Bariatric Surgery: An Opportunity to Improve Quality of Life and Healthy Habits
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Questionnaires
2.4.1. Short Form Survey-36 (SF-36)
2.4.2. Fourteen-Item Questionnaires That Assessed Adherence to a MedDiet
2.4.3. Rapid Assessment of Physical Activity (RAPA) Questionnaire
2.4.4. Beck’s Depression Inventory-II
2.5. Outcomes
2.6. Statistical Methods
3. Results
3.1. Participants Characteristics
3.2. Weight Loss Outcomes
3.3. Changes in MedDiet Adherence
3.4. Changes in Physical Activity
3.5. Changes in Depression
3.6. Changes in HRQoL
3.7. Predictive Factors for Improvement in PSC and MSC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; Regional Office for Europe. WHO European Regional Obesity: Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kyle, T.K.; Dhurandhar, E.J.; Allison, D.B. Regarding Obesity as a Disease: Evolving Policies and Their Implications. Endocrinol. Metab. Clin. N. Am. 2016, 45, 511–520. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of Overweight on the Risk of Developing Common Chronic Diseases During a 10-Year Period. Arch. Intern. Med. 2001, 161, 1581–1586. Available online: http://archinte.jamanetwork.com/ (accessed on 1 February 2024). [CrossRef]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of Hypertension, Diabetes, Dyslipidemia, and Metabolic Syndrome with Obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y.; Song, Y.S.; Hong, S.; Won, H.; Kim, W.J.; Kwon, Y.; Ha, J.; Fiedorowicz, J.G.; Solmi, M.; et al. Association of bariatric surgery with indicated and unintended outcomes: An umbrella review and meta-analysis for risk–benefit assessment. Obes. Rev. 2024, 25, e13670. [Google Scholar] [CrossRef]
- Coulman, K.D.; Blazeby, J.M. Health-Related Quality of Life in Bariatric and Metabolic Surgery. Curr. Obes. Rep. 2020, 9, 307–314. [Google Scholar] [CrossRef]
- Sierżantowicz, R.; Ładny, J.R.; Lewko, J. Quality of Life after Bariatric Surgery—A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9078. [Google Scholar] [CrossRef]
- Busutil, R.; Espallardo, O.; Torres, A.; Martínez-Galdeano, L.; Zozaya, N.; Hidalgo-Vega, Á. The impact of obesity on health-related quality of life in Spain. Health Qual. Life Outcomes 2017, 15, 197. [Google Scholar] [CrossRef]
- Fontaine, K.R.; Barofsky, I. Obesity and health-related quality of life. Obes. Rev. 2001, 2, 173–182. [Google Scholar] [CrossRef]
- Ballantyne, G.H. Measuring Outcomes following Bariatric Surgery: Weight Loss Parameters, Improvement in Co-morbid Conditions, Change in Quality of Life and Patient Satisfaction. Obes. Surg. 2003, 13, 954–964. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Wadden, T.A.; Moore, R.H.; Eisenberg, M.H.; Raper, S.E.; Williams, N.N. Changes in quality of life and body image after gastric bypass surgery. Surg. Obes. Relat. Dis. 2010, 6, 608–614. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Asokkumar, R.; Lacruz, T.; Rull, A.; Beltran, L.; Bautista-Castaño, I. The effect of weight loss and exercise on Health-Related Quality of Life (HRQOL) following Endoscopic Bariatric Therapies (EBT) for obesity. Health Qual. Life Outcomes 2020, 18, 130. [Google Scholar] [CrossRef]
- Rogers, M.; Lemstra, M. Improving health-related quality of life through an evidence-based obesity reduction program: The Healthy Weights Initiative. J. Multidiscip. Healthc. 2016, 9, 103–109. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, Obesity, and Depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Johnston, E.; Johnson, S.; McLeod, P.; Johnston, M. The Relation of Body Mass Index to Depressive Symptoms. Can. J. Public Health 2004, 95, 179–183. [Google Scholar] [CrossRef]
- Preiss, K.; Brennan, L.; Clarke, D. A systematic review of variables associated with the relationship between obesity and depression. Obes. Rev. 2013, 14, 906–918. [Google Scholar] [CrossRef]
- Dussaillant, C.; Echeverría, G.; Urquiaga, I.; Velasco, N.; Rigotti, A. Evidencia actual sobre los beneficios de la dieta mediterránea en salud. Rev. Méd. Chile 2016, 144, 1044–1052. [Google Scholar] [CrossRef]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef]
- Henríquez Sánchez, P.; Ruano, C.; de Irala, J.; Ruiz-Canela, M.; Martínez-González, M.A.; Sánchez-Villegas, A. Adherence to the Mediterranean diet and quality of life in the SUN Project. Eur. J. Clin. Nutr. 2012, 66, 360–368. [Google Scholar] [CrossRef]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vazquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. Adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 2. Propiedades psicométricas en población general. Clín. Salud 2003, 14, 249–280. [Google Scholar]
- Guirao, J.A. Elaboración y Validación de la Versión en Español Europeo de la Escala de Valoración Rápida de Actividad Física (RAPA). Ph.D. Thesis, Universidad de Alicante, Licante, Spain, 2012. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=64908 (accessed on 30 April 2024).
- Vilagut, G.; María Valderas, J.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Guallar-Castillón, P.; Garcia-Esquinas, E.; Rodríguez-Artalejo, F. Metabolically healthy obesity and health-related quality of life: A prospective cohort study. Clin. Nutr. 2017, 36, 853–860. [Google Scholar] [CrossRef]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Walwick, J.; Patrick, M.B. Peer Reviewed: The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev. Chronic Dis. 2006, 3, A118. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory: Second Edition Manual; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Friedman, M.A.; Brownell, K.D. Psychological Correlates of Obesity: Moving to the Next Research Generation. Psychol. Bull. 1995, 117, 3–20. [Google Scholar] [CrossRef]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Preoperative predictors of weight loss following bariatric surgery: Systematic review. Obes. Surg. 2012, 22, 70–89. [Google Scholar] [CrossRef]
- Müller, A.; Mitchell, J.E.; Sondag, C.; De Zwaan, M. Psychiatric aspects of bariatric surgery topical collection on eating disorders. Curr. Psychiatry Rep. 2013, 15, 397. [Google Scholar] [CrossRef]
- Barcones-Molero, M.F.; Sánchez-Villegas, A.; Martínez-González, M.A.; Bes-Rastrollo, M.; Martínez-Urbistondo, M.; Santabárbara, J.; Martínez, J.A. Influencia de la obesidad y la ganancia de peso sobre la calidad de vida según el SF-36 en individuos de la cohorte dinámica Seguimiento Universidad de Navarra. Rev. Clin. Esp. 2018, 218, 408–416. [Google Scholar] [CrossRef]
- Doll, H.A.; Petersen, S.E.K.; Stewart-Brown, S.L. Obesity and Physical and Emotional Well-Being: Associations between Body Mass Index, Chronic Illness, and the Physical and Mental Components of the SF-36 Questionnaire. Obes. Res. 2000, 8, 160–170. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Hayden, M.J.; Murphy, K.D.; Brown, W.A.; O’Brien, P.E. Axis I Disorders in Adjustable Gastric Band Patients: The Relationship Between Psychopathology and Weight Loss. Obes. Surg. 2014, 24, 1469–1475. [Google Scholar] [CrossRef]
- Hayden, M.J.; Dixon, J.B.; Dixon, M.E.; Shea, T.L.; O’Brien, P.E. Characterization of the Improvement in Depressive Symptoms Following Bariatric Surgery. Obes. Surg. 2011, 21, 328–335. [Google Scholar] [CrossRef]
- Booth, H.; Khan, O.; Prevost, A.T.; Reddy, M.; Charlton, J.; Gulliford, M.C. Impact of bariatric surgery on clinical depression. Interrupted time series study with matched controls. J. Affect. Disord. 2015, 174, 644–649. [Google Scholar] [CrossRef]
- Andersen, J.R.; Aasprang, A.; Bergsholm, P.; Sletteskog, N.; Våge, V.; Natvig, G.K. Predictors for health-related quality of life in patients accepted for bariatric surgery. Surg. Obes. Relat. Dis. 2009, 5, 329–333. [Google Scholar] [CrossRef]
- Brunault, P.; Frammery, J.; Couet, C.; Delbachian, I.; Bourbao-Tournois, C.; Objois, M.; Cosson, P.; Réveillère, C.; Ballon, N. Predictors of changes in physical, psychosocial, sexual quality of life, and comfort with food after obesity surgery: A 12-month follow-up study. Qual. Life Res. 2015, 24, 493–501. [Google Scholar] [CrossRef]
- Dawes, A.J.; Maggard-Gibbons, M.; Maher, A.R.; Booth, M.J.; Miake-Lye, I.; Beroes, J.M.; Shekelle, P.G. Mental Health Conditions Among Patients Seeking and Undergoing Bariatric Surgery. JAMA 2016, 315, 150–163. [Google Scholar] [CrossRef]
- Lier, H.Ø.; Biringer, E.; Stubhaug, B.; Tangen, T. Prevalence of psychiatric disorders before and 1 year after bariatric surgery: The role of shame in maintenance of psychiatric disorders in patients undergoing bariatric surgery. Nord. J. Psychiatry 2013, 67, 89–96. [Google Scholar] [CrossRef]
- Kolotkin, R.L.; Andersen, J.R. A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef]
- Kroes, M.; Osei-Assibey, G.; Baker-Searle, R.; Huang, J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: A systematic review. Curr. Med. Res. Opin. 2016, 32, 485–508. [Google Scholar] [CrossRef]
- Strain, G.W.; Kolotkin, R.L.; Dakin, G.F.; Gagner, M.; Inabnet, W.B.; Christos, P.; Saif, T.; Crosby, R.; Pomp, A. The effects of weight loss after bariatric surgery on health-related quality of life and depression. Nutr. Diabetes 2014, 4, e132. [Google Scholar] [CrossRef]
- Tettero, O.M.; Aronson, T.; Wolf, R.J.; Nuijten, M.A.H.; Hopman, M.T.E.; Janssen, I.M.C. Increase in Physical Activity After Bariatric Surgery Demonstrates Improvement in Weight Loss and Cardiorespiratory Fitness. Obes. Surg. 2018, 28, 3950–3957. [Google Scholar] [CrossRef]
- van Gemert, W.A.M.; van der Palen, J.; Monninkhof, E.M.; Rozeboom, A.; Peters, R.; Wittink, H.; Schuit, A.J.; Peeters, P.H. Quality of Life after Diet or Exercise-Induced Weight Loss in Overweight to Obese Postmenopausal Women: The SHAPE-2 Randomised Controlled Trial. PLoS ONE 2015, 10, e0127520. [Google Scholar] [CrossRef]
- Biter, L.U.; van Buuren, M.M.A.; Mannaerts, G.H.H.; Apers, J.A.; Dunkelgrün, M.; Vijgen, G.H.E.J. Quality of Life 1 Year After Laparoscopic Sleeve Gastrectomy Versus Laparoscopic Roux-en-Y Gastric Bypass: A Randomized Controlled Trial Focusing on Gastroesophageal Reflux Disease. Obes. Surg. 2017, 27, 2557–2565. [Google Scholar] [CrossRef]
- Kolotkin, R.L.; Crosby, R.D.; Gress, R.E.; Hunt, S.C.; Adams, T.D. Two-year changes in health-related quality of life in gastric bypass patients compared with severely obese controls. Surg. Obes. Relat. Dis. 2009, 5, 250–256. [Google Scholar] [CrossRef][Green Version]
- Albarrán-Sánchez, A.; Ramírez-Rentería, C.; Ferreira-Hermosillo, A.; Rodríguez-Pérez, V.; Espinosa-Cárdenas, E.; Molina-Ayala, M.; Boscó-Gárate, I.; Mendoza-Zubieta, V. Quality of life evaluation in Mexican patients with severe obesity before and after bariatric surgery. Gac. Med. Mex. 2023, 157, 64–69. [Google Scholar] [CrossRef]
- Canetti, L.; Bachar, E.; Bonne, O. Deterioration of mental health in bariatric surgery after 10 years despite successful weight loss. Eur. J. Clin. Nutr. 2016, 70, 17–22. [Google Scholar] [CrossRef]
- Paczkowska, A.; Hoffmann, K.; Raakow, J.; Pross, M.; Berghaus, R.; Michalak, M.; Bryl, W.; Marzec, K.; Kopciuch, D.; Zaprutko, T.; et al. Impact of bariatric surgery on depression, anxiety and stress symptoms among patients with morbid obesity: International multicentre study in Poland and Germany. BJPsych Open 2022, 8, e32. [Google Scholar] [CrossRef]
- O’Brien, P.E. Bariatric surgery: Mechanisms, indications and outcomes. J. Gastroenterol. Hepatol. 2010, 25, 1358–1365. [Google Scholar] [CrossRef]
- Andersen, J.R.; Aasprang, A.; Karlsen, T.I.; Karin Natvig, G.; Våge, V.; Kolotkin, R.L. Health-related quality of life after bariatric surgery: A systematic review of prospective long-term studies. Surg. Obes. Relat. Dis. 2015, 11, 466–473. [Google Scholar] [CrossRef]
- Hachem, A.; Brennan, L. Quality of Life Outcomes of Bariatric Surgery: A Systematic Review. Obes. Surg. 2016, 26, 395–409. [Google Scholar] [CrossRef]
- Rausa, E.; Kelly, M.E.; Galfrascoli, E.; Aiolfi, A.; Cavalcoli, F.; Turati, L.; Bonavina, L.; Sgroi, G. Quality of Life and Gastrointestinal Symptoms Following Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: A Systematic Review. Obes. Surg. 2019, 29, 1397–1402. [Google Scholar] [CrossRef]
- Duval, K.; Marceau, P.; Pérusse, L.; Lacasse, Y. An overview of obesity-specific quality of life questionnaires. Obes. Rev. 2006, 7, 347–360. [Google Scholar] [CrossRef]
- Forhan, M.; Vrkljan, B.; MacDermid, J. A systematic review of the quality of psychometric evidence supporting the use of an obesity-specific quality of life measure for use with persons who have class III obesity: Diagnostic in Obesity and Complications. Obes. Rev. 2010, 11, 222–228. [Google Scholar] [CrossRef]
- Camolas, J.; Ferreira, A.; Mannucci, E.; Mascarenhas, M.; Carvalho, M.; Moreira, P.; do Carmo, I.; Santos, O. Assessing quality of life in severe obesity: Development psychometric properties of the ORWELL-R. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2016, 21, 277–288. [Google Scholar] [CrossRef]
| Variables | Patients (n = 56) |
|---|---|
| Ages years (mean (SD) | 43.8 (13.1) |
| Age groups | |
| ≤43 years (n (%)) | 29 (51.8%) |
| >43 years (n (%)) | 27 (48.2%) |
| Female | 41 (73.2%) |
| Initial weight kg (mean (SD) | 128.9 (23.5) |
| Initial BMI kg/m2 (mean (SD) | 47.7 (6.4) |
| Initial BMI group | |
| 35–39.9 (n (%)) | 8 (14.3%) |
| ≥40 (n (%)) | 48 (85.7%) |
| Variable | Preprocedure | Postprocedure | p |
|---|---|---|---|
| MedDiet adherence group (n (%)) | |||
| Low | 32 (60.4) | 17 (32.1%) | 0.04 |
| Medium | 19 (35.8) | 32 (60.4%) | |
| High | 2 (3.8) | 4 (7.5%) | |
| Physical activity level aerobic | |||
| Inactive | 43 (79.6) | 24 (44.5) | NS |
| Active | 11 (20.4) | 30 (55.5) | |
| Physical activity level anaerobic | |||
| None | 51 (94.4) | 45 (84.9) | NS |
| Muscle strength | 3 (5.6) | 6 (11.3) | |
| Flexibility | 0 | 2 (3.8) | |
| Depression | |||
| Minimal | 36 (67.9) | 38 (71.7) | NS |
| Mild–severe | 17 (32.1) | 15 (28.3) | |
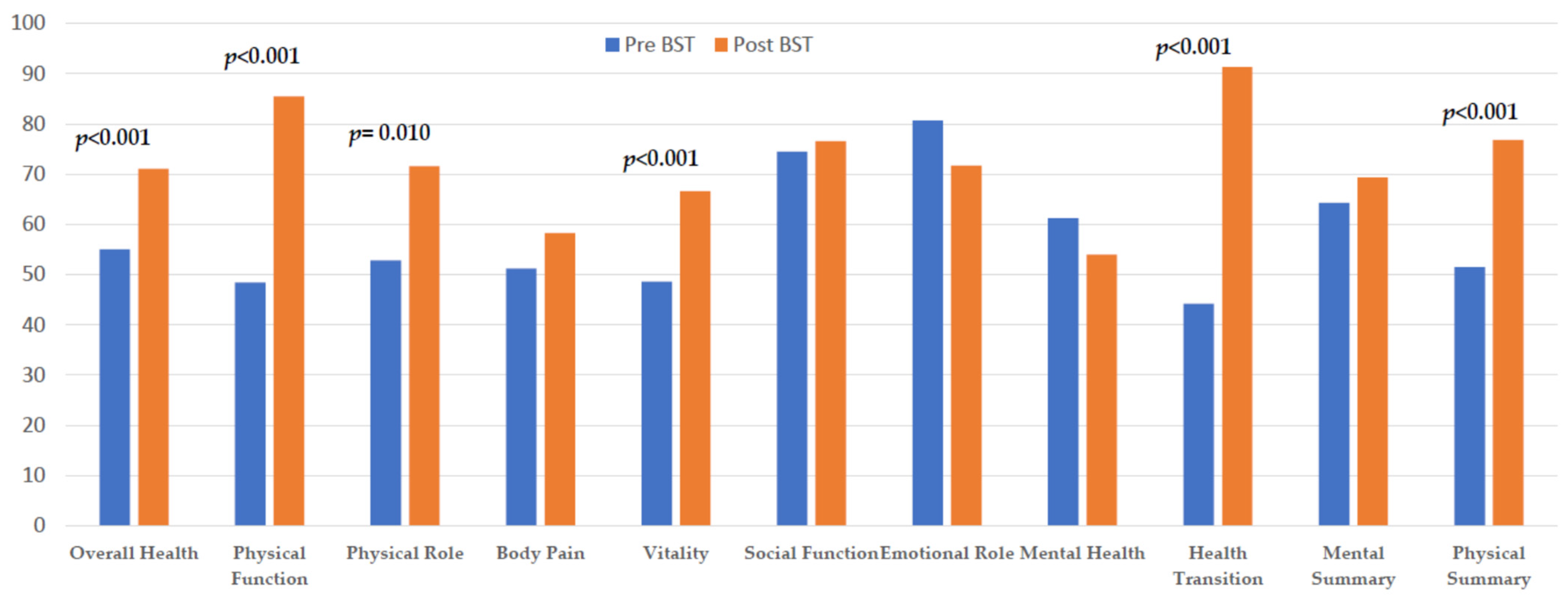
| SF-36 Domains | Spanish Population Mean | Preprocedure Mean | Postprocedure Mean | Mean Difference | p | |
|---|---|---|---|---|---|---|
| Functional Status | Overall health | 58.3 | 55.0 | 71.1 | 14.2 | <0.001 |
| Physical function | 84.7 | 48.4 | 85.5 | 37.3 | <0.001 | |
| Physical role | 83.2 | 52.8 | 71.6 | 18.6 | 0.010 | |
| Body pain | 79.0 | 51.2 | 58.3 | 7.5 | NS | |
| Emotional Status | Vitality | 66.9 | 48.6 | 66.6 | 17.3 | <0.001 |
| Social function | 90.1 | 74.5 | 76.6 | 0.98 | NS | |
| Emotional role | 86.6 | 80.7 | 71.7 | −7.1 | NS | |
| Mental health | 73.3 | 61.2 | 54.0 | −6.8 | 0.051 | |
| Health transition | --- | 44.2 | 91.3 | 52.4 | <0.001 | |
| MCS (mental component summary) | 64.3 | 69.3 | 5.8 | NS | ||
| PCS (physical component summary) | 51.5 | 76.8 | 25.1 | <0.001 | ||
| Physical Summary Component (Mean) | Mental Summary Component (Mean) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Δ Change | Baseline | Follow-Up | Δ Change | |
| Age | ||||||
| <43 years | 59.6 | 78.1 | 18.1 | 68.9 | 66 | −0.82 |
| ≥43 years | 42.7 | 75.6 | 33.1 | 59.3 | 73 | 13.8 |
| p-value | 0.010 | NS | NS | NS | NS | 0.045 |
| Gender | ||||||
| Male | 61.1 | 79.2 | 17.4 | 68 | 69.3 | 1.2 |
| Female | 48.1 | 76.1 | 27.9 | 63.1 | 69.4 | 7.6 |
| p-value | NS | NS | NS | NS | NS | NS |
| Initial BMI | ||||||
| <40 kg/m2 | 57.4 | 64.8 | 7.3 | 70.1 | 60.7 | −1.3 |
| ≥40 kg/m2 | 50.5 | 78.8 | 28.0 | 63.6 | 70.7 | 6.9 |
| p-value | NS | NS | NS | NS | NS | NS |
| %TBWL | ||||||
| ≤25 | 56.1 | 62.2 | 6.1 | 56.6 | 57.9 | 1.3 |
| 25.1–39.9 | 53.3 | 84.0 | 30.8 | 68.4 | 83.0 | 16.5 |
| ≥40 | 47.4 | 77.8 | 30.3 | 65.2 | 62.3 | −0.7 |
| p-value | NS | 0.011 | 0.020 | NS | 0.001 | NS |
| Procedure Type | ||||||
| Restrictive | 54.5 | 78.6 | 25.8 | 66.4 | 72.6 | 8.4 |
| Malabsortive | 49.7 | 73.8 | 23.8 | 60.1 | 63.4 | 1.3 |
| p-value | NS | NS | NS | NS | NS | NS |
| Mediterranean Diet Adherence n (%) | ||||||
| Low | 52.6 | 77.6 | 24.2 | 62.1 | 69.1 | 9.0 |
| Medium–High | 50.1 | 75.9 | 26.4 | 67.7 | 69.9 | 1.1 |
| p-value | NS | NS | NS | NS | NS | NS |
| Baseline RAPA Aerobic activities n (%) | ||||||
| Inactive | 48.9 | 77.4 | 28.1 | 62.7 | 71.7 | 10.2 |
| Active | 61.7 | 75.0 | 13.1 | 70.9 | 60.8 | −10.6 |
| p-value | NS | NS | NS | NS | NS | 0.019 |
| Depression (Beck) | ||||||
| None or Minimal | 58.9 | 79.5 | 20.3 | 73.7 | 75.0 | 1.35 |
| Mild–Severe | 36.9 | 70.3 | 35.0 | 45.1 | 57.0 | 15.8 |
| p-value | 0.001 | NS | NS | <0.001 | 0.041 | NS |
| Variable | Physical HRQoL (PSC Change) | 95% CI | p-Value |
|---|---|---|---|
| Coefficient β | |||
| %TBWL | 0.313 | 0.126 to 1.151 | 0.015 |
| Sex | 0.043 | −10.574 to 15.866 | 0.689 |
| Age | 0.009 | −0.565 to 0.602 | 0.949 |
| Initial PSC score | −0.697 | −1.144 to 0.447 | 0.000 |
| Initial Depression | −0.167 | −23.397 to 4267 | 0.170 |
| Variable | Mental HRQoL (MSC change) | 95% CI | p-value |
| Coefficient β | |||
| %TBWL | 0.106 | −0.359 to 0.751 | 0.480 |
| Sex | −0.043 | −17.804 to 11.985 | 0.737 |
| Age | 0.114 | −0.315 to 0.758 | 0.409 |
| Initial MSC score | −0.677 | −1.135 to −0.382 | 0.000 |
| Initial Depression | −0.191 | −29,249 to 8.566 | 0.246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-González, B.V.; Bautista-Castaño, I.; Hernández García, E.; Cornejo Torre, J.; Hernández Hernández, J.R.; Serra-Majem, L. Bariatric Surgery: An Opportunity to Improve Quality of Life and Healthy Habits. Nutrients 2024, 16, 1466. https://doi.org/10.3390/nu16101466
Díaz-González BV, Bautista-Castaño I, Hernández García E, Cornejo Torre J, Hernández Hernández JR, Serra-Majem L. Bariatric Surgery: An Opportunity to Improve Quality of Life and Healthy Habits. Nutrients. 2024; 16(10):1466. https://doi.org/10.3390/nu16101466
Chicago/Turabian StyleDíaz-González, Beatriz Vanessa, Inmaculada Bautista-Castaño, Elisabeth Hernández García, Judith Cornejo Torre, Juan Ramón Hernández Hernández, and Lluis Serra-Majem. 2024. "Bariatric Surgery: An Opportunity to Improve Quality of Life and Healthy Habits" Nutrients 16, no. 10: 1466. https://doi.org/10.3390/nu16101466
APA StyleDíaz-González, B. V., Bautista-Castaño, I., Hernández García, E., Cornejo Torre, J., Hernández Hernández, J. R., & Serra-Majem, L. (2024). Bariatric Surgery: An Opportunity to Improve Quality of Life and Healthy Habits. Nutrients, 16(10), 1466. https://doi.org/10.3390/nu16101466








