Gender Differences in the Impact of a High-Fat, High-Sugar Diet in Skeletal Muscles of Young Female and Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Body Mass Index (BMI) and Homeostasis Model Assessment of Insulin Resistance HOMA-IR (HOMA-IR) Calculation
2.3. Cryostat Sectioning
2.4. Myosin Heavy Chains Determination and cross-Sectional Area (CSA) Measurement
2.5. Periodic Acid-Schiff (PAS) Reaction for Glycogen Detection
2.6. Succinate Dehydrogenase Staining (SDH)
2.7. Sudan Black (SB) Staining
2.8. Alkaline Phosphatase Stain for Capillaries
2.9. Statistical Analyses
3. Results
3.1. Body Mass Index (BMI) and Homeostasis Model Assessment Insulin Resistance (HOMA-IR) Index Are Affected by a 20-Week HFHS Diet
3.2. Myosin Heavy Chain Expression and Cross-Sectional Area (CSA) Are Differently Altered in Female and Male Mice Fed with an HFHS Diet
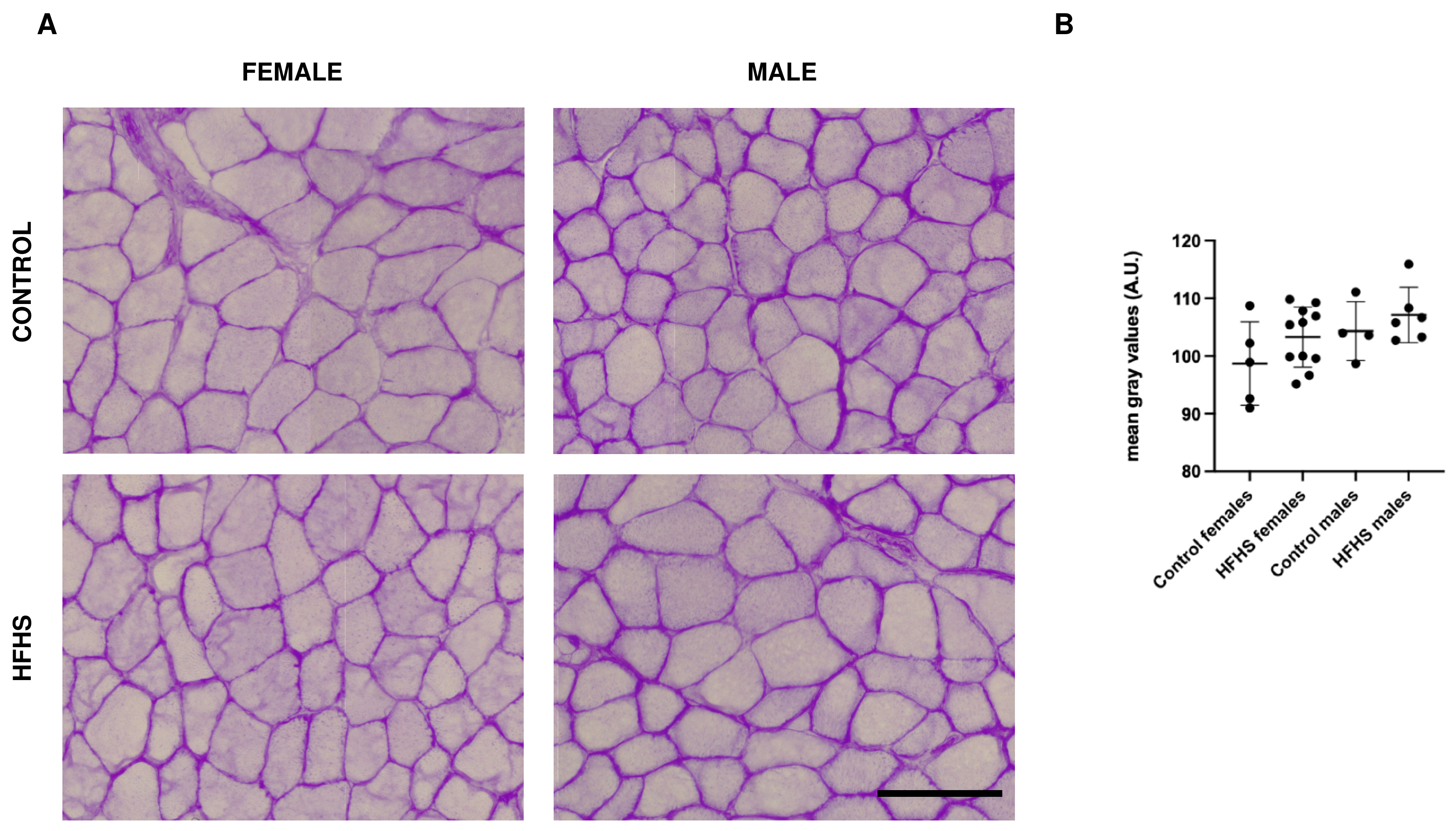
3.3. Glycogen Content Is Not Affected by HFHS Diet
3.4. Succinate Dehydrogenase (SDH) Assay Reveals an Increase in Mitochondrial Activity in HFHS-Fed Males
3.5. Sudan Black Stain Reveals an Increase in Intramyocellular Lipids (IMCLs) in HFHS-Fed Males
3.6. Skeletal Muscle Capillarization Is Altered in HFHS-Fed Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales Camacho, W.J.; Molina Díaz, J.M.; Plata Ortiz, S.; Plata Ortiz, J.E.; Morales Camacho, M.A.; Calderón, B.P. Childhood Obesity: Aetiology, Comorbidities, and Treatment. Diabetes/Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Atkins, J.L. Muscle Loss and Obesity: The Health Implications of Sarcopenia and Sarcopenic Obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic Obesity—Definition, Etiology and Consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of Sarcopenia and the Relationship with Fat Mass: Descriptive Review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef] [PubMed]
- Sack, C.; Ferrari, N.; Friesen, D.; Haas, F.; Klaudius, M.; Schmidt, L.; Torbahn, G.; Wulff, H.; Joisten, C. Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne). J. Clin. Med. 2022, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Zembura, M.; Czepczor-Bernat, K.; Dolibog, P.; Dolibog, P.T.; Matusik, P. Skeletal Muscle Mass, Muscle Strength, and Physical Performance in Children and Adolescents with Obesity. Front. Endocrinol. 2023, 14, 1252853. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolf, D. Skeletal Muscle Lipid Accumulation in Obesity, Insulin Resistance, and Type 2 Diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, Obesity, and Sex Effects on Insulin Sensitivity and Skeletal Muscle Mitochondrial Function. Diabetes 2009, 59, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E. Gender Differences in Fat Metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex Differences in Human Adipose Tissues—The Biology of Pear Shape. Biol. Sex. Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Clegg, D.J. Sex Differences in the Regulation of Body Weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.; Lees, B.; Stevenson, J. Sex- and Menopause-Associated Changes in Body-Fat Distribution. Am. J. Clin. Nutr. 1992, 55, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Tiidus, P.M. The Influence of Estrogen on Skeletal Muscle. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pataky, M.W.; Dasari, S.; Michie, K.L.; Sevits, K.J.; Kumar, A.A.; Klaus, K.A.; Heppelmann, C.J.; Robinson, M.M.; Carter, R.E.; Lanza, I.R.; et al. Impact of Biological Sex and Sex Hormones on Molecular Signatures of Skeletal Muscle at Rest and in Response to Distinct Exercise Training Modes. Cell Metab. 2023, 35, 1996–2010.e6. [Google Scholar] [CrossRef]
- Campbell, M.D.; Djukovic, D.; Raftery, D.; Marcinek, D.J. Age-Related Changes of Skeletal Muscle Metabolic Response to Contraction Are Also Sex-Dependent. J. Physiol. 2023; early view. [Google Scholar] [CrossRef]
- Bray, G.A. Medical Consequences of Obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Dyerberg, J.; Selleck, M.; Stender, S. Nutrition Transition and Its Relationship to the Development of Obesity and Related Chronic Diseases. Obes. Rev. 2008, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western Dietary Pattern Is Prospectively Associated with Cardio-Metabolic Traits and Incidence of the Metabolic Syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.d.F.; Andrade, J.M.O.; Almenara, C.C.P.; Broseguini-Filho, G.B.; Mill, J.G.; Baldo, M.P. High Fructose Intake and the Route towards Cardiometabolic Diseases. Life Sci. 2020, 259, 118235. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Rosso, N.; Dal Ben, M.; Raseni, A.; Boschelle, M.; Degrassi, C.; Nemeckova, I.; Nachtigal, P.; Avellini, C.; Tiribelli, C.; et al. An Animal Model for the Juvenile Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. PLoS ONE 2016, 11, e0158817. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Kirby, M.; Xanthakos, S.A.; Softic, S.; Feldstein, A.E.; Saxena, V.; Tang, P.H.; Miles, L.; Miles, M.V.; Balistreri, W.F.; et al. High-Fructose Medium-Chain-Trans-Fat Diet Induces Liver Fibrosis & Elevates Plasma Coenzyme Q9 in a Novel Murine Model of Obesity and NASH. Hepatology 2010, 52, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Peelor, R.; Miller, B.F.; Shankar, K.; Rudolph, M.C.; Reusch, J.E.B.; Scalzo, R.L. Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats. Nutrients 2023, 15, 4438. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bravo, M.; Hart, D.A.; Reimer, R.A.; Herzog, W. Alterations in Skeletal Muscle Morphology and Mechanics in Juvenile Male Sprague Dawley Rats Exposed to a High-Fat High-Sucrose Diet. Sci. Rep. 2023, 13, 12013. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Waters-Banker, C.; Sibole, S.C.; Herzog, W. High-Fat High-Sucrose Diet Leads to Dynamic Structural and Inflammatory Alterations in the Rat Vastus Lateralis Muscle. J. Orthop. Res. 2016, 34, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Crea, E.; Torelli, L.; Bergamo, A.; Reggiani, C.; Sava, G.; Toniolo, L. Age Dependent Modification of the Metabolic Profile of the Tibialis Anterior Muscle Fibers in C57BL/6J Mice. Int. J. Mol. Sci. 2020, 21, 3923. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Lefaucheur, L.; Čandek, M. Comparative Study of Two Classifications of Muscle Fibres: Consequences for the Photometric Determination of Glycogen According to Fibre Type in Red and White Muscle of the Pig. Meat Sci. 1995, 41, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Toniolo, L.; Crea, E.; Giacomello, E. A Short-Term Treatment with Resveratrol Improves the Inflammatory Conditions of Middle-Aged Mice Skeletal Muscles. Int. J. Food Sci. Nutr. 2022, 73, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Formoso, L.; Torelli, L.; Crea, E.; Bergamo, A.; Sava, G.; Giacomello, E. Long-Term Resveratrol Treatment Improves the Capillarization in the Skeletal Muscles of Ageing C57BL/6J Mice. Int. J. Food Sci. Nutr. 2020, 72, 37–44. [Google Scholar] [CrossRef]
- DeNies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-Induced Obesity Alters Skeletal Muscle Fiber Types of Male but Not Female Mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef] [PubMed]
- Messa, G.A.M.; Piasecki, M.; Hurst, J.; Hill, C.; Tallis, J.; Degens, H. The Impact of a High-Fat Diet in Mice Is Dependent on Duration and Age, and Differs between Muscles. J. Exp. Biol. 2020, 223, jeb217117. [Google Scholar] [CrossRef] [PubMed]
- Kho, A.T.; Kang, P.B.; Kohane, I.S.; Kunkel, L.M. Transcriptome-Scale Similarities between Mouse and Human Skeletal Muscles with Normal and Myopathic Phenotypes. BMC Musculoskelet. Disord. 2006, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human Skeletal Muscle ®bres: Molecular and Functional Diversity. Mol. Biol. 2000, 73, 195–262. [Google Scholar]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms Modulating Skeletal Muscle Phenotype. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1645–1687. ISBN 978-0-470-65071-4. [Google Scholar]
- Bloemberg, D.; Quadrilatero, J. Rapid Determination of Myosin Heavy Chain Expression in Rat, Mouse, and Human Skeletal Muscle Using Multicolor Immunofluorescence Analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Kammoun, M.; Cassar-Malek, I.; Meunier, B.; Picard, B. A Simplified Immunohistochemical Classification of Skeletal Muscle Fibres in Mouse. Eur. J. Histochem. 2014, 58, 2254. [Google Scholar] [CrossRef]
- Giacomello, E.; Toniolo, L. Editorial: The Fiber Profile of Skeletal Muscles as a Fingerprint of Muscle Quality. Front. Physiol. 2022, 13, 1105252. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Mandarino, L.J. Fuel Selection in Human Skeletal Muscle in Insulin Resistance: A Reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. Ser. A 2016, 71, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Seebacher, F. The Effects of Obesity on Skeletal Muscle Contractile Function. J. Exp. Biol. 2018, 221, jeb163840. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, J.; Neumann-Haefelin, C.; Belz, U.; Kramer, W.; Juretschke, H.-P.; Herling, A.W. Correlation between Insulin Resistance and Intramyocellular Lipid Levels in Rats. Magn. Reson. Med. 2005, 53, 1275–1282. [Google Scholar] [CrossRef]
- Randle, P.J. Regulatory Interactions between Lipids and Carbohydrates: The Glucose Fatty Acid Cycle after 35 Years. Diabetes/Metab. Rev. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C. Signalling Aspects of Insulin Resistance in Skeletal Muscle: Mechanisms Induced by Lipid Oversupply. Cell. Signal. 2000, 12, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Moriggi, M.; Belloli, S.; Barbacini, P.; Murtaj, V.; Torretta, E.; Chaabane, L.; Canu, T.; Penati, S.; Malosio, M.L.; Esposito, A.; et al. Skeletal Muscle Proteomic Profile Revealed Gender-Related Metabolic Responses in a Diet-Induced Obesity Animal Model. Int. J. Mol. Sci. 2021, 22, 4680. [Google Scholar] [CrossRef] [PubMed]
- Paavonsalo, S.; Hariharan, S.; Lackman, M.H.; Karaman, S. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells 2020, 9, 2683. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; DeRosa, G.; Gennaro, F.D.; Forcada, P. Angiotensin-Converting Enzyme Inhibition and Angiogenesis in Myocardium of Obese Zucker Rats. Am. J. Hypertens. 2004, 17, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.L.P.; Fernandes, T.; Soci, U.P.R.; Silveira, A.C.; Barretti, D.L.M.; Negrão, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxidative Med. Cell. Longev. 2017, 2017, e2415246. [Google Scholar] [CrossRef] [PubMed]
- Huxley, V.H.; Kemp, S.S. Sex-Specific Characteristics of the Microcirculation. Adv. Exp. Med. Biol. 2018, 1065, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Behnke, B.J.; Prisby, R.D.; Lesniewski, L.A.; Donato, A.J.; Olin, H.M.; Delp, M.D. Influence of Ageing and Physical Activity on Vascular Morphology in Rat Skeletal Muscle. J. Physiol. 2006, 575, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Muller-Delp, J. Microvascular Adaptations to Muscle Stretch: Findings from Animals and the Elderly. Front. Physiol. 2022, 13, 939459. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Whitelaw, M.L.; Peet, D.J. The Hypoxia-Inducible Factors: Key Transcriptional Regulators of Hypoxic Responses. CMLS Cell. Mol. Life Sci. 2003, 60, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Fanò, G.; Mecocci, P.; Vecchiet, J.; Belia, S.; Fulle, S.; Polidori, M.C.; Felzani, G.; Senin, U.; Vecchiet, L.; Beal, M.F. Age and Sex Influence on Oxidative Damage and Functional Status in Human Skeletal Muscle. J. Muscle Res. Cell Motil. 2001, 22, 345–351. [Google Scholar] [CrossRef]
- Roberts, B.M.; Lavin, K.M.; Many, G.M.; Thalacker-Mercer, A.; Merritt, E.K.; Bickel, C.S.; Mayhew, D.L.; Tuggle, S.C.; Cross, J.M.; Kosek, D.J.; et al. Human Neuromuscular Aging: Sex Differences Revealed at the Myocellular Level. Exp. Gerontol. 2018, 106, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Doyel, R.; Widule, C.; Hunter, S.K. Sex Differences with Aging in the Fatigability of Dynamic Contractions. Exp. Gerontol. 2015, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Belin de Chantemèle, E.J. Sex Hormones, Aging and Cardiometabolic Syndrome. Biol. Sex. Differ. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Stanko, P.; Berkowitz, L.N.; Parnell, N.; Zuppe, A.; Bale, T.L.; Ziolek, T.; Epperson, C.N. Inclusion of Sex and Gender in Biomedical Research: Survey of Clinical Research Proposed at the University of Pennsylvania. Biol. Sex. Differ. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]




| Control Females (n = 5) | HFHS Females (n = 11) | Control Males (n = 5) | HFHS Males (n = 6) | |
|---|---|---|---|---|
| Body weight (mean ± standard deviation) | 25.78 ± 1.475 | 34.82 ± 3.844 *** | 31.43 ± 1.279 **** | 48.06 ± 3.005 |
| Body length (mean ± standard deviation) | 8.72 ± 0.1789 | 9.145 ± 0.2296 ** | 9.18 ± 0.2049 | 9.5 ± 0.2757 |
| BMI (mean ± standard deviation) | 34 ± 1 | 41.64 ± 4456 ** | 37.60 ± 1.817 | 50 ± 3.619 *** |
| Fasting glucose (mean ± standard deviation) | 281.6 ± 33.23 | 293 ± 59.12 | 255.8 ± 38.28 | 390.3 ± 71.74 ** |
| Fasting insulin (mean ± standard deviation) | 1.150 ± 0.1957 | 2.356 ± 0.5885 *** | 1.524 ± 0.2737 | 2.716 ± 1.344 |
| HOMA-IR (mean ± standard deviation) | 0.8120 ± 0.2239 | 1.7 ± 0.5287 ** | 0.9780 ± 0.2905 | 3.613 ± 2.616 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toniolo, L.; Gazzin, S.; Rosso, N.; Giraudi, P.; Bonazza, D.; Concato, M.; Zanconati, F.; Tiribelli, C.; Giacomello, E. Gender Differences in the Impact of a High-Fat, High-Sugar Diet in Skeletal Muscles of Young Female and Male Mice. Nutrients 2024, 16, 1467. https://doi.org/10.3390/nu16101467
Toniolo L, Gazzin S, Rosso N, Giraudi P, Bonazza D, Concato M, Zanconati F, Tiribelli C, Giacomello E. Gender Differences in the Impact of a High-Fat, High-Sugar Diet in Skeletal Muscles of Young Female and Male Mice. Nutrients. 2024; 16(10):1467. https://doi.org/10.3390/nu16101467
Chicago/Turabian StyleToniolo, Luana, Silvia Gazzin, Natalia Rosso, Pablo Giraudi, Deborah Bonazza, Monica Concato, Fabrizio Zanconati, Claudio Tiribelli, and Emiliana Giacomello. 2024. "Gender Differences in the Impact of a High-Fat, High-Sugar Diet in Skeletal Muscles of Young Female and Male Mice" Nutrients 16, no. 10: 1467. https://doi.org/10.3390/nu16101467
APA StyleToniolo, L., Gazzin, S., Rosso, N., Giraudi, P., Bonazza, D., Concato, M., Zanconati, F., Tiribelli, C., & Giacomello, E. (2024). Gender Differences in the Impact of a High-Fat, High-Sugar Diet in Skeletal Muscles of Young Female and Male Mice. Nutrients, 16(10), 1467. https://doi.org/10.3390/nu16101467







