Abstract
This review aimed to synthesise existing literature on the efficacy of personalised or precision nutrition (PPN) interventions, including medical nutrition therapy (MNT), in improving outcomes related to glycaemic control (HbA1c, post-prandial glucose [PPG], and fasting blood glucose), anthropometry (weight, BMI, and waist circumference [WC]), blood lipids, blood pressure (BP), and dietary intake among adults with prediabetes or metabolic syndrome (MetS). Six databases were systematically searched (Scopus, Medline, Embase, CINAHL, PsycINFO, and Cochrane) for randomised controlled trials (RCTs) published from January 2000 to 16 April 2023. The Academy of Nutrition and Dietetics Quality Criteria were used to assess the risk of bias. Seven RCTs (n = 873), comprising five PPN and two MNT interventions, lasting 3–24 months were included. Consistent and significant improvements favouring PPN and MNT interventions were reported across studies that examined outcomes like HbA1c, PPG, and waist circumference. Results for other measures, including fasting blood glucose, HOMA-IR, blood lipids, BP, and diet, were inconsistent. Longer, more frequent interventions yielded greater improvements, especially for HbA1c and WC. However, more research in studies with larger sample sizes and standardised PPN definitions is needed. Future studies should also investigate combining MNT with contemporary PPN factors, including genetic, epigenetic, metabolomic, and metagenomic data.
1. Introduction
Prediabetes is a metabolic state characterised by disruptions in glucose regulation and insulin resistance, wherein blood glucose levels exceed normal thresholds but do not reach the diagnostic criteria for Type 2 Diabetes Mellitus (T2DM) [1,2,3]. Conversely, metabolic syndrome (MetS) is a cluster of metabolic abnormalities that includes hypertension, central obesity, insulin resistance, and atherogenic dyslipidaemia [4,5]. As of 2022, the global prevalence of impaired fasting glucose is estimated at 10.6% (541 million individuals) [6], while MetS prevalence ranges from 12.5% to 31.4% [7].
The pathology for both conditions is complicated, with lifestyle, environmental, and genetic factors involved in disease progression [2,8,9]. Shared lifestyle risk factors for prediabetes and MetS include poor dietary habits, sedentary behaviour, obesity, smoking, and inadequate sleep [10]. If left untreated, prediabetes stands out as a pivotal risk factor for the eventual development of T2DM, with around 70% of individuals progressing from prediabetes to T2DM [11]. Similarly, MetS amplifies the risk not only for T2DM but also for cardiovascular disease (CVD), stroke, and myocardial infarction [2,12]. Consequently, early interventions addressing these shared risk factors are imperative to prevent adverse health outcomes associated with these conditions.
A healthy diet is widely recognised as a crucial factor in reducing the risk of prediabetes, MetS, and other non-communicable diseases [13,14]. However, current approaches to providing universal dietary recommendations or guidelines do not consider individual variations in dietary response. Personalised and precision nutrition approaches aim to improve health and well-being by leveraging dietary interventions that accommodate human variability [15]. For example, research has shown that individuals consuming the same meal may experience different glycaemic responses, highlighting the limitations of generic approaches [16]. Machine learning algorithms have also been developed to accurately predict personalised post-prandial glucose response to foods [16]. The algorithm was evaluated using a dietary intervention RCT that demonstrated a significantly lower post-prandial blood glucose response in participants after consuming lower carbohydrate, higher fibre, or higher fat-to-carbohydrate ratio meals, but this response was not consistent between individuals [16].
Currently, there is no universally agreed-upon definition for personalised and precision nutrition, and these terms are often used interchangeably. Efforts have been made to clarify these terms, with personalised nutrition defined as incorporating various information, including genetics, phenotypic, medical, nutritional, and other relevant information, to provide tailored nutritional guidance for individuals [15]. It also allows for interventions to be tailored based on an individual’s behaviour, preferences, lifestyle, and health objectives. These principles align with Medical Nutrition Therapy (MNT) [17], a category of personalised nutrition provided exclusively by registered and accredited practising dietitians. MNT involves a nutritional diagnosis and counselling services to facilitate lifestyle changes. On the other hand, precision nutrition is suggested to take a more dynamic approach, integrating genetic, metabolic, and environmental factors to develop comprehensive recommendations for individuals or subpopulation groups, utilising cutting-edge technologies such as metabolomics, metagenomics, and epigenetics [15]. In the context of this review, personalised and precision nutrition (PPN) are used as an umbrella term to encompass approaches that utilise one or more of the abovementioned components to tailor interventions to individuals.
To date, no systematic review has summarised the evidence of MNT and PPN interventions in adults with prediabetes or MetS. Therefore, the aim of this systematic review is to consolidate current literature from randomised controlled trials investigating the effectiveness of PPN interventions, including MNT, on outcomes related to glycaemic control, anthropometry, blood lipids, blood pressure, and dietary intake among individuals with prediabetes or MetS. Findings from this review may inform future treatment and research in prediabetes or MetS through the use of PPN and/or MNT.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Table S1) [18]. The protocol for this systematic review was registered on Open Science Frame (OSF) (https://doi.org/10.17605/OSF.IO/9Z8TE, accessed on 20 April 2024) [19].
2.2. Database and Search
The search strategy was developed with the help of a research librarian. Medical Subject Headings and keywords were used, including terms like “prediabetes” or “risk of diabetes” and “nutrition therapy” or “personalised diet”. The search was carried out systematically across six databases (Scopus, Medline, Embase, CINAHL, PsycINFO, and Cochrane) and included articles published between January 2000 and 16 April 2023 to account for significant advancements made in PPN and MNT methods within this time and ensuring that the results of this review reflect the most up-to-date knowledge available. The search was also restricted to include only randomised controlled trials (RCTs), articles published in English, and studies involving human subjects. The complete search string for all databases can be found in Supplementary Materials, Figures S1–S6.
2.3. Study Selection Criteria
The inclusion of studies was determined according to the Population, Intervention, Comparison, Outcomes, and Study (PICOS) framework (as detailed in Table 1). The study population comprised adults diagnosed with prediabetes or MetS who participated in an RCT that reported the effect of a personalised nutrition-based dietary intervention, including MNT. Valid comparator groups comprised those receiving usual or standard care or engaging in non-personalised dietary interventions.

Table 1.
PICOS criteria for inclusion of final studies in this systematic review.
The primary outcome measures were focused on glycaemic control indicators, including HbA1c levels, fasting blood glucose concentrations, post-prandial glucose/results from oral glucose tolerance tests (OGTT), and more (Table 1). The secondary outcome measures encompassed anthropometric parameters (weight, waist circumference, and body mass index [BMI]) as well as assessments of blood lipids, blood pressure, and dietary intake.
2.4. Study Selection
Studies from the search results were managed using the Covidence 2.0 platform [Covidence systematic review software, Melbourne] [20]. Duplicates were removed before at least two reviewers independently screened titles, abstracts, and full texts for inclusion in this review. Discrepancies were resolved by consensus or adjudication by other research team members.
2.5. Risk of Bias and Study Quality Assessment
Two independent reviewers assessed the methodological quality of the included full-text articles. The risk of bias was assessed using the Academy of Nutrition and Dietetics Quality Criteria checklist [21]. This tool was selected because it has a higher inter-observer agreement than ROB 2.0 [22]. Differences were resolved by discussion and consensus.
2.6. Data Extraction
A standardised template, implemented within a Microsoft Excel (Version 2402; Build 16.0.17328.20282) spreadsheet, was used to extract data from the included articles. The standardised template was first piloted with three articles to ensure that all information relevant to this systematic review was collected. Data extraction was carried out by a singular reviewer (SR), and accuracy was confirmed by a second reviewer.
2.7. Synthesis of Results
Due to the heterogeneity in study design, population, and interventions, a meta-analysis was not performed. The results were synthesised narratively and summarised by study characteristics (study design, intervention, and control type) and outcome measure results. Wherever possible, results for outcome measures included both the confidence interval and p-value for within-group or between-group differences. The summary also included the consistency of the reported significant differences (p < 0.05) between the intervention and comparator groups for each outcome, whether the differences were increases or decreases.
3. Results
3.1. Search Results
The initial search yielded 7396 studies. After removing 1186 duplicates, 6040 articles were excluded based on their title and abstract. A total of 170 full-text articles were retrieved, and 163 studies were excluded during screening. The primary reason for exclusion was incorrect intervention type (n = 85), with most excluded interventions lacking personalisation or comprehensive lifestyle interventions involving both diet and physical activity that could not identify separate dietary impacts (Figure 1). A total of seven articles met all inclusion criteria and were included in this review [23,24,25,26,27,28,29].
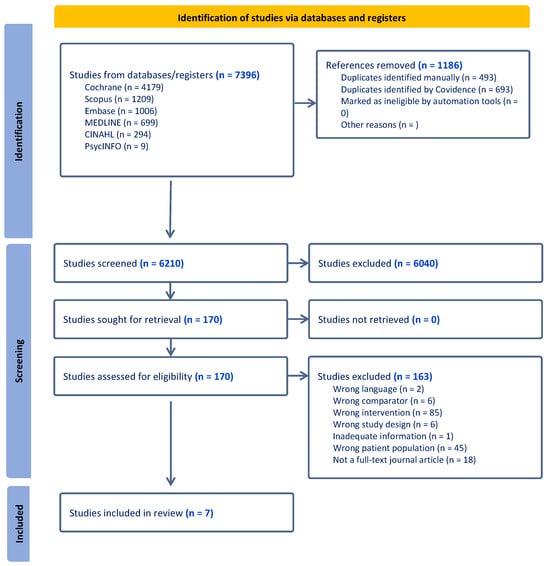
Figure 1.
PRISMA flow diagram for the literature search and the study selection process [30].
3.2. Characteristics of the Included Studies
Characteristics of the included studies are presented in Table 2. All seven of the included papers were parallel RCTs [23,24,25,26,27,28,29]. The sample size of participants varied from 46 [26] to 225 [24], with a mean age range of 50 to 60 years. Across the included articles, there was a total of 873 participants [23,24,25,26,27,28,29]. The portion of males ranged from 28% [27] to 100% [23], and in total, across the included studies, 462 participants were male, and 411 were female [23,24,25,26,27,28,29]. Two studies were based in the USA [27,28], while others were from Brazil [29], Finland [26], Italy [25], Israel [24], and Japan [23]. Two papers met the inclusion criteria for prediabetes [24,28], two papers included individuals with MetS [25,26], two papers included those with impaired glucose tolerance [23,29], and one included participants with an HbA1c range of 6–6.9% [27].

Table 2.
Study characteristics and results summary of studies included in the systematic review examining the effect of PPN and MNT on adults with prediabetes.
3.3. Quality Assessment of Studies
The results of the quality assessment are summarised in Table S2 in Supplementary Materials. Most evaluations (n = 6) received positive ratings, with just one study rated as neutral. The neutral assessment was attributed to factors such as the absence of a power calculation and lack of blinding in the study design.
3.4. Interventions
Personalisation varied between studies, with four studies personalising based on dietary and nutritional information [23,25,26,27], one employed a published algorithm, which integrates clinical and gut microbiome features to predict personal post-prandial glycaemic responses to meals [24], and two were MNT interventions (Table 2) [28,29]. Four interventions were conducted by nutritionists [23,25,26,29], two by dietitians [24,28], and one by an interventionist with unreported qualifications [27]. Four interventions were delivered face-to-face [25,26,28,29], one used a combination of face-to-face and phone [27], another used a combination of face-to-face and mail [23], and one used a combination of face-to-face, email, and phone [24]. The length of the included studies ranged from 6 months [27] to 2 years [25]. The frequency of each intervention session ranged from once-off [23] to 36 sessions [29], while the duration of interventions ranged from 3 months [28] to 2 years [25]. One study compared individual MNT (considered the intervention) to group MNT (considered the comparator) [28].
3.5. Comparators
The comparator groups also varied among the studies (Table 2). The most common control group was a usual care comparator or standardised generic information [23,25,27]. Another study implemented a Mediterranean diet, which included specific nutrient targets based on percentage energy intake. Additionally, meals were assessed and scored based on the recommendations of four independent dietitians, and participants’ dietary preferences were also considered [24]. Among the remaining studies, one encouraged participants of the control group to continue with their usual diet and physical activity [26], and another had participants attend group nutrition sessions facilitated by a dietitian [28]. One study provided insufficient details regarding the control group, with the authors stating that participants did not receive any advice [29].
3.6. Outcome Measures
3.6.1. Glycaemic Control Outcomes
All included studies evaluated blood glucose levels (BGLs), of which six studies reported examining fasting BGLs [23,24,26,27,28,29]. Two studies used continuous glucose monitors (CGMs) to examine mean glucose [24,27], while three examined post-prandial glucose (Table 2 and Table S3) [23,24,29].
Four studies examining fasting BGLs reported significant decreases (p < 0.05) in the intervention group compared to baseline [26,27,28,29]. Two of these papers reported significant differences between the intervention and comparison groups (Figure 2) [27,29]. Another study also reported a significant difference in BGLs between the intervention and control groups but did not specify whether these measures were taken in a fasted state [25].

Figure 2.
Number of studies that reported statistically significant differences between the intervention and comparison groups for each outcome of interest. The blue bars represent reported increases in the intervention group compared to the control group, while the red bars denote decreases. Studies that examined the outcome but did not report a significant between-group difference are not included in this figure (see Table S3 for further information). Esposito et al. [25] did not specify if blood/plasma glucose levels (counted under BGL outcome) were measured in a fasted state. Pimentel et al. [29] did not report the timing for when post-prandial glucose or insulin were measured, while Ben-Yacov et al. [24] calculated post-prandial glucose from continuous glucose monitor (CGM) data. BGL (Blood Glucose Level), BMI (Body Mass Index), HbA1c (Glycated Haemoglobin), HOMA-IR (Homeostatic Model Assessment of Insulin Resistance).
Both papers that measured mean glucose using a CGM reported significant differences between groups in favour of the intervention groups [24,27]. Interestingly, Dorans et al. also noted significant improvements in CGM night-time glucose in the intervention relative to the control [27].
Among the three studies that evaluated the impact on post-prandial glucose, all reported a significant difference between the intervention and control groups, although at varying times (Table 2, Figure 2, and Table S3). One study found a significant difference at 2 h after a 75 g oral glucose challenge but not at 1 h [23], while the other did not specify a time frame or dose [29]. The third study, which used CGM data, noted a difference between groups at 5 h but not 2 h following an at-home 75 g oral glucose challenge [24].
Four included studies examined HbA1c, of which three reported significant between-group differences in favour of the intervention group (Figure 2 and Table S3) [24,27,29]. Notably, in the one study that reported no difference, the intervention involved only two sessions with participants [28] compared to interventions with 8 [24], 10 [27], and 36 [29] sessions in the other studies. Ben-Yacovet et al. [24] was the only study that measured fructosamine (a shorter-term measure reflecting 2–3 week changes in blood glucose) and found a significant decrease in the intervention compared to the control group.
Multiple papers reported outcomes related to insulin, including HOMA-IR and fasting insulin (Table 2 and Table S3) [24,25,26,27,29]. Four studies examined HOMA-IR using the homeostasis model of assessment [24,25,27,29], of which two reported a significantly greater decrease in the intervention group compared to the control group (Figure 2) [25,27]. Similarly, two of the five studies examining fasting insulin levels [24,25,26,27,29] also reported a significant between-group difference in favour of the intervention [25,27]. Pimentel et al. [29] also reported a significant decrease in post-prandial insulin levels in the intervention compared to the control group but did not indicate the timing of these measurements.
3.6.2. Anthropometric Outcomes
Six of the included studies reported anthropometric data (Table 2) [24,25,26,27,28,29]. All six studies examined body weight [24,25,26,27,28,29]. Three of these studies reported significant between-group differences in favour of the intervention group (Figure 2) [25,26,27]. Four studies examined waist circumference changes [24,25,26,27], three of which reported significant differences between the intervention and control groups [25,26,27]. Five studies examined changes in BMI [24,25,26,28,29], two of which reported a significant decrease in the intervention group and a significant difference between the intervention and control groups [25,26].
3.6.3. Blood Lipids
Five studies examined changes in blood lipid levels (Table 2) [24,25,27,28,29]. All five studies reported changes in total cholesterol [24,25,27,28,29]. Of those five, two reported significant differences between the intervention and control groups (Figure 2) [25,29]. All five studies reported changes in HDL cholesterol [24,25,27,28,29]. Of those five studies, two reported a significantly greater increase in HDL in the intervention group compared to the control group [24,25]. Four papers examined LDL cholesterol, and all papers reported non-significant between-group differences [24,27,28,29]. Four papers reported changes in triglyceride levels [24,25,28], and two reported a significant decrease in the intervention compared to control groups [24,25].
3.6.4. Blood Pressure
Five of the included studies examined changes in systolic and diastolic blood pressure (Table 2) [24,25,26,27,28]. Only one study reported significant differences in systolic and diastolic blood pressure between the intervention and control groups, with the intervention group showing a significant decrease (Figure 2) [25]. Another study reported a significant difference between the intervention and control groups at the midway assessment (3 months) but not at the final follow-up [27].
3.6.5. Dietary Outcomes
The methods for assessing dietary outcomes and adherence varied among the studies (Table 2). One study employed daily food logs via a smartphone app [24], offering a selection of over 7000 foods. One paper utilised a food frequency questionnaire (FFQW65) [23], while another used 24-h recalls [27]. However, the most common method across studies was the use of food records [25,26,29], typically spanning 3 [25] to 7 [29] days.
Among the seven studies, five evaluated nutrient composition (Table 2) [24,25,26,27,29]. Of these, only one study investigated data at the food group level between groups [25]. Additionally, one study reported the top 10 most logged foods by participants based on whether they received the intervention or control intervention but did not report or quantify differences in intake between the groups [24]. Another study solely reported on the absolute value of the proportion of over/under intake fraction for estimated total energy [23].
4. Discussion
This review summarises the current evidence on the effectiveness of PPN and MNT in improving various outcomes related to glycaemic control, anthropometry, blood lipids, blood pressure, and diet among adults with prediabetes or MetS. Comparing interventions to standard care or non-personalised approaches showed evidence supporting PPN and MNT in improving certain glycaemic response outcomes like HbA1c, post-prandial glucose, and waist circumference, where the majority of studies (at least 75%) investigating these outcomes reported a significant between-group difference favouring the intervention (Figure 2, Table S3). However, mixed results were found for other outcomes such as fasting BGL, HOMA-IR, fasting insulin, BMI, weight, blood pressure, and blood lipids (Figure 2 and Table S3). Positive findings were identified for certain outcomes, such as mean CGM glucose, but were measured in only two studies. Variations in study design, including the types of PPN interventions and comparison groups utilised, as well as the frequency and duration of interventions, appeared to influence the magnitude of the reported changes.
The findings suggest that more intense and longer interventions seemed to have a greater positive effect, especially on outcomes like HbA1c and waist circumference. For instance, interventions with eight or more sessions or lasting 6 months or longer showed significant differences in HbA1c levels [24,27,29] and waist circumference [25,26,27] compared to less intensive and shorter studies. These results are not surprising, as HbA1c is a long-term marker of blood sugar levels, and longer interventions are expected to have a more notable impact on HbA1c results. Previous research has demonstrated that multiple encounters of MNT interventions are necessary to achieve desired outcomes in individuals with diabetes, such as HbA1c levels [31]. Similar findings have been observed for waist circumference, with longer interventions and more frequent sessions leading to greater weight loss [25,26,27]. These results are consistent with previous studies indicating that more than 28 sessions resulted in significantly better improvements in weight, BMI, waist circumference, HbA1c, and fasting blood glucose levels compared to those who received fewer sessions [32]. Given that obesity is a major risk factor for prediabetes and weight loss can reduce the risk of developing type 2 diabetes, personalised interventions and MNT may be effective in managing prediabetes and preventing its progression. However, more longer-term studies are necessary to better understand the relationship between the dose of intervention and its response in treating prediabetes.
Studies that compared mean CGM glucose levels [24,27] showed a significant decrease in favour of the PPN intervention groups. However, for other outcomes such as HOMA-IR, fasting insulin, fasting BGL, weight, BMI, total cholesterol, and blood pressure, significant differences favouring the intervention were reported in some studies, but the results were more mixed, as not all studies reported significant results. These findings are similar to a systematic review focusing on MNT and prediabetes, which found that MNT compared to standard care significantly improved HbA1c, fasting BGL, anthropometric measures, cholesterol levels, and blood pressure [33]. A study using MNT provided by dietitians reported a significant decrease within the intervention group in fasting BGL, weight, and HbA1c [28]. Differences in comparison groups and the level of precision and personalisation in the intervention were major sources of heterogeneity and may explain the inconsistencies across the studies in this review. As expected, studies that used standard care or generic information as the comparator tended to report a greater magnitude of difference in favour of the intervention group [23,25,26,27,29]. This is in contrast to studies where comparators, involving some level of guidance from a dietitian or personalisation, reported smaller differences between the control and intervention groups [24,28]. For instance, comparison groups that received small group sessions facilitated by a dietitian [28], or a personalised Mediterranean diet but did not receive the same level of precision and personalisation relating to the microbiome and other clinical or biological markers as the intervention group [24]. Additionally, the purpose of the intervention seemed to impact the outcomes differently. For example, a low-carbohydrate diet [27] successfully produced significant improvements in glycaemic and anthropometric-related measures relative to the comparison group but not for other clinical measures, such as blood pressure and blood lipids. This was in contrast to a study that investigated a tailored Mediterranean diet [25] provided by a nutritionist, which produced significant improvements in all glycaemic, anthropometric, and clinical measures and is likely explained by the manipulation of multiple dietary components in the diet, resulting in a more widespread effect.
4.1. Strengths and Limitations
A major strength of the current review is the inclusion of only RCTs, which are the highest-ranked study designs in terms of evidence hierarchy. To minimise confounding effects on the results, studies were excluded if participants were also given medications, supplements, and physical activity as part of the intervention, thereby focusing solely on the effectiveness of dietary interventions. Another strength is that six out of the seven studies had positive ratings regarding quality assessment [23,24,25,26,27,28], therefore having a lower risk of bias. The limited evidence base and considerable variation in study design, interventions, comparison groups, and sample populations pose a challenge for drawing definitive conclusions and generalising results. For example, only one included paper personalised intake on factors other than diet and health data [24].
4.2. Recommendations
Further research is needed to determine the effectiveness of PPN among diverse population groups, especially since only two studies have investigated PPN interventions in individuals with MetS [25,26]. Moreover, different approaches to precision and personalisation need to be further explored. For example, only one study integrated advanced precision data, such as clinical and gut microbiome information, to predict individual post-prandial glycaemic responses to meals and further tailor their dietary intervention [24]. Advances in multi-omic technologies, including genomics and metabolomics, coupled with sophisticated data analysis techniques, have improved our understanding of individual variability and led to the identification of novel disease subgroups (subphenotypes) that impact clinical practice and disease understanding [2,34]. However, this review highlights the need for exploration and validation in the use of this information to guide PPN for people with pre-diabetes and MetS. At the same time, behavioural, psychological, and sociocultural factors are essential components of dietary prescription, which are core to MNT and key determinants of patient adherence and should not be underestimated in future PPN interventions [35]. Future studies should aim for larger sample sizes to enhance study power and detect statistically significant between-group differences more effectively. This will improve the generalisability of findings to a broader population. Finally, a major challenge identified during the paper selection process was lack of a standardised definition for PPN interventions. Establishing a universal definition for PPN will promote consistency and comparability across research within this field.
5. Conclusions
The current systematic review suggests that the use of PPN and MNT for managing prediabetes and MetS is promising, especially for interventions of longer duration and that offer more frequent sessions or contact with interventionists. More consistent improvements favouring PPN and MNT interventions were reported across studies examining outcomes such as HbA1c, post-prandial glucose, and waist circumference. However, further research is essential to enhance our understanding of PPN as a treatment for prediabetes and MetS. Different approaches to precision and personalisation should be further explored, for example, through combining MNT with contemporary factors such as genetic, epigenetic, metabolomic, and metagenomic data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16101479/s1, Table S1: PRISMA Reporting Guidelines Checklist; Table S2: Academy of Nutrition and Dietetic Quality criteria checklist for included studies in the systematic review examining the effect of PN and MNT for adults with prediabetes; Table S3: Summary of between-group differences between the intervention and comparison groups for outcomes of interest (Source data for Figure 2 in the manuscript). Tables S4–S9: Search string for each database.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Given that this was a systematic review, all data used for this study are already published and publicly available.
Acknowledgments
The authors would like to express their gratitude to Research Librarian Nicole Faull-Brown for her assistance in refining the search strategy.
Conflicts of Interest
C.E.C. is funded by an Australian National Health and Medical Research Council Leadership Investigator Grant (APP2009340). The authors declare that they have no conflicts of interest to disclose.
References
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Schulze, M.B. Metabolic health and cardiometabolic risk clusters: Implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. 2023, 11, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of β-Cell Dysfunction and Insulin Resistance to the Pathogenesis of Impaired Glucose Tolerance and Impaired Fasting Glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired Fasting Glucose and Impaired Glucose Tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef]
- International Diabetes Federation. Annual Report 2021; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Mambiya, M.; Shang, M.; Wang, Y.; Li, Q.; Liu, S.; Yang, L.; Zhang, Q.; Zhang, K.; Liu, M.; Nie, F.; et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Front. Public Health 2019, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Thor, S.M.; Ramadas, A. Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence. Nutrients 2020, 12, 2990. [Google Scholar] [CrossRef] [PubMed]
- Dolui, M.; Sarkar, S.; Ghosh, P.; Hossain, M. Dietary diversity and association with non-communicable diseases (NCDs) among adult men (15-54 years): A cross-sectional study using National Family and Health Survey, India. PLoS Glob. Public Health 2023, 3, e0001775. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Ramos-Lopez, O.; Pérusse, L.; Kato, H.; Ordovas, J.M.; Martínez, J.A. Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci. Technol. 2022, 128, 253–264. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Academy of Nutrition and Dietetics. How an RDN Can Help with Diabetes. Available online: https://www.eatright.org/health/health-conditions/diabetes/how-an-rdn-can-help-with-diabetes (accessed on 25 March 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Foster, E.D. Open Science Framework (OSF). J. Med. Libr. Assoc. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 30 May 2023).
- Ao, N. Dietetics. In Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2012. [Google Scholar]
- Handu, D. Comparison of the Academy of Nutrition and Dietetics updated Quality Criteria Checklist and Cochrane’s ROB 2.0 as risk of bias tools. Cochrane Database Syst. Rev. 2018, 9. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamaoka, K.; Yokotsuka, M.; Tango, T.; Watanabe, M.; Yamaoka, K.; Yokotsuka, M.; Tango, T. Randomized controlled trial of a new dietary education program to prevent type 2 diabetes in a high-risk group of Japanese male workers. Diabetes Care 2003, 26, 3209–3214. [Google Scholar] [CrossRef]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Kolobkov, D.; Koren, N.; Cohen Dolev, N.; Travinsky Shmul, T.; Wolf, B.C.; Kosower, N.; et al. Personalized Postprandial Glucose Response–Targeting Diet Versus Mediterranean Diet for Glycemic Control in Prediabetes. Diabetes Care 2021, 44, 1980–1991. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Salopuro, T.; Schwab, U.S.; Kekäläinen, J.; Kallio, P.; Laaksonen, D.E.; Pulkkinen, L.; Lindi, V.I.; Sivenius, K.; Mager, U.; et al. Weight reduction modulates expression of genes involved in extracellular matrix and cell death: The GENOBIN study. Int. J. Obes. 2008, 32, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Dorans, K.S.; Bazzano, L.A.; Qi, L.; He, H.; Chen, J.; Appel, L.J.; Chen, C.-S.; Hsieh, M.-H.; Hu, F.B.; Mills, K.T.; et al. Effects of a Low-Carbohydrate Dietary Intervention on Hemoglobin A1c: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2238645. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.E.; Boyer, K.M.; Spanbauer, S.M.; Sprague, D.; Bingham, M. Effectiveness of Prediabetes Nutrition Shared Medical Appointments: Prevention of Diabetes. Diabetes Educ. 2013, 39, 344–353. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Portero-McLellan, K.C.; Oliveira, E.P.; Spada, A.P.M.; Oshiiwa, M.; Zemdegs, J.C.S.; Barbalho, S.M. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr. Res. 2010, 30, 186–190. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Razaz, J.M.; Rahmani, J.; Varkaneh, H.K.; Thompson, J.; Clark, C.; Abdulazeem, H.M. The health effects of medical nutrition therapy by dietitians in patients with diabetes: A systematic review and meta-analysis: Nutrition therapy and diabetes. Prim. Care Diabetes 2019, 13, 399–408. [Google Scholar] [CrossRef]
- Singh, N.; Stewart, R.A.H.; Benatar, J.R. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: A meta-analysis of randomised trials. BMJ Open 2019, 9, e029966. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, J.M.; Senkus, K.E.; Evert, A.B.; Raynor, H.A.; Rozga, M.; Handu, D.; Moloney, L.M. The effectiveness of medical nutrition therapy provided by a dietitian in adults with prediabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2023, 118, 892–910. [Google Scholar] [CrossRef]
- Reddy, K.; Sinha, P.; O’Kane, C.M.; Gordon, A.C.; Calfee, C.S.; McAuley, D.F. Subphenotypes in critical care: Translation into clinical practice. Lancet Respir. Med. 2020, 8, 631–643. [Google Scholar] [CrossRef]
- Shannon, C.E.; Ní Chathail, M.B.; Mullin, S.M.; Meehan, A.; McGillicuddy, F.C.; Roche, H.M. Precision nutrition for targeting pathophysiology of cardiometabolic phenotypes. Rev. Endocr. Metab. Disord. 2023, 24, 921–936. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).