Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function
Abstract
1. Introduction
2. Discussion
2.1. The Atherosclerosis as Main Target of Dietetic Regimen
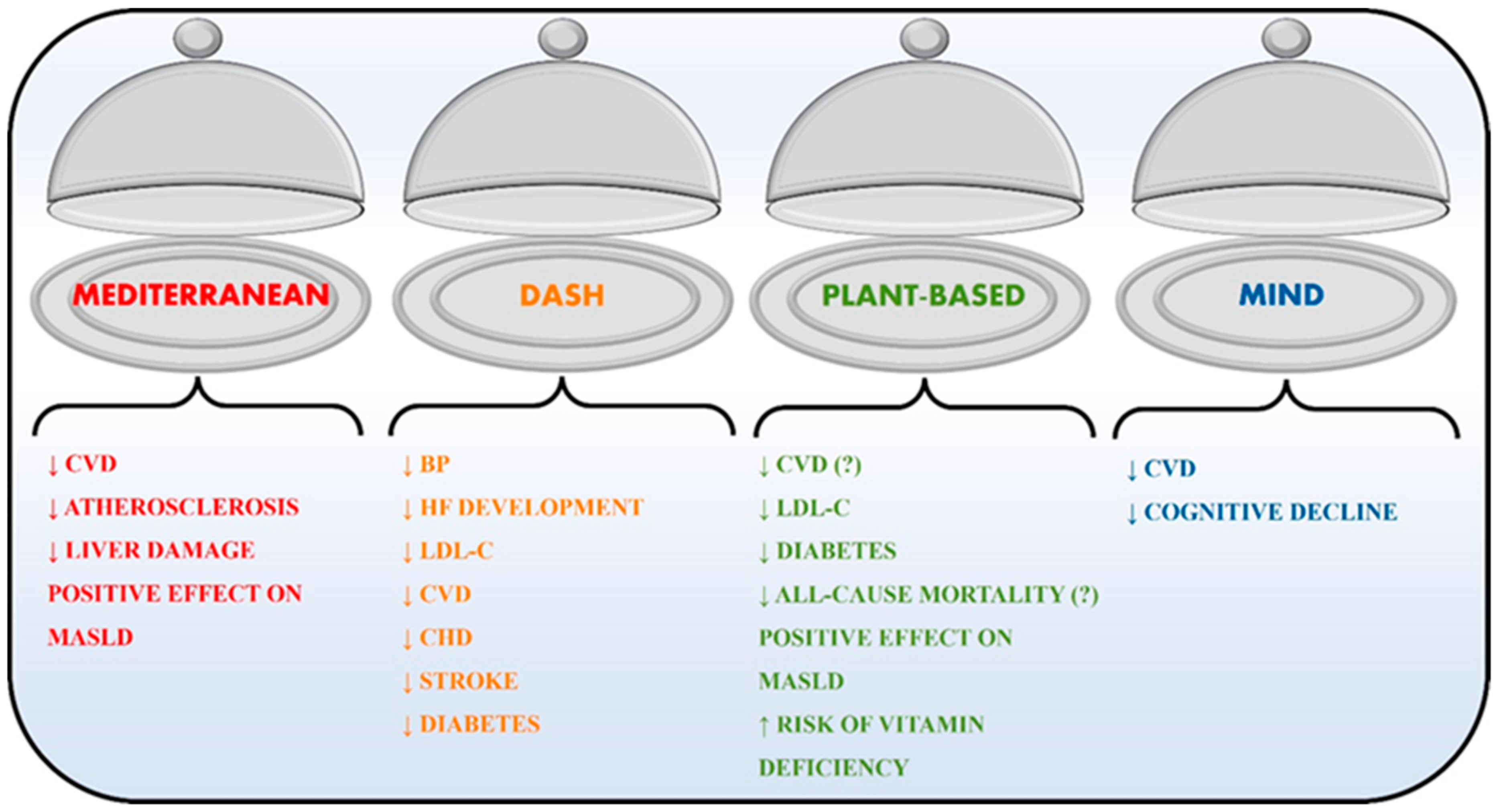
2.2. Dietetic Regimen
2.2.1. Mediterranean Diet
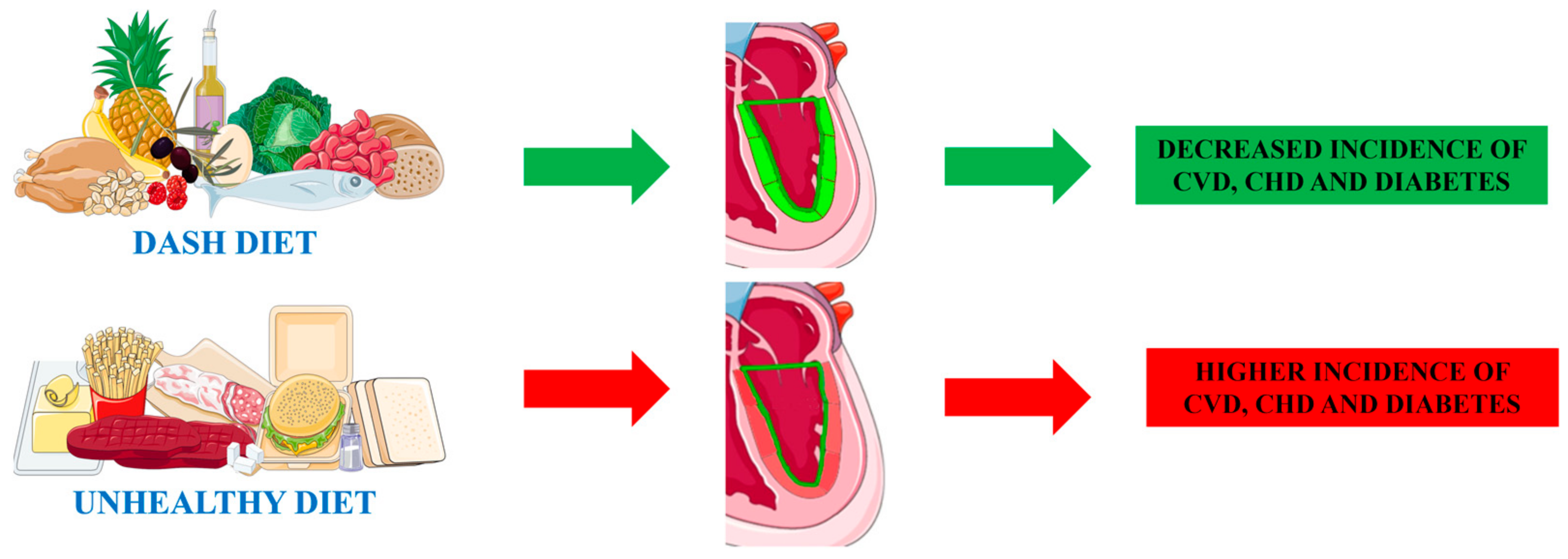
2.2.2. Dietary Approaches to Stop Hypertension (DASH)
2.2.3. Plant-Based Diets
2.2.4. Mediterranean–DASH Intervention Diet for Neurodegenerative Delay (MIND)
2.3. Cardiac Evaluation Regarding Diet in MASLD and Cancer
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Szczepańska, E.; Białek-Dratwa, A.; Janota, B.; Kowalski, O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)-Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients 2022, 14, 2649. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional Recommendations for Cardiovascular Disease Prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef]
- Torres, N.; Guevara-Cruz, M.; Velázquez-Villegas, L.A.; Tovar, A.R. Nutrition and Atherosclerosis. Arch. Med. Res. 2015, 46, 408–426. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Pazoki, R.; Dehghan, A.; Evangelou, E.; Warren, H.; Gao, H.; Caulfield, M.; Elliott, P.; Tzoulaki, I. Genetic Predisposition to High Blood Pressure and Lifestyle Factors: Associations with Midlife Blood Pressure Levels and Cardiovascular Events. Circulation 2018, 137, 653–661. [Google Scholar] [CrossRef]
- Romano, S.; Rigon, G.; Albrigi, M.; Tebaldi, G.; Sartorio, A.; Cristin, L.; Burrei, G.; Fava, C.; Minuz, P. Hypertension, Uncontrolled Hypertension and Resistant Hypertension: Prevalence, Comorbidities and Prescribed Medications in 228,406 Adults Resident in Urban Areas. A Population-Based Observational Study. Intern. Emerg. Med. 2023, 18, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N. The Seven Countries Study: 2289 Deaths in 15 Years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Plaz Torres, M.C.; Aghemo, A.; Lleo, A.; Bodini, G.; Furnari, M.; Marabotto, E.; Miele, L.; Giannini, E.G. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients 2019, 11, 2971. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P.T. Diet, Nutrition and the Prevention of Excess Weight Gain and Obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef]
- Abou, R.; van der Bijl, P.; Bax, J.J.; Delgado, V. Global Longitudinal Strain: Clinical Use and Prognostic Implications in Contemporary Practice. Heart 2020, 106, 1438–1444. [Google Scholar] [CrossRef]
- Leitman, M.; Lysyansky, P.; Sidenko, S.; Shir, V.; Peleg, E.; Binenbaum, M.; Kaluski, E.; Krakover, R.; Vered, Z. Two-Dimensional Strain-a Novel Software for Real-Time Quantitative Echocardiographic Assessment of Myocardial Function. J. Am. Soc. Echocardiogr. 2004, 17, 1021–1029. [Google Scholar] [CrossRef]
- Karlsen, S.; Dahlslett, T.; Grenne, B.; Sjøli, B.; Smiseth, O.; Edvardsen, T.; Brunvand, H. Global Longitudinal Strain Is a More Reproducible Measure of Left Ventricular Function than Ejection Fraction Regardless of Echocardiographic Training. Cardiovasc. Ultrasound 2019, 17, 18. [Google Scholar] [CrossRef]
- Cho, G.-Y.; Marwick, T.H.; Kim, H.-S.; Kim, M.-K.; Hong, K.-S.; Oh, D.-J. Global 2-Dimensional Strain as a New Prognosticator in Patients with Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 618–624. [Google Scholar] [CrossRef]
- Delgado-Montero, A.; Tayal, B.; Goda, A.; Ryo, K.; Marek, J.J.; Sugahara, M.; Qi, Z.; Althouse, A.D.; Saba, S.; Schwartzman, D.; et al. Additive Prognostic Value of Echocardiographic Global Longitudinal and Global Circumferential Strain to Electrocardiographic Criteria in Patients with Heart Failure Undergoing Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2016, 9, e004241. [Google Scholar] [CrossRef]
- Ohara, Y.; Fukuoka, Y.; Tabuchi, I.; Sahara, S.; Hosogi, S.; Nishimoto, M.; Yamamoto, K. The Impairment of Endocardial Radial Strain Is Related to Aortic Stenosis Severity in Patients with Aortic Stenosis and Preserved LV Ejection Fraction Using Two-Dimensional Speckle Tracking Echocardiography. Echocardiography 2012, 29, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, N.; Kirigaya, J.; Gohbara, M.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Minamimoto, Y.; Kimura, Y.; et al. Global Strain Measured by Three-Dimensional Speckle Tracking Echocardiography Is a Useful Predictor for 10-Year Prognosis After a First ST-Elevation Acute Myocardial Infarction. Circ. J. 2021, 85, 1735–1743. [Google Scholar] [CrossRef]
- Luis, S.A.; Yamada, A.; Khandheria, B.K.; Speranza, V.; Benjamin, A.; Ischenko, M.; Platts, D.G.; Hamilton-Craig, C.R.; Haseler, L.; Burstow, D.; et al. Use of Three-Dimensional Speckle-Tracking Echocardiography for Quantitative Assessment of Global Left Ventricular Function: A Comparative Study to Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 285–291. [Google Scholar] [CrossRef]
- Bhore, A.; Shah, P.; Hardas, S.; Asawa, M. Myocardial Strain Analysis by 4D-Speckle Tracking Echocardiography for Prediction of Coronary Artery Disease Severity in Patients with Stable Angina Pectoris. Indian Heart J. 2023, 75, 177–184. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Schoeneck, M.; Iggman, D. The Effects of Foods on LDL Cholesterol Levels: A Systematic Review of the Accumulated Evidence from Systematic Reviews and Meta-Analyses of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef]
- Pan, J.; Han, W.; Jiang, Y.; Wu, J.; Zhou, X. Association of Dietary Cholesterol and Dyslipidemia in Chinese Health Examinees. J. Health Popul. Nutr. 2022, 41, 15. [Google Scholar] [CrossRef]
- Jahns, L.; Davis-Shaw, W.; Lichtenstein, A.H.; Murphy, S.P.; Conrad, Z.; Nielsen, F. The History and Future of Dietary Guidance in America. Adv. Nutr. 2018, 9, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef]
- Liu, X.; Morris, M.C.; Dhana, K.; Ventrelle, J.; Johnson, K.; Bishop, L.; Hollings, C.S.; Boulin, A.; Laranjo, N.; Stubbs, B.J.; et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Study: Rationale, Design and Baseline Characteristics of a Randomized Control Trial of the MIND Diet on Cognitive Decline. Contemp. Clin. Trials 2021, 102, 106270. [Google Scholar] [CrossRef]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a Healthy Dietary Pattern Results in Significant Reductions in C-Reactive Protein Levels in Adults: A Meta-Analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.L.; Thomas, C.J.; Tierney, A.C.; Kucianski, T.; George, E.S.; Ruiz-Canela, M.; Hebert, J.R.; Shivappa, N.; Itsiopoulos, C. Randomization to 6-Month Mediterranean Diet Compared with a Low-Fat Diet Leads to Improvement in Dietary Inflammatory Index Scores in Patients with Coronary Heart Disease: The AUSMED Heart Trial. Nutr. Res. 2018, 55, 94–107. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Torres-Peña, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuña, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean Diet Improves Endothelial Function in Patients with Diabetes and Prediabetes: A Report from the CORDIOPREV Study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a Traditional Mediterranean Diet on Lipoprotein Oxidation: A Randomized Controlled Trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of Seven Popular Structured Dietary Programmes and Risk of Mortality and Major Cardiovascular Events in Patients at Increased Cardiovascular Risk: Systematic Review and Network Meta-Analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-Term Secondary Prevention of Cardiovascular Disease with a Mediterranean Diet and a Low-Fat Diet (CORDIOPREV): A Randomised Controlled Trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-Style Diet for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Rundek, T.; Wright, C.B.; Gu, Y.; Scarmeas, N.; Homma, S.; Russo, C.; Elkind, M.S.V.; Sacco, R.L.; Di Tullio, M.R. A Mediterranean-Style Diet and Left Ventricular Mass (from the Northern Manhattan Study). Am. J. Cardiol. 2015, 115, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Bacharaki, D.; Petrakis, I.; Kyriazis, P.; Markaki, A.; Pleros, C.; Tsirpanlis, G.; Theodoridis, M.; Balafa, O.; Georgoulidou, A.; Drosataki, E.; et al. Adherence to the Mediterranean Diet Is Associated with a More Favorable Left Ventricular Geometry in Patients with End-Stage Kidney Disease. J. Clin. Med. 2022, 11, 5746. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Ahmed, A.; Arnett, D.K.; Polak, J.F.; Hundley, W.G.; Bluemke, D.A.; Heckbert, S.R.; Jacobs, D.R.; Nettleton, J.A. Mediterranean Diet Score and Left Ventricular Structure and Function: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2016, 104, 595–602. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Pitsavos, C.; Metallinos, G.; Antoniou, C.; Oikonomou, E.; Kotroyiannis, I.; Tsantilas, A.; Tsitsinakis, G.; Tousoulis, D.; Panagiotakos, D.B.; et al. Cross-Sectional Relationship of a Mediterranean Type Diet to Diastolic Heart Function in Chronic Heart Failure Patients. Heart Vessel. 2012, 27, 576–584. [Google Scholar] [CrossRef]
- Sanches Machado d’Almeida, K.; Ronchi Spillere, S.; Zuchinali, P.; Corrêa Souza, G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients 2018, 10, 58. [Google Scholar] [CrossRef]
- Ternacle, J.; Wan, F.; Sawaki, D.; Surenaud, M.; Pini, M.; Mercedes, R.; Ernande, L.; Audureau, E.; Dubois-Rande, J.-L.; Adnot, S.; et al. Short-Term High-Fat Diet Compromises Myocardial Function: A Radial Strain Rate Imaging Study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, S.; Wu, M.; Niu, Y.; Wang, K.; Zhu, H.; Yuan, J. Impact High Fat Diet on Myocardial Strain in Mice by 2D Speckle Tracking Imaging. Obes. Res. Clin Pract. 2021, 15, 133–137. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller, E.R.; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bertoni, A.G.; Nettleton, J.A.; Bluemke, D.A.; Levitan, E.B.; Burke, G.L. DASH Eating Pattern Is Associated with Favorable Left Ventricular Function in the Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Nutr. 2012, 31, 401–407. [Google Scholar] [CrossRef]
- Hummel, S.L.; Seymour, E.M.; Brook, R.D.; Sheth, S.S.; Ghosh, E.; Zhu, S.; Weder, A.B.; Kovács, S.J.; Kolias, T.J. Low-Sodium DASH Diet Improves Diastolic Function and Ventricular-Arterial Coupling in Hypertensive Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2013, 6, 1165–1171. [Google Scholar] [CrossRef]
- Yi, S.-Y.; Steffen, L.M.; Haring, B.; Rebholz, C.M.; Mosley, T.H.; Shah, A.M. Associations of the Dietary Approaches to Stop Hypertension Dietary Pattern with Cardiac Structure and Function. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3345–3351. [Google Scholar] [CrossRef]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a Vegan Diet for Health Benefits and Risks—An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef]
- Thompson, A.S.; Tresserra-Rimbau, A.; Karavasiloglou, N.; Jennings, A.; Cantwell, M.; Hill, C.; Perez-Cornago, A.; Bondonno, N.P.; Murphy, N.; Rohrmann, S.; et al. Association of Healthful Plant-Based Diet Adherence with Risk of Mortality and Major Chronic Diseases Among Adults in the UK. JAMA Netw. Open 2023, 6, e234714. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between Plant-Based Dietary Patterns and Risks of Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality—A Systematic Review and Meta-Analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant Based Diet and Its Effect on Cardiovascular Disease. Int. J. Env. Res. Public Health 2023, 20, 3337. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Papier, K.; Tong, T.Y.N. Plant-Based Diets and Long-Term Health: Findings from the EPIC-Oxford Study. Proc. Nutr. Soc. 2022, 81, 190–198. [Google Scholar] [CrossRef]
- Landry, M.J.; Ward, C.P.; Cunanan, K.M.; Durand, L.R.; Perelman, D.; Robinson, J.L.; Hennings, T.; Koh, L.; Dant, C.; Zeitlin, A.; et al. Cardiometabolic Effects of Omnivorous vs Vegan Diets in Identical Twins: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2344457. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Bazzano, L.A.; He, J.; Whelton, S.P.; Fernandez, C.; Ley, S.; Qi, L.; Krousel-Wood, M.; Harlan, T.S.; Kelly, T.N. Consumption of Animal and Plant Foods and Risk of Left Ventricular Diastolic Dysfunction: The Bogalusa Heart Study. ESC Heart Fail. 2020, 7, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- Król, W.; Price, S.; Śliż, D.; Parol, D.; Konopka, M.; Mamcarz, A.; Wełnicki, M.; Braksator, W. A Vegan Athlete’s Heart-Is It Different? Morphology and Function in Echocardiography. Diagnostics 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Li, H.; Wang, Y.; Barnes, L.; Schneider, J.A.; Bennett, D.A.; Morris, M.C. Relation of DASH- and Mediterranean-like Dietary Patterns to Cognitive Decline in Older Persons. Neurology 2014, 83, 1410–1416. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.-L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.L.; Hänninen, T.; Laakso, M.P.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife Vascular Risk Factors and Late-Life Mild Cognitive Impairment: A Population-Based Study. Neurology 2001, 56, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Kåreholt, I.; Ngandu, T.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Serum Cholesterol Changes after Midlife and Late-Life Cognition: Twenty-One-Year Follow-up Study. Neurology 2007, 68, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean Diet with Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Slows Cognitive Decline with Aging. Alzheimers Dement 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Mirmiran, P.; Azizi, F. Adherence to the MIND Diet and the Risk of Cardiovascular Disease in Adults: A Cohort Study. Food Funct. 2022, 13, 1651–1658. [Google Scholar] [CrossRef]
- Walker, M.E.; O’Donnell, A.A.; Himali, J.J.; Rajendran, I.; Melo van Lent, D.; Ataklte, F.; Jacques, P.F.; Beiser, A.S.; Seshadri, S.; Vasan, R.S.; et al. Associations of the Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay Diet with Cardiac Remodelling in the Community: The Framingham Heart Study. Br. J. Nutr. 2021, 126, 1888–1896. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis of Paired-Biopsy Studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Ning, H.; Lewis, C.E.; Carr, J.J.; Rinella, M.E.; Shah, S.J.; Lima, J.A.C.; Lloyd-Jones, D.M. Longitudinal Association of Non-Alcoholic Fatty Liver Disease with Changes in Myocardial Structure and Function: The CARDIA Study. J. Am. Heart Assoc. 2020, 9, e014279. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver Clinical Practice Guidelines for the Diagnosis and Management of Metabolic Associated Fatty Liver Disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Datz, C.; Reiberger, T.; Trauner, M. Diet and Exercise in NAFLD/NASH: Beyond the Obvious. Liver Int. 2021, 41, 2249–2268. [Google Scholar] [CrossRef]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean Diet and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease-Related Risk of Cardiovascular Disease and Other Cardiac Complications. Diabetes Obes. Metab. 2022, 24 (Suppl. S2), 28–43. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña, J.D.; Arenas-de Larriva, A.P.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients 2023, 15, 1483. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, Nutrition, and Cancer Risk: What Do We Know and What Is the Way Forward? BMJ 2020, m511. [Google Scholar] [CrossRef]
- Takachi, R.; Inoue, M.; Shimazu, T.; Sasazuki, S.; Ishihara, J.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Iso, H.; Tsubono, Y.; et al. Consumption of Sodium and Salted Foods in Relation to Cancer and Cardiovascular Disease: The Japan Public Health Center-Based Prospective Study. Am. J. Clin. Nutr. 2010, 91, 456–464. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L. Soy Consumption and Prostate Cancer Risk in Men: A Revisit of a Meta-Analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Yarmand, S.; Abdollahi, N.; Nejad, E.T.; Souni, F.; Vali, M.; Nouri, M.; Shateri, Z.; Rashidkhani, B. Association between Adherence to a Dietary Approach to Stop Hypertension and the Mediterranean Diets and Risk of Colorectal Cancer: A Matched Case-Control Study. Clin. Nutr. ESPEN 2024, 60, 195–202. [Google Scholar] [CrossRef]
- González-Palacios Torres, C.; Barrios-Rodríguez, R.; Muñoz-Bravo, C.; Toledo, E.; Dierssen, T.; Jiménez-Moleón, J.J. Mediterranean Diet and Risk of Breast Cancer: An Umbrella Review. Clin. Nutr. 2023, 42, 600–608. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Asselin, C.Y.; Lam, A.; Cheung, D.Y.C.; Eekhoudt, C.R.; Zhu, A.; Mittal, I.; Mayba, A.; Solati, Z.; Edel, A.; Austria, J.A.; et al. The Cardioprotective Role of Flaxseed in the Prevention of Doxorubicin- and Trastuzumab-Mediated Cardiotoxicity in C57BL/6 Mice. J. Nutr. 2020, 150, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Rogan, K.J.; Sung, M.M.; Zordoky, B.N.; Haykowsky, M.J.; Young, M.E.; Jones, L.W.; Dyck, J.R.B. Both Aerobic Exercise and Resveratrol Supplementation Attenuate Doxorubicin-Induced Cardiac Injury in Mice. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E243–E253. [Google Scholar] [CrossRef] [PubMed]
- McNally, B.; Griffin, J.L.; Roberts, L.D. Dietary Inorganic Nitrate: From Villain to Hero in Metabolic Disease? Mol. Nutr. Food Res. 2016, 60, 67–78. [Google Scholar] [CrossRef]
- Turpin, V.-R.G.; Lovoy, G.M.; Parr, S.K.; Hammond, S.T.; Post, H.K.; Caldwell, J.T.; Banister, H.R.; Scheuermann, B.C.; Colburn, T.D.; Ade, C.J. Inorganic Nitrate Supplementation May Improve Diastolic Function and the O2 Cost of Exercise in Cancer Survivors: A Pilot Study. Support Care Cancer 2022, 31, 63. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Participants | Study Design | Dietetic Regimen | Observed Effect on Heart of Considered Diet | Strain | LVEF |
|---|---|---|---|---|---|---|---|
| Gardener, H. et al. [46] | 2015 | 1937 Adults >40 years old, without IS | cross-sectional | Mediterranean diet | lower LV mass | NE | NE |
| Bacharaki, D. et al. [47] | 2022 | 127 Adults in dialysis | cross-sectional | Mediterranean diet | lower incidence of LVH | NE | NE |
| Levitan, E.B. et al. [48] | 2016 | 4497 Adults 45–84 years old, without CVD | cross-sectional | Mediterranean diet | higher LV mass, LVEF and stroke volume | NE | Y |
| Chrysohoou, C. et al. [49] | 2012 | 372 Adults with HFrEF | cross-sectional | Mediterranean diet | better left ventricular filling pattern | NE | N |
| Nguyen, H.T. et al. [58] | 2012 | 4506 Adults 45–84 years old, without CVD | cross-sectional | DASH | better end-diastolic volume and stroke volume | NE | N |
| Hummel, S.L. et al. [59] | 2013 | 13 Adults with hypertension and HFpEF | prospective cohort | DASH | better LVEF and stroke volume | NE | Y |
| Yi, S.Y. et al. [60] | 2021 | 4651 Adults 45–64 years old | prospective cohort | DASH | higher longitudinal strain lower left ventricle mean wall thickness | Y | N |
| Razavi, A.C. et al. [68] | 2020 | 456 Adults and children with preserved EF | prospective cohort | Plant-based diet | lower risk LVDD | NE | N |
| Król, W. et al. [69] | 2020 | 22 Adult amateur runners | case-control | Vegan diet | lower LV mass, relative wall thickness and higher GLS | Y | NE |
| Walker, M.E. et al. [79] | 2021 | 2512 Adults | prospective cohort | MIND | higher LV mass but not LVH. Higher LEVF and better GLS and GCS. After adjustment only better GCS was confirmed | Y * | N * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartorio, A.; Dal Pont, C.; Romano, S. Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function. Nutrients 2024, 16, 1471. https://doi.org/10.3390/nu16101471
Sartorio A, Dal Pont C, Romano S. Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function. Nutrients. 2024; 16(10):1471. https://doi.org/10.3390/nu16101471
Chicago/Turabian StyleSartorio, Andrea, Chiara Dal Pont, and Simone Romano. 2024. "Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function" Nutrients 16, no. 10: 1471. https://doi.org/10.3390/nu16101471
APA StyleSartorio, A., Dal Pont, C., & Romano, S. (2024). Standard and New Echocardio Techniques, Such as Global Longitudinal Strain, to Monitor the Impact of Diets on Cardiovascular Diseases and Heart Function. Nutrients, 16(10), 1471. https://doi.org/10.3390/nu16101471






