Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies—A Narrative Review
Abstract
1. Introduction
2. The Role of Micronutrients and the Effects of Their Deficiency in the Human Diet
| Element Deficiency | Se | Fe | Zn | I |
|---|---|---|---|---|
| Cardiovascular system | Keshan’s disease, atherosclerosis, hypertension, and congestive heart failure | Heart failure | As a result of its antioxidant properties, deficiency may be correlated with the development of cardiovascular diseases, including atherosclerosis | Indirectly, iodine deficiency leading to hypothyroidism can cause arrhythmias such as bradycardia and atrioventricular block, impaired systolic function, increased left ventricular (LV) diastolic filling, diastolic dysfunction with impaired cardiac relaxation, and atrial stiffness |
| Nervous system | Muscular dystrophy (multinodular myopathy, rigid spinal muscular dystrophy and desmin-associated myopathy with Mallory bodies), disorders of mental status | Deficient myelinization of the brain and impaired metabolism of monoamines, resulting in deficits in memory/learning and motor skills, but also in emotional and psychological disorders | Maternal zinc deficiency has been associated with severe fetal congenital malformations of the central nervous system and increased maternal morbidity Acrodermatitis enteropathica | Iodine deficiency can lead to hypothyroidism, which negatively affects the development of the fetal renal system. Children of iodine-deficient mothers are at risk of cognitive impairment, with cretinism being one of the most serious symptoms |
| Gastrointestinal system | Hepatopathies | Constipation and bowel problems | Appetite disorders and diarrhea Acrodermatitis enteropathica | Increased risk of atrophic gastritis and gastric cancer |
| Endocrine system | Autoimmune thyroiditis | Iron deficiency anemia (IDA) impairs the metabolism of tar-its (hypothyroidism) | Reduces the concentration of circulating insulin-like growth factor 1 (IGF-1), contributing to growth retardation and hypogonadism Acrodermatitis enteropathica | Hypothyroidism, thyroid goiter |
| References | [44,45] | [46,47,48,49,50,51] | [52,53] | [54,55,56] |
3. Biofortification of Plants and Plant Products
4. Biofortification of Food of Animal Origin
5. Bioavailability of Minerals from Biofortified Raw Materials
6. Commercialization and Restrictions on the Introduction of Biofortified Raw Materials
7. Biofortification as a Tool and Not a Remedy for the Problem of Hidden Hunger
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.K. Livestock Production: Recent Trends, Future Prospects. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Hasanaliyeva, G.; Sufar, E.K.; Wang, J.; Rempelos, L.; Volakakis, N.; Iversen, P.O.; Leifert, C. Effects of Agricultural Intensification on Mediterranean Diets: A Narrative Review. Foods 2023, 12, 3779. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Papers; FAO: Rome, Italy, 2012. [Google Scholar]
- Prom-u-thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous Biofortification of Rice With Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef] [PubMed]
- Consentino, B.B.; Ciriello, M.; Sabatino, L.; Vultaggio, L.; Baldassano, S.; Vasto, S.; Rouphael, Y.; La Bella, S.; De Pascale, S. Current Acquaintance on Agronomic Biofortification to Modulate the Yield and Functional Value of Vegetable Crops: A Review. Horticulturae 2023, 9, 219. [Google Scholar] [CrossRef]
- Kobylińska, M.; Antosik, K.; Decyk, A.; Kurowska, K. Malnutrition in Obesity: Is It Possible? Obes. Facts 2021, 15, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Rojas, P.; Basfi-fer, K.; Carrasco, F.; Inostroza, J.; Codoceo, J.; Valencia, A.; Papapietro, K.; Csendes, A.; Ruz, M. Micronutrient Deficiencies in Morbidly Obese Women Prior to Bariatric Surgery. Obes. Surg. 2016, 26, 361–368. [Google Scholar] [CrossRef]
- Knazicka, Z.; Bihari, M.; Janco, I.; Harangozo, L.; Arvay, J.; Kovacik, A.; Massanyi, P.; Galik, B.; Saraiva, J.M.A.; Habanova, M. Blood Concentration of Macro- and Microelements in Women Who Are Overweight/Obesity and Their Associations with Serum Biochemistry. Life 2024, 14, 465. [Google Scholar] [CrossRef]
- Malden, S.; Gillespie, J.; Hughes, A.; Gibson, A.-M.; Farooq, A.; Martin, A.; Summerbell, C.; Reilly, J.J. Obesity in Young Children and Its Relationship with Diagnosis of Asthma, Vitamin D Deficiency, Iron Deficiency, Specific Allergies and Flat-Footedness: A Systematic Review and Meta-Analysis. Obes. Rev. 2021, 22, e13129. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Milanta, C.; Agostinelli, M.; Todisco, C.F.; Bona, F.; Dolor, J.; La Mendola, A.; Tosi, M.; Zuccotti, G. Micronutrient Deficiency in Children and Adolescents with Obesity—A Narrative Review. Children 2023, 10, 695. [Google Scholar] [CrossRef]
- García, O.P.; Long, K.Z.; Rosado, J.L. Impact of Micronutrient Deficiencies on Obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef]
- Sharma, A.; Subramaniam, P. Association of Salivary Zinc Levels to Dental Caries and Body Mass Index. A Comparative Study. J. Clin. Pediatr. Dent. 2021, 45, 265–268. [Google Scholar] [CrossRef]
- Jaksic, M.; Martinovic, M.; Gligorovic-Barhanovic, N.; Vujacic, A.; Djurovic, D.; Nedovic-Vukovic, M. Association between Inflammation, Oxidative Stress, Vitamin D, Copper and Zinc with Pre-Obesity and Obesity in School Children from the City of Podgorica, Montenegro. J. Pediat. Endocrinol. Metab. 2019, 32, 951–957. [Google Scholar] [CrossRef]
- Prasad, R. Micro Mineral Nutrient Deficiencies in Humans, Animals and Plants and Their Amelioration. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 225–233. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and Child Undernutrition: Global and Regional Exposures and Health Consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and Their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main Nutritional Deficiencies. J. Prev. Med. Hyg. 2022, 63, E93–E101. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.R. Declining Fruit and Vegetable Nutrient Composition: What Is the Evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Pressman, P.; Clemens, R.A.; Hayes, A.W. Bioavailability of Micronutrients Obtained from Supplements and Food: A Survey and Case Study of the Polyphenols. Toxicol. Res. Appl. 2017, 1, 2397847317696366. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food Synergy: The Key to a Healthy Diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P.; Christian, P. Micronutrient Deficiencies in Pregnancy Worldwide: Health Effects and Prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Freeland-Graves, J.H.; Sanjeevi, N.; Lee, J.J. Global Perspectives on Trace Element Requirements. J. Trace Elem. Med. Biol. 2015, 31, 135–141. [Google Scholar] [CrossRef]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.d.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.d.C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef]
- Kiely, M.E.; McCarthy, E.K.; Hennessy, Á. Iron, Iodine and Vitamin D Deficiencies during Pregnancy: Epidemiology, Risk Factors and Developmental Impacts. Proc. Nutr. Soc. 2021, 80, 290–302. [Google Scholar] [CrossRef]
- Ahmed, T.; Hossain, M.; Sanin, K.I. Global Burden of Maternal and Child Undernutrition and Micronutrient Deficiencies. Ann. Nutr. Metab. 2013, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Gonmei, Z.; Toteja, G.S. Micronutrient Status of Indian Population. Indian J. Med. Res. 2018, 148, 511–521. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Ouyang, Y.; Huang, F.; Ding, G.; Zhang, B. Do Chinese Children Get Enough Micronutrients? Nutrients 2017, 9, 397. [Google Scholar] [CrossRef]
- Luo, H.; Zyba, S.J.; Webb, P. Measuring Malnutrition in All Its Forms: An Update of the Net State of Nutrition Index to Track the Global Burden of Malnutrition at Country Level. Glob. Food Secur. 2020, 26, 100453. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global Trends in Dietary Micronutrient Supplies and Estimated Prevalence of Inadequate Intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- WHO. Trace Elements in Human Nutrition and Health. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Agnew, U.M.; Slesinger, T.L. Zinc Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Acute Human Toxicity and Mortality after Selenium Ingestion: A Review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium Biochemistry and Its Role for Human Health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Jankowska, E.A.; von Haehling, S.; Anker, S.D.; Macdougall, I.C.; Ponikowski, P. Iron Deficiency and Heart Failure: Diagnostic Dilemmas and Therapeutic Perspectives. Eur. Heart J. 2013, 34, 816–829. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron Deficiency and Cognitive Functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Lozoff, B.; De Andraca, I.; Castillo, M.; Smith, J.B.; Walter, T.; Pino, P. Behavioral and Developmental Effects of Preventing Iron-Deficiency Anemia in Healthy Full-Term Infants. Pediatrics 2003, 112, 846–854. [Google Scholar] [CrossRef]
- Bruner, A.B.; Joffe, A.; Duggan, A.K.; Casella, J.F.; Brandt, J. Randomised Study of Cognitive Effects of Iron Supplementation in Non-Anaemic Iron-Deficient Adolescent Girls. Lancet 1996, 348, 992–996. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and Mechanisms of Emotional Behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef]
- Ashraf, T.S.; De Sanctis, V.; Yassin, M.; Wagdy, M.; Soliman, N. Chronic Anemia and Thyroid Function. Acta Biomed. 2017, 88, 119–127. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health. 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Floriani, C.; Gencer, B.; Collet, T.-H.; Rodondi, N. Subclinical Thyroid Dysfunction and Cardiovascular Diseases: 2016 Update. Eur. Heart J. 2018, 39, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The Role of Iodine in Human Growth and Development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C.D. Iodine: Health Implications of Deficiency. J. Evid. Based Complement. Altern. Med. 2011, 16, 190–194. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Bhushan, B.; Kokkiligadda, A.; Kumar, V.; Behare, P.; Tomar, S.K. Vitamin B12 Biofortification of Soymilk through Optimized Fermentation with Extracellular B12 Producing Lactobacillus Isolates of Human Fecal Origin. Curr. Res. Food Sci. 2021, 4, 646–654. [Google Scholar] [CrossRef] [PubMed]
- de Lourdes Samaniego-Vaesken, M.; Alonso-Aperte, E.; Varela-Moreiras, G. Vitamin Food Fortification Today. Food Nutr. Res. 2012, 56, 5459. [Google Scholar] [CrossRef]
- Oh, S.; Cave, G.; Lu, C. Vitamin B12 (Cobalamin) and Micronutrient Fortification in Food Crops Using Nanoparticle Technology. Front. Plant Sci. 2021, 12, 668819. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Fernie, A.R.; Sreenivasulu, N. Meeting Human Dietary Vitamin Requirements in the Staple Rice via Strategies of Biofortification and Post-Harvest Fortification. Trends Food Sci. Technol. 2021, 109, 65–82. [Google Scholar] [CrossRef]
- Li, J.; Scarano, A.; Gonzalez, N.M.; D’Orso, F.; Yue, Y.; Nemeth, K.; Saalbach, G.; Hill, L.; de Oliveira Martins, C.; Moran, R.; et al. Biofortified Tomatoes Provide a New Route to Vitamin D Sufficiency. Nat. Plants 2022, 8, 611–616. [Google Scholar] [CrossRef]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic Food Fortification with a Balanced Ratio of Dietary ω-3/ω-6 Omega Fatty Acids for the Prevention of Lifestyle Diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Feizollahi, E.; Hadian, Z.; Honarvar, Z. Food Fortification with Omega-3 Fatty Acids; Microencapsulation as an Addition Method. Curr. Nutr. Food Sci. 2018, 14, 90–103. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 301899. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Microelements and Their Role in Human Health. In Soil Components and Human Health; Nieder, R., Benbi, D.K., Reichl, F.X., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2018; pp. 317–374. ISBN 978-94-024-1222-2. [Google Scholar]
- Taskin, M.B.; Gunes, A. Iron Biofortification of Wheat Grains by Foliar Application of Nano Zero-Valent Iron (nZVI) and Other Iron Sources with Urea. J. Soil Sci. Plant Nutr. 2022, 22, 4642–4652. [Google Scholar] [CrossRef]
- Buturi, C.V.; Coelho, S.R.M.; Cannata, C.; Basile, F.; Giuffrida, F.; Leonardi, C.; Mauro, R.P. Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System. Horticulturae 2022, 8, 858. [Google Scholar] [CrossRef]
- Kougia, E.; Ioannou, E.; Roussis, V.; Tzovenis, I.; Chentir, I.; Markou, G. Iron (Fe) Biofortification of Arthrospira Platensis: Effects on Growth, Biochemical Composition and in Vitro Iron Bioaccessibility. Algal Res. 2023, 70, 103016. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Yang, X. Biofortification and Bioavailability of Rice Grain Zinc as Affected by Different Forms of Foliar Zinc Fertilization. PLoS ONE 2012, 7, e45428. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison Study of Zinc Nanoparticles and Zinc Sulphate on Wheat Growth: From Toxicity and Zinc Biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Dávila Rangel, I.E.; Trejo Téllez, L.I.; Ortega Ortiz, H.; Juárez Maldonado, A.; González Morales, S.; Companioni González, B.; Cabrera De la Fuente, M.; Benavides Mendoza, A. Comparison of Iodide, Iodate, and Iodine-Chitosan Complexes for the Biofortification of Lettuce. Appl. Sci. 2020, 10, 2378. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Huang, Y.-Z.; Hu, Y.; Liu, Y.-X. Iodine Uptake by Spinach (Spinacia oleracea L.) Plants Grown in Solution Culture: Effects of Iodine Species and Solution Concentrations. Environ. Int. 2003, 29, 33–37. [Google Scholar] [CrossRef]
- Krzemińska, J.; Smoleń, S.; Kowalska, I.; Pitala, J.; Sularz, O.; Koronowicz, A. Effect of Biofortification with Iodine by 8-Hydroxy-7-Iodo-5-Quinolinesulfonic Acid and 5-Chloro-7-Iodo-8-Quinolinol on the Chemical Composition and Antioxidant Properties of Potato Tubers (Solanum tuberosum L.) in a Pot Experiment. Appl. Sci. 2023, 13, 4659. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine Biofortification of Crops: Agronomic Biofortification, Metabolic Engineering and Iodine Bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Lidon, F.C.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; et al. Selenium Biofortification Of Rice Through Foliar Application With Selenite And Selenate. Exp. Agric. 2019, 55, 528–542. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and Bio-Transformation of Selenium Nanoparticles by Wheat Seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of Biofortification with Iodine and Selenium of Lettuce Cultivated in the NFT Hydroponic System. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Dyląg, A.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Sularz, O.; Krzemińska, J.; Pitala, J.; Koronowicz, A. Evaluation of the Chemical Composition and Nutritional Value of Lettuce (Lactuca sativa L.) Biofortified in Hydroponics with Iodine in the Form of Iodoquinolines. Front. Plant Sci. 2023, 14, 1288773. [Google Scholar] [CrossRef]
- Şimşek, O.; Çelik, H. Effects of Iron Fortification on Growth and Nutrient Amounts of Spinach (Spinacia oleracea L.). J. Plant Nutr. 2021, 44, 2770–2782. [Google Scholar] [CrossRef]
- Francini, A.; Quattrini, E.; Giuffrida, F.; Ferrante, A. Biofortification of Baby Leafy Vegetables Using Nutrient Solution Containing Selenium. J. Sci. Food Agric. 2023, 103, 5472–5480. [Google Scholar] [CrossRef]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Simultaneous Biofortification of Vitamin C and Mineral Nutrients in Arugula Microgreens. Food Chem. 2024, 440, 138180. [Google Scholar] [CrossRef]
- Tavan, M.; Wee, B.; Fuentes, S.; Pang, A.; Brodie, G.; Viejo, C.G.; Gupta, D. Biofortification of Kale Microgreens with Selenate-Selenium Using Two Delivery Methods: Selenium-Rich Soilless Medium and Foliar Application. Sci. Hortic. 2024, 323, 112522. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus Spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Palacios-Rojas, N.; Meng, E.; Pixley, K.; Trethowan, R.; Peña, R.J. Enhancing the Mineral and Vitamin Content of Wheat and Maize through Plant Breeding. J. Cereal Sci. 2007, 46, 293–307. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Oparinde, A.; Andersson, M.S.; Asare-Marfo, D.; Diressie, M.T.; Gonzalez, C.; Lividini, K.; Moursi, M.; Zeller, M. Availability, Production, and Consumption of Crops Biofortified by Plant Breeding: Current Evidence and Future Potential. Ann. N. Y. Acad. Sci. 2017, 1390, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, V.V. Genotypic Variation for Micronutrient Content in Traditional and Improved Rice Lines and Its Role in Biofortification Programme. Indian J. Sci. Technol. 2014, 7, 1414–1425. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic Dissection of Grain Zinc Concentration in Spring Wheat for Mainstreaming Biofortification in CIMMYT Wheat Breeding. Sci. Rep. 2018, 8, 13526. [Google Scholar] [CrossRef]
- George, T.S.; French, A.S.; Brown, L.K.; Karley, A.J.; White, P.J.; Ramsay, L.; Daniell, T.J. Genotypic Variation in the Ability of Landraces and Commercial Cereal Varieties to Avoid Manganese Deficiency in Soils with Limited Manganese Availability: Is There a Role for Root-Exuded Phytases? Physiol. Plant. 2014, 151, 243–256. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Owens, G.; Thakur, J.K.; Manna, M.C.; Niazi, N.K.; Jayaraman, S.; Patra, A.K. Impact of Genetically Modified Crops on Rhizosphere Microorganisms and Processes: A Review Focusing on Bt Cotton. Appl. Soil Ecol. 2020, 148, 103492. [Google Scholar] [CrossRef]
- Lucht, J.M. Public Acceptance of Plant Biotechnology and GM Crops. Viruses 2015, 7, 4254–4281. [Google Scholar] [CrossRef]
- Dubock, A. An Overview of Agriculture, Nutrition and Fortification, Supplementation and Biofortification: Golden Rice as an Example for Enhancing Micronutrient Intake. Agric. Food Secur. 2017, 6, 59. [Google Scholar] [CrossRef]
- Beyer, P. Golden Rice and ‘Golden’ Crops for Human Nutrition. New Biotech. 2010, 27, 478–481. [Google Scholar] [CrossRef]
- Tang, G.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Grusak, M.A. Golden Rice Is an Effective Source of Vitamin A23. Am. J. Clin. Nutr. 2009, 89, 1776–1783. [Google Scholar] [CrossRef]
- Goto, F.; Yoshihara, T.; Shigemoto, N.; Toki, S.; Takaiwa, F. Iron Fortification of Rice Seed by the Soybean Ferritin Gene. Nat. Biotechnol. 1999, 17, 282–286. [Google Scholar] [CrossRef]
- Yu, X.; Luo, Q.; Huang, K.; Yang, G.; He, G. Prospecting for Microelement Function and Biosafety Assessment of Transgenic Cereal Plants. Front. Plant Sci. 2018, 9, 326. [Google Scholar] [CrossRef]
- Drakakaki, G.; Christou, P.; Stöger, E. Constitutive Expression of Soybean Ferritin cDNA Intransgenic Wheat and Rice Results in Increased Iron Levels in Vegetative Tissues but Not in Seeds. Transgenic Res. 2000, 9, 445–452. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Cheng, K.-J.; Tseng, T.-H.; Wang, C.-S.; Liu, L.-F.; Yu, S.-M. Production of Two Highly Active Bacterial Phytases with Broad pH Optima in Germinated Transgenic Rice Seeds. Transgenic Res. 2004, 13, 29–39. [Google Scholar] [CrossRef]
- LeDuc, D.L.; AbdelSamie, M.; Móntes-Bayon, M.; Wu, C.P.; Reisinger, S.J.; Terry, N. Overexpressing Both ATP Sulfurylase and Selenocysteine Methyltransferase Enhances Selenium Phytoremediation Traits in Indian Mustard. Environ. Pollut. 2006, 144, 70–76. [Google Scholar] [CrossRef]
- LeDuc, D.L.; Tarun, A.S.; Montes-Bayon, M.; Meija, J.; Malit, M.F.; Wu, C.P.; AbdelSamie, M.; Chiang, C.-Y.; Tagmount, A.; deSouza, M.; et al. Overexpression of Selenocysteine Methyltransferase in Arabidopsis and Indian Mustard Increases Selenium Tolerance and Accumulation. Plant Physiol. 2004, 135, 377–383. [Google Scholar] [CrossRef]
- Woźniak, D.; Cichy, W.; Dobrzyńska, M.; Przysławski, J.; Drzymała-Czyż, S. Reasonableness of Enriching Cow’s Milk with Vitamins and Minerals. Foods 2022, 11, 1079. [Google Scholar] [CrossRef]
- Manoharan, A.; Chinnaraja, A. Physicochemical and Sensory Characteristics of Symbiotic Fruit Flavoured Yoghurt Fortified with Calcium and Vitamin D. Int. J. Chem. Stud. 2020, 8, 1059–1064. [Google Scholar] [CrossRef]
- Barnkob, L.L.; Argyraki, A.; Jakobsen, J. Naturally Enhanced Eggs as a Source of Vitamin D: A Review. Trends Food Sci. Technol. 2020, 102, 62–70. [Google Scholar] [CrossRef]
- Heck, R.T.; Lorenzo, J.M.; Dos Santos, B.A.; Cichoski, A.J.; de Menezes, C.R.; Campagnol, P.C.B. Microencapsulation of Healthier Oils: An Efficient Strategy to Improve the Lipid Profile of Meat Products. Curr. Opin. Food Sci. 2021, 40, 6–12. [Google Scholar] [CrossRef]
- Reddy, G.V.B.; Reddy, B.V.V.; Amaravathi, P.; Reddy, G.V.S.; Sen, A.R. Quality Characteristics of Functional Chicken Meat Sausages Enriched With Omega-3-Fatty Acids. Asian J. Dairy Food Res. 2022, 41, 329–334. [Google Scholar] [CrossRef]
- Batkowska, J.; Drabik, K.; Brodacki, A.; Czech, A.; Adamczuk, A. Fatty Acids Profile, Cholesterol Level and Quality of Table Eggs from Hens Fed with the Addition of Linseed and Soybean Oil. Food Chem. 2021, 334, 127612. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z.; Grčević, M.; Galović, O.; Hanžek, D.; Biazik, E. Fatty Acid Profile of Eggs Produced by Laying Hens Fed Diets Containing Different Shares of Fish Oil. Poult. Sci. 2021, 100, 101379. [Google Scholar] [CrossRef]
- Long, S.; Liu, S.; Wu, D.; Mahfuz, S.; Piao, X. Effects of Dietary Fatty Acids from Different Sources on Growth Performance, Meat Quality, Muscle Fatty Acid Deposition, and Antioxidant Capacity in Broilers. Animals 2020, 10, 508. [Google Scholar] [CrossRef]
- Clark, A.; Kuznesof, S.; Davies, S.; Waller, A.; Ritchie, A.; Wilson, S.; Harbord, L.; Hill, T. Egg Enrichment with Vitamin D: The Sunshine Eggs Projects. Nutr. Bull. 2021, 46, 332–338. [Google Scholar] [CrossRef]
- Ghasemi, H.A.; Hajkhodadadi, I.; Hafizi, M.; Fakharzadeh, S.; Abbasi, M.; Kalanaky, S.; Nazaran, M.H. Effect of Advanced Chelate Compounds-Based Mineral Supplement in Laying Hen Diet on the Performance, Egg Quality, Yolk Mineral Content, Fatty Acid Composition, and Oxidative Status. Food Chem. 2022, 366, 130636. [Google Scholar] [CrossRef]
- Brodacki, A.; Batkowska, J.; Stępniowska, A.; Blicharska, E.; Drabik, K. Quality and Mineral Composition of Eggs from Hens Supplemented with Copper-Lysine Chelate. Arch. Anim. Breed. 2018, 61, 109–113. [Google Scholar] [CrossRef]
- Sarlak, S.; Tabeidian, S.A.; Toghyani, M.; Shahraki, A.D.F.; Goli, M.; Habibian, M. Effects of Replacing Inorganic with Organic Iron on Performance, Egg Quality, Serum and Egg Yolk Lipids, Antioxidant Status, and Iron Accumulation in Eggs of Laying Hens. Biol. Trace Elem. Res. 2021, 199, 1986–1999. [Google Scholar] [CrossRef]
- Abedini, M.; Shariatmadari, F.; Karimi Torshizi, M.A.; Ahmadi, H. Effects of Zinc Oxide Nanoparticles on the Egg Quality, Immune Response, Zinc Retention, and Blood Parameters of Laying Hens in the Late Phase of Production. J. Anim. Physiol. Anim. Nutr. 2018, 102, 736–745. [Google Scholar] [CrossRef]
- Abedini, M.; Shariatmadari, F.; Torshizi, M.A.K.; Ahmadi, H. Effects of Zinc Oxide Nanoparticles on Performance, Egg Quality, Tissue Zinc Content, Bone Parameters, and Antioxidative Status in Laying Hens. Biol. Trace Elem. Res. 2018, 184, 259–267. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dobrzański, Z.; Górecki, H.; Zielińska, A.; Korczyński, M.; Opaliński, S. Effect of Macroalgae Enriched with Microelements on Egg Quality Parameters and Mineral Content of Eggs, Eggshell, Blood, Feathers and Droppings. J. Anim. Physiol. Anim. Nutr. 2011, 95, 374–387. [Google Scholar] [CrossRef]
- Witkowska, Z.; Świniarska, M.; Korczyński, M.; Opaliński, S.; Konkol, D.; Michalak, I.; Saeid, A.; Mironiuk, M.; Chojnacka, K. Biofortification of Hens’ Eggs with Microelements by Innovative Bio-Based Dietary Supplement. J. Anim. Physiol. Anim. Nutr. 2019, 103, 485–492. [Google Scholar] [CrossRef]
- Yang, X.-E.; Chen, W.-R.; Feng, Y. Improving Human Micronutrient Nutrition through Biofortification in the Soil–Plant System: China as a Case Study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef]
- Koronowicz, A.A.; Kopeć, A.; Master, A.; Smoleń, S.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Rakoczy, R.; Leszczyńska, T.; et al. Transcriptome Profiling of Caco-2 Cancer Cell Line Following Treatment with Extracts from Iodine-Biofortified Lettuce (Lactuca sativa L.). PLoS ONE 2016, 11, e0147336. [Google Scholar] [CrossRef]
- Utoiu, E.; Oancea, A.; Gaspar, A.; Seciu, A.-M. Selenium Biofortification Treatment Of Cauliflower Enhances Their Content In Chemopreventive Compounds And In Vitro Antitumoral Activity. Sci. Bull. Ser. F Biotechnol. 2017, XXI, 33–40. [Google Scholar]
- Tavares Antunes, P.; Vaz-Tostes, M.d.G.; Tomáz Sant’Ana, C.; Araújo de Faria, R.; Lopes Toledo, R.C.; Brunoro Costa, N.M. Bioavailability of Iron and the Influence of Vitamin a in Biofortified Foods. Agronomy 2019, 9, 777. [Google Scholar] [CrossRef]
- Tako, E.; Blair, M.W.; Glahn, R.P. Biofortified Red Mottled Beans (Phaseolus vulgaris L.) in a Maize and Bean Diet Provide More Bioavailable Iron than Standard Red Mottled Beans: Studies in Poultry (Gallus gallus) and an in Vitro Digestion/Caco-2 Model. Nutr. J. 2011, 10, 113. [Google Scholar] [CrossRef]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified Indica Rice Attains Iron and Zinc Nutrition Dietary Targets in the Field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef]
- Vaz-Tostes, M.d.G.; Verediano, T.A.; de Mejia, E.G.; Brunoro Costa, N.M. Evaluation of Iron and Zinc Bioavailability of Beans Targeted for Biofortification Using in Vitro and in Vivo Models and Their Effect on the Nutritional Status of Preschool Children. J. Sci. Food Agric. 2016, 96, 1326–1332. [Google Scholar] [CrossRef]
- Haas, J.D.; Luna, S.V.; Lung’aho, M.G.; Wenger, M.J.; Murray-Kolb, L.E.; Beebe, S.; Gahutu, J.-B.; Egli, I.M. Consuming Iron Biofortified Beans Increases Iron Status in Rwandan Women after 128 Days in a Randomized Controlled Feeding Trial123. J. Nutr. 2016, 146, 1586–1592. [Google Scholar] [CrossRef]
- Jou, M.-Y.; Du, X.; Hotz, C.; Lönnerdal, B. Biofortification of Rice with Zinc: Assessment of the Relative Bioavailability of Zinc in a Caco-2 Cell Model and Suckling Rat Pups. J. Agric. Food Chem. 2012, 60, 3650–3657. [Google Scholar] [CrossRef]
- Kodkany, B.S.; Bellad, R.M.; Mahantshetti, N.S.; Westcott, J.E.; Krebs, N.F.; Kemp, J.F.; Hambidge, K.M. Biofortification of Pearl Millet with Iron and Zinc in a Randomized Controlled Trial Increases Absorption of These Minerals above Physiologic Requirements in Young Children. J. Nutr. 2013, 143, 1489–1493. [Google Scholar] [CrossRef]
- do Nascimento da Silva, E.; Cadore, S. Bioavailability Assessment of Copper, Iron, Manganese, Molybdenum, Selenium, and Zinc from Selenium-Enriched Lettuce. J. Food Sci. 2019, 84, 2840–2846. [Google Scholar] [CrossRef]
- Gómez-Jacinto, V.; Navarro-Roldán, F.; Garbayo-Nores, I.; Vílchez-Lobato, C.; Borrego, A.A.; García-Barrera, T. In Vitro Selenium Bioaccessibility Combined with in Vivo Bioavailability and Bioactivity in Se-Enriched Microalga (Chlorella sorokiniana) to Be Used as Functional Food. J. Funct. Foods 2020, 66, 103817. [Google Scholar] [CrossRef]
- Hu, L.; Fan, H.; Wu, D.; Wan, J.; Wang, X.; Huang, R.; Liu, W.; Shen, F. Assessing Bioaccessibility of Se and I in Dual Biofortified Radish Seedlings Using Simulated in Vitro Digestion. Food Res. Int. 2019, 119, 701–708. [Google Scholar] [CrossRef]
- Delaqua, D.; Carnier, R.; Cadore, S.; Sanches, V.L.; Berton, R.S.; Corbi, F.C.A.; Coscione, A.R. In Vitro Bioaccessibility and Bioavailability of Selenium in Agronomic Biofortified Wheat. J. Food Compos. Anal. 2022, 105, 104253. [Google Scholar] [CrossRef]
- Cakmak, I.; Marzorati, M.; Van den Abbeele, P.; Hora, K.; Holwerda, H.T.; Yazici, M.A.; Savasli, E.; Neri, J.; Du Laing, G. Fate and Bioaccessibility of Iodine in Food Prepared from Agronomically Biofortified Wheat and Rice and Impact of Cofertilization with Zinc and Selenium. J. Agric. Food Chem. 2020, 68, 1525–1535. [Google Scholar] [CrossRef]
- Li, R.; Li, D.-W.; Yan, A.-L.; Hong, C.-L.; Liu, H.-P.; Pan, L.-H.; Song, M.-Y.; Dai, Z.-X.; Ye, M.-L.; Weng, H.-X. The Bioaccessibility of Iodine in the Biofortified Vegetables throughout Cooking and Simulated Digestion. J. Food Sci. Technol. 2018, 55, 366–375. [Google Scholar] [CrossRef]
- Tonacchera, M.; Dimida, A.; De Servi, M.; Frigeri, M.; Ferrarini, E.; De Marco, G.; Grasso, L.; Agretti, P.; Piaggi, P.; Aghini-Lombardi, F.; et al. Iodine Fortification of Vegetables Improves Human Iodine Nutrition: In Vivo Evidence for a New Model of Iodine Prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, E694–E697. [Google Scholar] [CrossRef]
- Piątkowska, E.; Kopeć, A.; Bieżanowska-Kopeć, R.; Pysz, M.; Kapusta-Duch, J.; Koronowicz, A.A.; Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; et al. The Impact of Carrot Enriched in Iodine through Soil Fertilization on Iodine Concentration and Selected Biochemical Parameters in Wistar Rats. PLoS ONE 2016, 11, e0152680. [Google Scholar] [CrossRef]
- Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Smoleń, S.; Skoczylas, Ł.; Leszczyńska, T.; Sady, W. The Iodine Content in Urine, Faeces and Selected Organs of Rats Fed Lettuce Biofortified with Iodine Through Foliar Application. Biol. Trace Elem. Res. 2016, 174, 347–355. [Google Scholar] [CrossRef]
- Granby, K.; Amlund, H.; Valente, L.M.P.; Dias, J.; Adoff, G.; Sousa, V.; Marques, A.; Sloth, J.J.; Larsen, B.K. Growth Performance, Bioavailability of Toxic and Essential Elements and Nutrients, and Biofortification of Iodine of Rainbow Trout (Onchorynchus mykiss) Fed Blends with Sugar Kelp (Saccharina latissima). Food Chem. Toxicol. 2020, 141, 111387. [Google Scholar] [CrossRef]
- Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; Smoleń, S.; Rakoczy, R.; Skoczylas, Ł.; Leszczyńska, T.; et al. Effect of Lettuce Biofortified with Iodine by Soil Fertilization on Iodine Concentration in Various Tissues and Selected Biochemical Parameters in Serum of Wistar Rats. J. Funct. Foods 2015, 14, 479–486. [Google Scholar] [CrossRef]
- Dias, D.M.; Costa, N.M.B.; Nutti, M.R.; Tako, E.; Martino, H.S.D. Advantages and Limitations of in Vitro and in Vivo Methods of Iron and Zinc Bioavailability Evaluation in the Assessment of Biofortification Program Effectiveness. Crit. Rev. Food Sci. Nutr. 2018, 58, 2136–2146. [Google Scholar] [CrossRef]
- Haas, J.D.; Beard, J.L.; Murray-Kolb, L.E.; del Mundo, A.M.; Felix, A.; Gregorio, G.B. Iron-Biofortified Rice Improves the Iron Stores of Nonanemic Filipino Women12. J. Nutr. 2005, 135, 2823–2830. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Mehta, S.; Udipi, S.A.; Ghugre, P.S.; Luna, S.V.; Wenger, M.J.; Murray-Kolb, L.E.; Przybyszewski, E.M.; Haas, J.D. A Randomized Trial of Iron-Biofortified Pearl Millet in School Children in India. J. Nutr. 2015, 145, 1576–1581. [Google Scholar] [CrossRef]
- Yadava, D.K.; Hossain, F.; Mohapatra, T. Nutritional Security through Crop Biofortification in India: Status & Future Prospects. Indian J. Med. Res. 2018, 148, 621. [Google Scholar] [CrossRef]
- Sheoran, S.; Kumar, S.; Ramtekey, V.; Kar, P.; Meena, R.S.; Jangir, C.K. Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview. Sustainability 2022, 14, 3301. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Nkundabombi, M.G.; Nakimbugwe, D.; Muyonga, J.H. Effect of Processing Methods on Nutritional, Sensory, and Physicochemical Characteristics of Biofortified Bean Flour. Food Sci. Nutr. 2016, 4, 384–397. [Google Scholar] [CrossRef]
- Ciccolini, V.; Pellegrino, E.; Coccina, A.; Fiaschi, A.I.; Cerretani, D.; Sgherri, C.; Quartacci, M.F.; Ercoli, L. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017, 65, 5443–5452. [Google Scholar] [CrossRef]
- Chiplonkar, S.; Kajale, N.A.; Sanwalka, N. A Review of Food-Based Intervention Strategies for Improving Micronutrient Status and Health During Childhood. Curr. Res. Nutr. Food Sci. 2022, 10, 407–426. [Google Scholar] [CrossRef]
- Islam, M.H.; Jubayer, A.; Nowar, A.; Nayan, M.M.; Islam, S. Dietary Diversity and Micronutrients Adequacy among the Women of Reproductive Age at St. Martin’s Island in Bangladesh. BMC Nutr. 2023, 9, 52. [Google Scholar] [CrossRef]
- Sauder, K.A.; Harte, R.N.; Ringham, B.M.; Guenther, P.M.; Bailey, R.L.; Alshawabkeh, A.; Cordero, J.F.; Dunlop, A.L.; Ferranti, E.P.; Elliott, A.J.; et al. Disparities in Risks of Inadequate and Excessive Intake of Micronutrients during Pregnancy. J. Nutr. 2021, 151, 3555–3569. [Google Scholar] [CrossRef]
- Neufeld, L.M.; Hendriks, S.; Hugas, M. Healthy Diet: A Definition for the United Nations Food Systems Summit 2021. In Science and Innovations for Food Systems Transformation; von Braun, J., Afsana, K., Fresco, L.O., Hassan, M.H.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 21–30. ISBN 978-3-031-15703-5. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- van Ginkel, M.; Cherfas, J. What Is Wrong with Biofortification. Glob. Food Secur. 2023, 37, 100689. [Google Scholar] [CrossRef]
- Leal, M.F.C.; Catarino, R.I.L.; Pimenta, A.M.; Souto, M.R.S. Roles of Metal Microelements in Neurodegenerative Diseases. Neurophysiology 2020, 52, 80–88. [Google Scholar] [CrossRef]
- Mwiti Kibiti, C.; Jide Afolayan, A. The Biochemical Role of Macro and Micro-Minerals in the Management of Diabetes Mellitus and Its Associated Complications: A Review. Int. J. Vitam. Nutr. Res. 2015, 85, 88–103. [Google Scholar] [CrossRef]
- Rotter, I.; Kosik-Bogacka, D.I.; Dołęgowska, B.; Safranow, K.; Kuczyńska, M.; Laszczyńska, M. Analysis of the Relationship between the Blood Concentration of Several Metals, Macro- and Micronutrients and Endocrine Disorders Associated with Male Aging. Environ. Geochem. Health 2016, 38, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Mohn, E.S.; Johnson, E.J. Nutrient Absorption in the Human Gastrointestinal Tract. In Nanotechnology and Functional Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 3–34. ISBN 978-1-118-46215-7. [Google Scholar]
- Muros, J.J.; Cabrera-Vique, C.; Briones, M.; Seiquer, I. Assessing the Dietary Intake of Calcium, Magnesium, Iron, Zinc and Copper in Institutionalised Children and Adolescents from Guatemala. Contribution of Nutritional Supplements. J. Trace Elem. Med. Biol. 2019, 53, 91–97. [Google Scholar] [CrossRef] [PubMed]
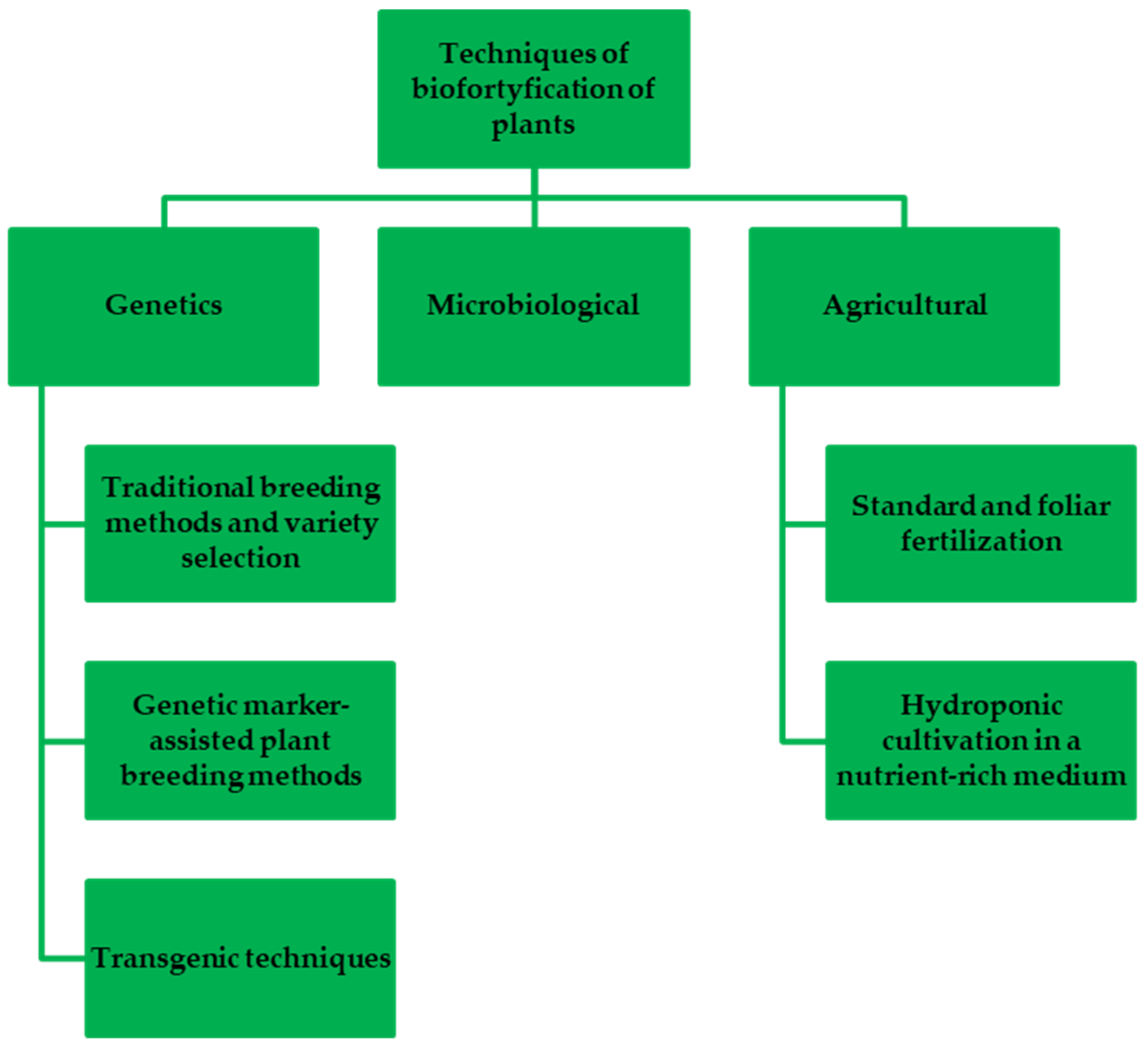
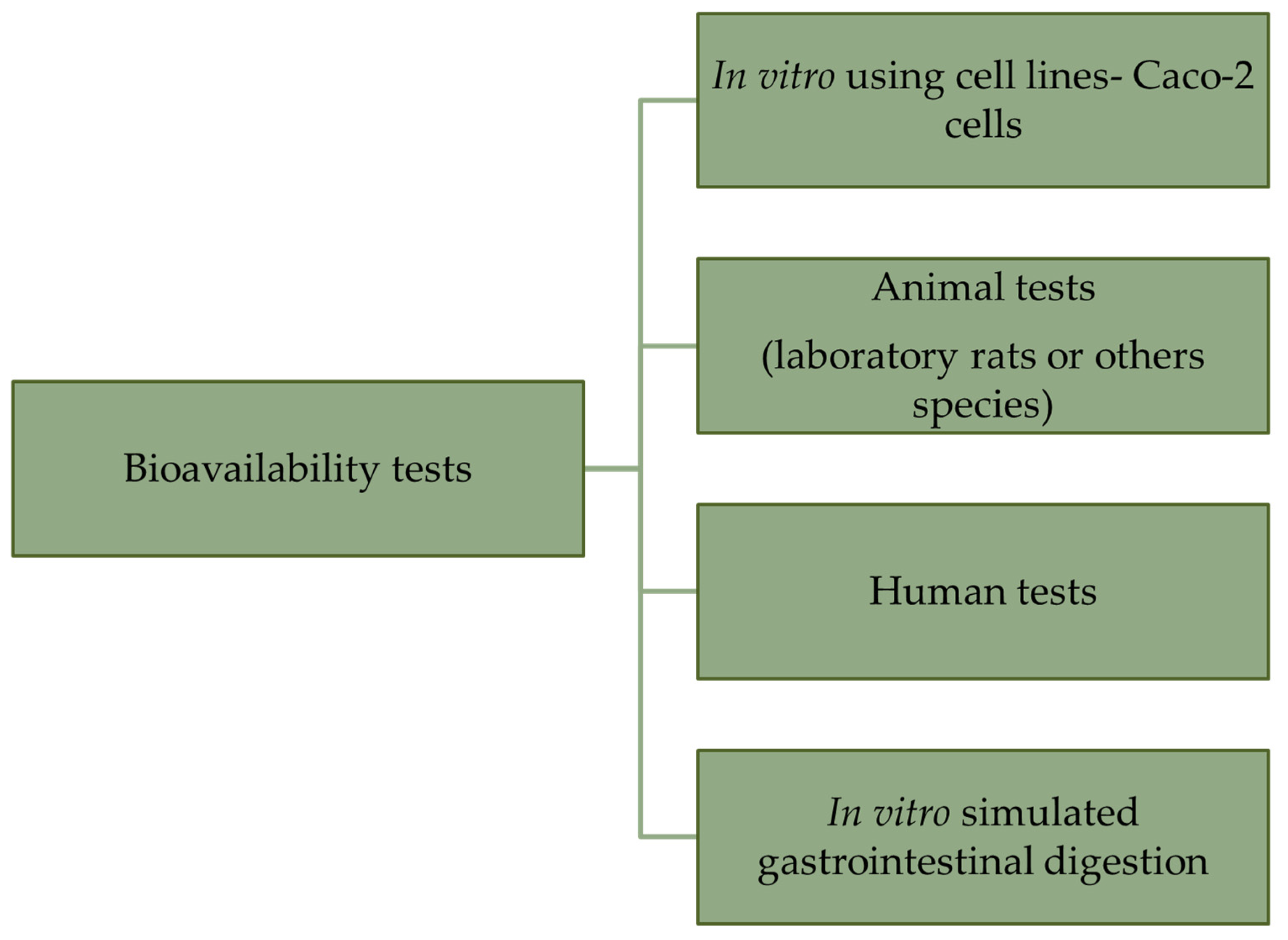
| Element | Forms Available for Plants | Chemicals Used in Biofortification | Source |
|---|---|---|---|
| Fe | Fe2+, Fe3+, iron chelates | FeSO4, nanoparticles, organic combinations (Fe-HBED, Fe-DTPA), iron citrate | [67,68,69] |
| Zn | Zn2+, zinc chelates, zinc in complex form—for example, in combination with amino acids (Zn + glycine) | Zn-EDTA, Zn + amino acids (ZN + AA), ZnSO4·7H2O, ZnO nanoparticles | [70,71] |
| I | I−, IO3−, organic combinations, CH3I | I−, IO3−, iodine–chitosan complex, solutions of iodoquinolines | [72,73,74,75] |
| Se | SeO42−, SeO32−, organic forms like selenocysteine, selenomethionine | SeO42−, SeO32−, synthesized and biosynthesized selenium nanoparticles | [76,77] |
| Microelement | Biofortified Material | Bioavailability Test | Result | Reference |
|---|---|---|---|---|
| Fe | Cowpea (Vigna unguiculata L. Walp) | Wistar rats | No differences in hemoglobin levels, but hemoglobin levels similar to the ferrous-sulfate-supplemented group | [121] |
| Red mottled beans (Phaseolus vulgaris L.) | Caco-2 cells/animal test on chickens | For Caco-2 cells, significantly higher ferritin levels were found after the use of biofortified beans with high iron concentrations For chickens, there was a significant increase in hemoglobin content for birds fed feed with biofortified iron, with no difference in liver ferritin levels for the groups tested | [122] | |
| Transgenic and wild-type indica rice | Caco-2 cells | Increased bioavailable iron levels in biofortified rice compared to wild type | [123] | |
| Carioca bean | Caco-2 cells/Wistar rats/humans | No differences in in vitro tests (ferritin levels); higher bioavailability of iron in biofortified beans than control in a study in rats; no effect on human nutrition | [124] | |
| Fe-biofortified beans | Humans | The group supplemented with biofortified beans had a significantly greater increase in hemoglobin and serum ferritin compared to the control group | [125] | |
| Zn | Rice (Oryza sativa L.) | Caco-2 cells/Sprague–Dawley rats | Increased zinc absorption from biofortified rice in rats | [126] |
| Pearl millet (Cenchrus americanus) | Humans | Higher zinc absorption from biofortified millet compared to non-biofortified group | [127] | |
| Canon bean and Pontal bean | Humans | No impact of zinc level in serum | [124] | |
| Se | Lettuce (Lactuca sativa L.) | Caco-2 cells | Improved Se assimilation from selenate biofortified lettuce. | [128] |
| Microalga (Chlorella sorokiniana) | Mice (Mus musculus)/in vitro simulated gastrointestinal digestion | An increase in Se bioavailability in mice was observed, but only at a low dose, and high levels in the kidneys, associated with excretion, in simulated digestion showed high (81%) Se availability, especially in the form of selenomethionine | [129] | |
| Radish seedlings | In vitro simulated gastrointestinal digestion | High bioavailability (exceeding 85%) in the test material after simulated in vitro digestion | [130] | |
| Wheat | Caco-2 cells | Despite the high Se content of biofortified wheat, only 19.6% of Se was absorbed by cells | [131] | |
| I | Rice and wheat | In vitro simulated gastrointestinal digestion | High release rate of iodine from the matrix in in vitro digestion | [132] |
| Celery and pak choi | In vitro simulated gastrointestinal digestion | The loss of iodine content during processing (soaking, cooking) was determined, and a high bioavailability was found after simulated digestion | [133] | |
| Biofortified vegetables (potatoes, carrots, cherry tomatoes, and green salad) | Humans | Increase in urinary iodine content as a diagnostic indicator in this study of iodine deficiency disorders | [134] | |
| Carrots | Wistar rats | Higher iodine levels in urine, feces, and tissues | [135] | |
| Lettuce | Wistar rats | Higher iodine levels in urine, feces, and tissues | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białowąs, W.; Blicharska, E.; Drabik, K. Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies—A Narrative Review. Nutrients 2024, 16, 1481. https://doi.org/10.3390/nu16101481
Białowąs W, Blicharska E, Drabik K. Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies—A Narrative Review. Nutrients. 2024; 16(10):1481. https://doi.org/10.3390/nu16101481
Chicago/Turabian StyleBiałowąs, Wojciech, Eliza Blicharska, and Kamil Drabik. 2024. "Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies—A Narrative Review" Nutrients 16, no. 10: 1481. https://doi.org/10.3390/nu16101481
APA StyleBiałowąs, W., Blicharska, E., & Drabik, K. (2024). Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies—A Narrative Review. Nutrients, 16(10), 1481. https://doi.org/10.3390/nu16101481







