Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Design
- Age between 40 and 75;
- LDL-C level between 70 and 190 mg/dL, confirmed in at least two sequential checks conducted within the last six months prior to signing the consent form.
- Personal history of cardiovascular disease or high risk (≥20%);
- Triglycerides (TG) ≥ 400 mg/dL;
- Body Mass Index ≥ 35 kg/m2;
- Assumption of lipid-lowering drugs or supplements affecting lipid metabolism within the last three months;
- Diabetes mellitus;
- Known severe or uncontrolled thyroid, liver, renal, or muscle diseases.
2.2. Study Endpoints
2.3. Sample Size Calculation and Statistical Power
2.4. Statistical Analyses
3. Results
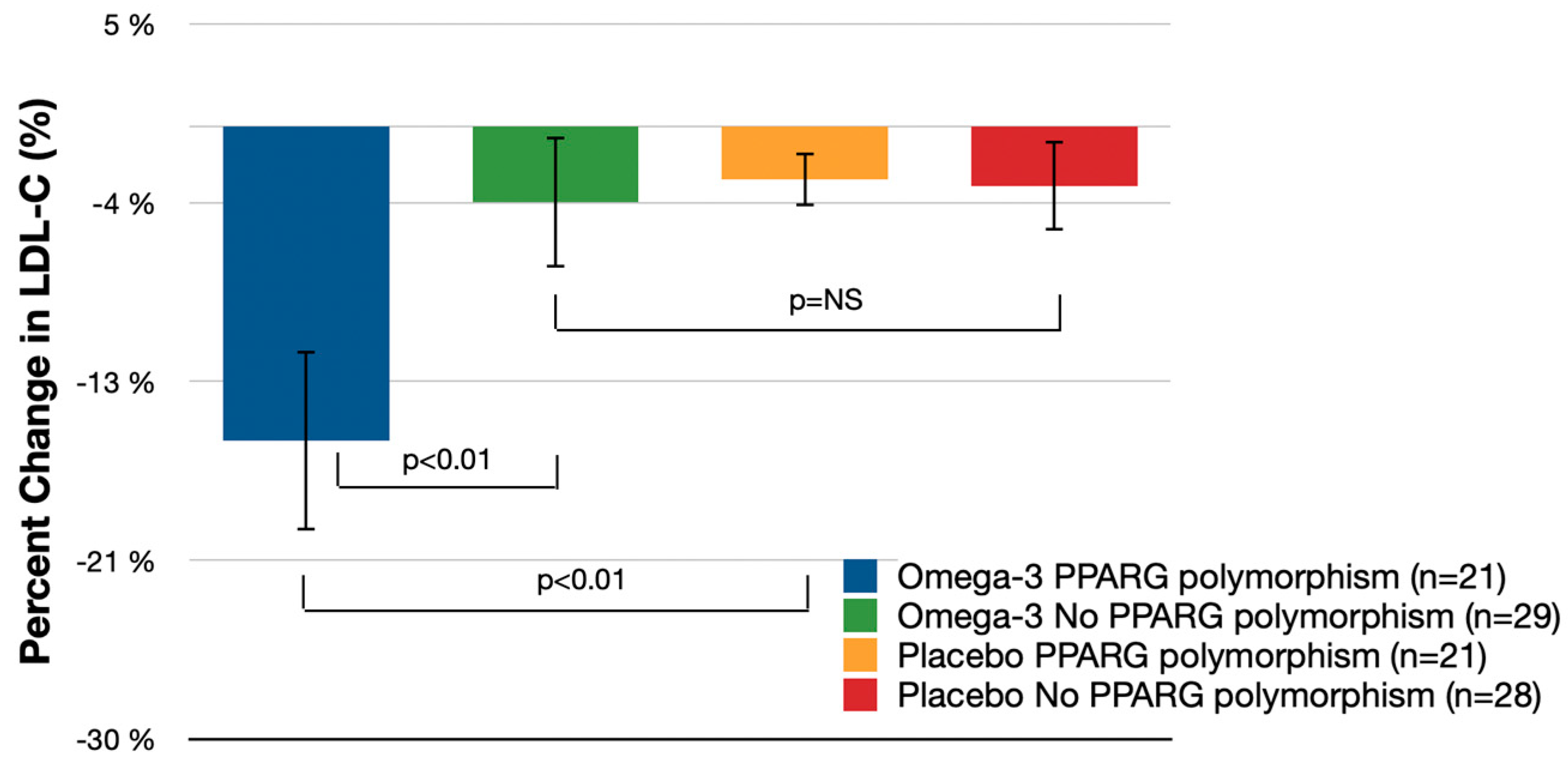
3.1. Primary Endpoint
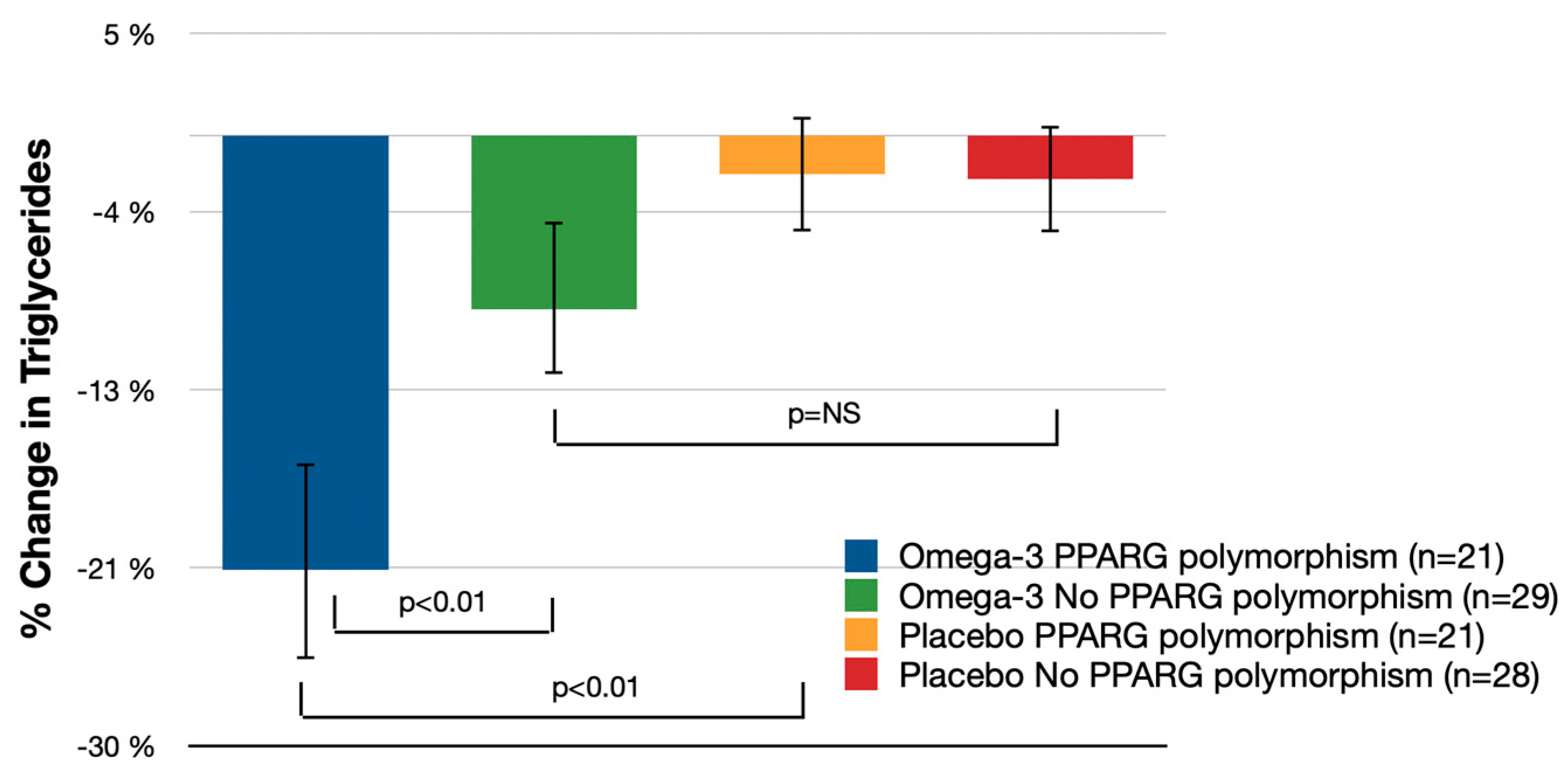
3.2. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019, Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, S.; He, C. PPARγ Gene Polymorphisms, Metabolic Disorders, and Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 9, 808929. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Von Schacky, C.; Harris, W.S. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc. Res. 2007, 73, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Sarkar, S.; Zaffar, D.; Dandona, P.; Kalyani, R.R. Omega-3 Fatty Acids in Cardiovascular Disease and Diabetes: A Review of Recent Evidence. Curr. Cardiol. Rep. 2023, 25, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Darestani, N.; Bahrami, A.; Mozafarian, M.R.; Esmalian Afyouni, N.; Akhavanfar, R.; Abouali, R.; Moradian, A.; Lorase, S. Association of Polyunsaturated Fatty Acid Intake on Inflammatory Gene Expression and Multiple Sclerosis: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4627. [Google Scholar] [CrossRef]
- Chaddha, A.; Eagle, K.A. Omega-3 Fatty Acids and Heart Health. Circulation 2015, 132, e350–e352. [Google Scholar] [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 69. [Google Scholar] [CrossRef]
- Li, S.; He, C.; Nie, H.; Pang, Q.; Wang, R.; Zeng, Z.; Song, Y. G Allele of the rs1801282 Polymorphism in PPARγ Gene Confers an Increased Risk of Obesity and Hypercholesterolemia, While T Allele of the rs3856806 Polymorphism Displays a Protective Role Against Dyslipidemia: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 919087. [Google Scholar] [CrossRef]
- Gu, S.J.; Guo, Z.R.; Zhou, Z.Y.; Hu, X.S.; Wu, M. PPAR α and PPAR γ Polymorphisms as Risk Factors for Dyslipidemia in a Chinese Han Population. Lipids Health Dis. 2014, 13, 23. [Google Scholar] [CrossRef]
- Deeb, S.S.; Fajas, L.; Nemoto, M.; Pihlajamäki, J.; Mykkänen, L.; Kuusisto, J.; Laakso, M.; Fujimoto, W.; Auwerx, J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat. Genet. 1998, 20, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Law, R.E. PPARgamma-mediated insulin sensitization: The importance of fat versus muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E287–E291. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Auwerx, J. PPAR(gamma) and glucose homeostasis. Annu. Rev. Nutr. 2002, 22, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARγ and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, Y. Role of Peroxisome Proliferator-Activated Receptor-γ in the Glucose-Sensing Apparatus of Liver and β-Cells. Diabetes 2004, 53 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef]
- Tavares, V.; Hirata, R.D.; Rodrigues, A.C.; Monte, O.; Salles, J.E.; Scalissi, N.; Speranza, A.C.; Hirata, M.H. Association between Pro12Ala polymorphism of the PPAR-gamma2 gene and insulin sensitivity in Brazilian patients with type-2 diabetes mellitus. Diabetes Obes. Metab. 2005, 7, 605–611. [Google Scholar] [CrossRef]
- Song, Y.; Raheel, T.M.; Jia, A.; Dai, G.; Liu, L.; Long, X.; He, C. rs10865710 polymorphism in PPARG promoter is associated with the severity of type 2 diabetes mellitus and coronary artery disease in a Chinese population. Postgrad. Med. J. 2022, 98, 778–787. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Allayee, H.; Gerszten, R.E.; Ideraabdullah, F.; Kris-Etherton, P.M.; Ordovás, J.M.; Rimm, E.B.; Wang, T.J.; Bennett, B.J.; American Heart Association Council on Functional Genomics and Translational Biology; et al. Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2016, 9, 291–313. [Google Scholar] [CrossRef]
- Haga, S.B.; Burke, W. Pharmacogenetic testing: Not as simple as it seems. Genet. Med. 2008, 10, 391–395. [Google Scholar] [CrossRef]
- Eslick, G.D.; Howe, P.R.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Stein, E.A.; Bays, H.E.; Maki, K.C.; Doyle, R.T.; Shalwitz, R.A.; Ballantyne, C.M.; Ginsberg, H.N.; COMBination of Prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2007, 29, 1354–1367. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Glaser, R.; Christian, L.M. Omega-3 fatty acids and stress-induced immune dysregulation: Implications for wound healing. Mil. Med. 2014, 179 (Suppl. S11), 129–133. [Google Scholar] [CrossRef] [PubMed]
- Mahdieh, N.; Heshmatzad, K.; Rabbani, B. A systematic review of LDLR, PCSK9, and APOB variants in Asia. Atherosclerosis 2020, 305, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Weng, R.; Gu, X.; Zhong, Z. Apolipoprotein E gene polymorphism and the risk of cardiovascular disease and type 2 diabetes. BMC Cardiovasc. Disord. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Aboulaghras, S.; Aanniz, T.; Benali, T.; El Omari, N.; El-Shazly, M.; Lee, L.H.; Mustafa, S.K.; Sahib, N.; Rebezov, M.; et al. Effects of Mediterranean diets and nutrigenomics on cardiovascular health. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Tikkanen, E.; Gustafsson, S.; Ingelsson, E. Associations of Fitness, Physical Activity, Strength, and Genetic Risk With Cardiovascular Disease: Longitudinal Analyses in the UK Biobank Study. Circulation 2018, 137, 2583–2591. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.; Home, P. Carotid intima-media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab. Syndr. Obes. 2011, 4, 23–34. [Google Scholar] [CrossRef]
- Hulthe, J.; Wikstrand, J.; Fagerberg, B. Relationship between C-reactive protein and intima-media thickness in the carotid and femoral arteries and to antibodies against oxidized low-density lipoprotein in healthy men: The Atherosclerosis and Insulin Resistance (AIR) study. Clin. Sci. 2001, 100, 371–378. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R.; et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef]
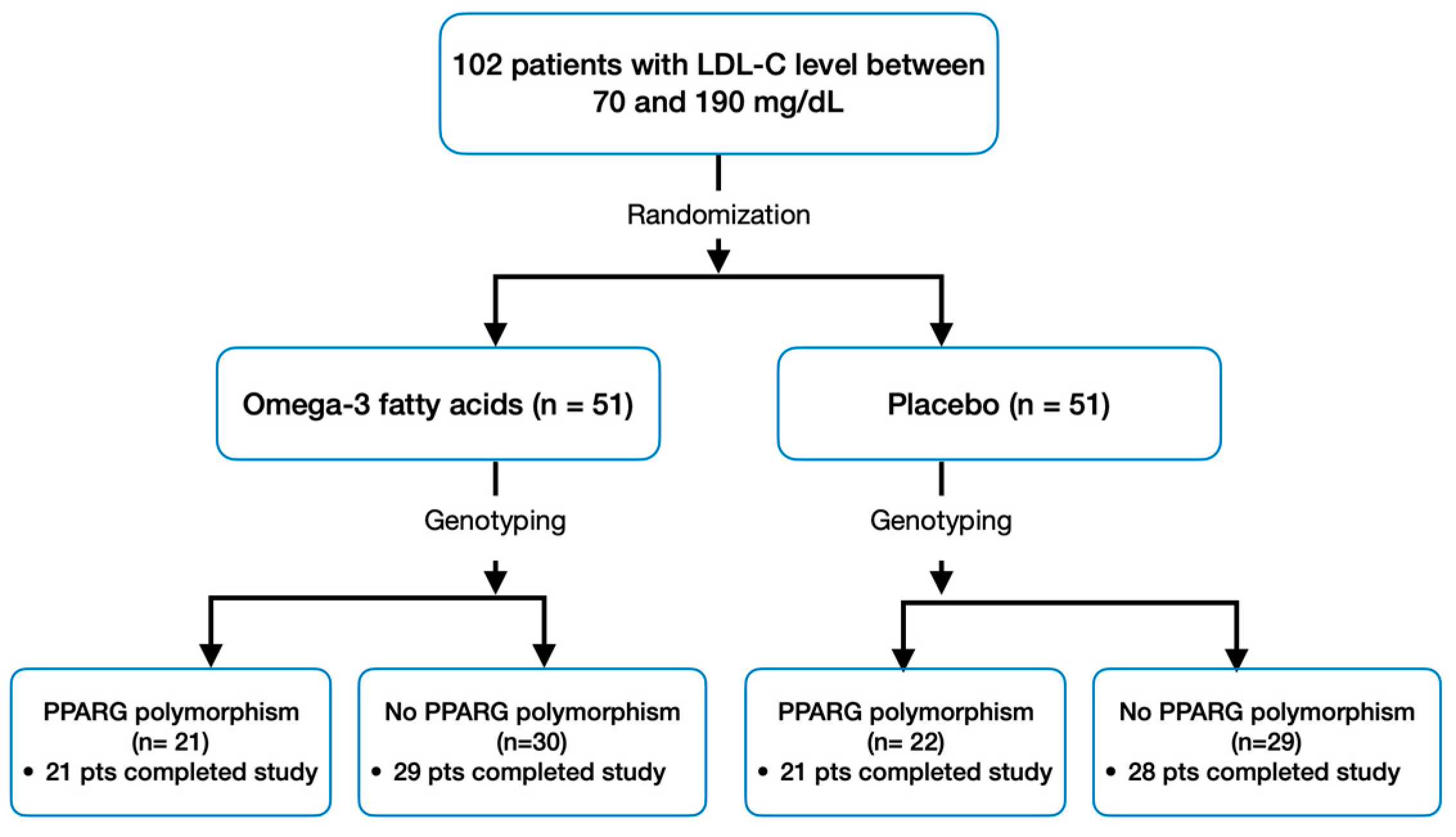
| Omega-3 Fatty Acids (n = 50) | p-Value | Placebo (n = 49) | p-Value | |||
|---|---|---|---|---|---|---|
| PPARG Polymorphism (n = 21) | No PPARG Polymorphism (n = 29) | PPARG Polymorphism (n = 21) | No PPARG Polymorphism (n = 28) | |||
| Age, y | 62.7 ± 6.0 | 63.5 ± 10.1 | p = 0.76 | 60.8 ± 10.4 | 59.5 ± 10.2 | p = 0.69 |
| Women, % | 57.1 | 55.2 | p = 0.71 | 54.5 | 60.7 | p = 0.28 |
| Body mass index, kg/m2 | 28.5 ± 3.2 | 27.9 ± 3.0 | p = 0.68 | 28.7 ± 3.4 | 27.8 ± 2.9 | p = 0.70 |
| 10-y risk ASCVD risk, % | 7.9 | 7.5 | p = 0.75 | 8.2 | 7.3 | p = 0.61 |
| Total cholesterol, mg/dL | 215 ± 25 | 200 ± 22 | p = 0.05 | 220 ± 28 | 198 ± 20 | p = 0.03 |
| LDL-C, mg/dL | 135.3 ± 23.2 | 123.7 ± 19.3 | p = 0.04 | 139.2 ± 27.1 | 119.9 ± 17.8 | p = 0.02 |
| HDL-C, mg/dL | 52.4 ± 13.5 | 61.9 ± 16.6 | p = 0.03 | 51.5 ± 11.6 | 59.2 ± 15.1 | p = 0.05 |
| Triglycerides, mg/dL | 160 ± 35 | 140 ± 25 | p = 0.03 | 165 ± 40 | 135 ± 20 | p = 0.02 |
| hsCRP, mg/L | 2.5 ± 1.7 | 1.9 ± 1.3 | p = 0.20 | 2.6 ± 1.8 | 1.8 ± 1.2 | p = 0.19 |
| Omega-3 Fatty Acids (n = 50) (% Change from Baseline (95% CI)) | Omega-3 Fatty Acids PPARG Polymorphism vs. Omega-3 Fatty Acids No PPARG Polymorphism | Placebo (n = 49) (% Change From Baseline (95% CI)) | Omega-3 Fatty Acids PPARG Polymorphism vs. Placebo PPARG Polymorphism | Omega-3 Fatty Acids No PPARG Polymorphism vs. Placebo No PPARG Polymorphism | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPARG Polymorphism (n = 21) | No PPARG Polymorphism (n = 29) | % Difference (95% CI) | p-Value | PPARG Polymorphism (n = 21) | No PPARG Polymorphism (n = 28) | % Difference (95% CI) | p-Value | % Difference (95% CI) | p-Value | |
| LDL-C | −15.4% (−19.8%, −11.0%) | −3.7% (−6.9%, −0.6%) | −11.6% (−19.3%, −4.0%) | p < 0.01 | −2.6% (−4.1%, −1.1%) | −2.9% (−5.1%, −0.8%) | −12.8% (−21.7%, −3.9%) | p < 0.01 | 0.2% (−5.0%, 5.4%) | p = 0.28 |
| Total cholesterol | −6.2% (−10.9%, −1.5%) | −1.4% (−3.4%, 0.7%) | −4.9% (−8.6%, −1.1%) | p = 0.23 | −1.4% (−3.3%, 0.4%) | −1.5% (−3.8%, 0.8%) | −4.8% (−13.6%, 4.1%) | p = 0.29 | 0.13% (−5.06%, 5.32%) | p = 0.61 |
| HDL-C | 6.7% (2.1%, 11.3%) | 4.4% (0.1%, 8.6%) | 2.3% (−3.8%, 8.4%) | p = 0.21 | 3.23% (−1.3%, 7.3%) | 5.0% (0.6%, 9.5%) | 3.4% (−5.4%, 12.3%) | p = 0.18 | −0.7% (−5.9%, 4.5%) | p = 0.14 |
| Triglycerides | −21.3% (−26.5%, −16.2%) | −8.5% (−12.8%, −4.3%) | −12.8% (−22.2%, 3.4%) | p < 0.01 | −1.9% (−4.7%, 0.9%) | −2.1% (−4.8%, 0.5%) | −13.4% (−22.1%, −4.8%) | p < 0.01 | −7.4% (−14.0%, −0.8%) | p = 0.21 |
| hsCRP | −5.8% (−32.5%, 20.9%) | −2.1%(−24.1%, 19.9%) | −3.7% (−8.4%, 1.0%) | p = 0.56 | −3.9%(−21.6%, 13.8%) | −6.1% (−23.9%, 11.7%) | −1.9% (−46.3%, 42.5%) | p = 0.82 | −4.0% (−32.3%, 24.3%) | p = 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokushalov, E.; Ponomarenko, A.; Bayramova, S.; Garcia, C.; Pak, I.; Shrainer, E.; Voronina, E.; Sokolova, E.; Johnson, M.; Miller, R. Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 97. https://doi.org/10.3390/nu16010097
Pokushalov E, Ponomarenko A, Bayramova S, Garcia C, Pak I, Shrainer E, Voronina E, Sokolova E, Johnson M, Miller R. Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(1):97. https://doi.org/10.3390/nu16010097
Chicago/Turabian StylePokushalov, Evgeny, Andrey Ponomarenko, Sevda Bayramova, Claire Garcia, Inessa Pak, Evgenya Shrainer, Elena Voronina, Ekaterina Sokolova, Michael Johnson, and Richard Miller. 2024. "Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 1: 97. https://doi.org/10.3390/nu16010097
APA StylePokushalov, E., Ponomarenko, A., Bayramova, S., Garcia, C., Pak, I., Shrainer, E., Voronina, E., Sokolova, E., Johnson, M., & Miller, R. (2024). Evaluating the Impact of Omega-3 Fatty Acid (SolowaysTM) Supplementation on Lipid Profiles in Adults with PPARG Polymorphisms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(1), 97. https://doi.org/10.3390/nu16010097






