) | 0.54 (0.46, 0.64) | 0.79 (0.67, 0.93) |
| Q4 | 0.48 (0.40, 0.57) | 0.49 (0.41, 0.58) | 0.53 (0.44, 0.62) | 0.78 (0.66, 0.93) |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| Low HDL-C | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.82 (0.67, 1.00) | 0.83 (0.68, 1.02) | 0.89 (0.74, 1.06) | 1.08 (0.89, 1.30) |
| Q3 | 0.62 (0.51, 0.76) | 0.63 (0.51, 0.77) | 0.82 (0.67, 0.99) | 0.92 (0.75, 1.12) |
| Q4 | 0.47 (0.38, 0.57) | 0.48 (0.39, 0.59) | 0.78 (0.64, 0.95) | 0.87 (0.71, 1.07) |
| p-trend | <0.001 | <0.001 | <0.001 | 0.002 |
| Hypertension | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.88 (0.71, 1.09) | 0.89 (0.74, 1.08) | 0.08 (0.07, 0.10) | 0.53 (0.44, 0.63) |
| Q3 | 0.86 (0.69, 1.07) | 0.92 (0.76, 1.12) | 0.20 (0.16, 0.25) | 0.60 (0.50, 0.72) |
| Q4 | 0.81 (0.64, 1.01) | 0.92 (0.76, 1.12) | 0.16 (0.13, 0.20) | 0.49 (0.41, 0.59) |
| p-trend | 0.001 | 0.147 | <0.001 | <0.001 |
| Hyperglycemia | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.82 (0.70, 0.97) | 0.81 (0.69, 0.94) | 0.27 (0.23, 0.31) | 0.88 (0.76, 1.01) |
| Q3 | 0.78 (0.66, 0.93) | 0.80 (0.68, 0.93) | 0.41 (0.35, 0.48) | 0.80 (0.69, 0.93) |
| Q4 | 0.65 (0.54, 0.77) | 0.70 (0.59, 0.82) | 0.37 (0.31, 0.43) | 0.75 (0.64, 0.87) |
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 |
| Variables | N | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Total | New Cases | HR (95% CI) * | p-Trend | HR (95% CI) * | p-Trend | |
| Metabolic syndrome | 8146 | 761 | 0.014 | 0.007 | ||
| Q1 | 1719 | 140 | 1.00 | 1.00 | ||
| Q2 | 2125 | 213 | 1.15 (0.93, 1.42) | 1.15 (0.93, 1.42) | ||
| Q3 | 2157 | 218 | 1.07 (0.87, 1.32) | 1.09 (0.88, 1.34) | ||
| Q4 | 2145 | 190 | 0.80 (0.64, 0.99) | 0.80 (0.65, 1.00) | ||
| Abdominal obesity | 8146 | 786 | 0.003 | 0.002 | ||
| Q1 | 1719 | 148 | 1.00 | 1.00 | ||
| Q2 | 2125 | 206 | 1.11 (0.90, 1.38) | 1.11 (0.90, 1.37) | ||
| Q3 | 2157 | 225 | 0.98 (0.79, 1.20) | 0.98 (0.80, 1.21) | ||
| Q4 | 2145 | 207 | 0.77 (0.63, 0.96) | 0.77 (0.63, 0.96) | ||
| High triglycerides | 8146 | 872 | 0.013 | 0.011 | ||
| Q1 | 1719 | 177 | 1.00 | 1.00 | ||
| Q2 | 2125 | 211 | 0.94 (0.77, 1.15) | 0.94 (0.77, 1.14) | ||
| Q3 | 2157 | 263 | 1.04 (0.86, 1.25) | 1.02 (0.85, 1.24) | ||
| Q4 | 2145 | 221 | 0.76 (0.63, 0.93) | 0.76 (0.62, 0.92) | ||
| Low HDL-C | 8146 | 575 | <0.001 | <0.001 | ||
| Q1 | 1719 | 137 | 1.00 | 1.00 | ||
| Q2 | 2125 | 143 | 0.80 (0.63, 1.01) | 0.80 (0.64, 1.02) | ||
| Q3 | 2157 | 150 | 0.75 (0.60, 0.95) | 0.76 (0.61, 0.96) | ||
| Q4 | 2145 | 145 | 0.63 (0.50, 0.80) | 0.64 (0.50, 0.81) | ||
| Hypertension | 8146 | 824 | 0.323 | 0.328 | ||
| Q1 | 1719 | 154 | 1.00 | 1.00 | ||
| Q2 | 2125 | 217 | 1.07 (0.87, 1.32) | 1.06 (0.86, 1.31) | ||
| Q3 | 2157 | 239 | 1.15 (0.94, 1.40) | 1.16 (0.95, 1.43) | ||
| Q4 | 2145 | 214 | 0.89 (0.72, 1.09) | 0.89 (0.73, 1.10) | ||
| Hyperglycemia | 8146 | 1027 | 0.753 | 0.733 | ||
| Q1 | 1719 | 204 | 1.00 | 1.00 | ||
| Q2 | 2125 | 254 | 1.01 (0.84, 1.21) | 1.00 (0.83, 1.21) | ||
| Q3 | 2157 | 284 | 1.01 (0.85, 1.21) | 1.02 (0.85, 1.23) | ||
| Q4 | 2145 | 285 | 0.96 (0.80, 1.15) | 0.97 (0.81, 1.16) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, M.; Xi, Y.; Wu, J.; Zhuo, L.-B.; Yan, Y.; Yang, Y.-D.; Feng, Y.-Y.; Tan, H.-Q.; Yang, H.-F.; Chen, Y.-M. Relationship between Circulating 25-Hydroxyvitamin D and Metabolic Syndrome in Chinese Adults: A Large Nationwide Longitudinal Study. Nutrients 2024, 16, 1480. https://doi.org/10.3390/nu16101480
Shu M, Xi Y, Wu J, Zhuo L-B, Yan Y, Yang Y-D, Feng Y-Y, Tan H-Q, Yang H-F, Chen Y-M. Relationship between Circulating 25-Hydroxyvitamin D and Metabolic Syndrome in Chinese Adults: A Large Nationwide Longitudinal Study. Nutrients. 2024; 16(10):1480. https://doi.org/10.3390/nu16101480
Chicago/Turabian StyleShu, Mi, Yue Xi, Jie Wu, Lai-Bao Zhuo, Yan Yan, Yi-Duo Yang, Yue-Yue Feng, Hua-Qiao Tan, Hui-Fang Yang, and Yu-Ming Chen. 2024. "Relationship between Circulating 25-Hydroxyvitamin D and Metabolic Syndrome in Chinese Adults: A Large Nationwide Longitudinal Study" Nutrients 16, no. 10: 1480. https://doi.org/10.3390/nu16101480
APA StyleShu, M., Xi, Y., Wu, J., Zhuo, L.-B., Yan, Y., Yang, Y.-D., Feng, Y.-Y., Tan, H.-Q., Yang, H.-F., & Chen, Y.-M. (2024). Relationship between Circulating 25-Hydroxyvitamin D and Metabolic Syndrome in Chinese Adults: A Large Nationwide Longitudinal Study. Nutrients, 16(10), 1480. https://doi.org/10.3390/nu16101480







