Diet Quality and Mortality among Chinese Adults: Findings from the China Health and Nutrition Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Assessment of Dietary Intake
2.3. Calculation of Scores of Three Dietary Indices
2.4. Ascertainment of Deaths
2.5. Assessment of Covariates
2.6. Statistical Analysis
3. Results
3.1. Associations between Dietary Indices and All-Cause Mortality
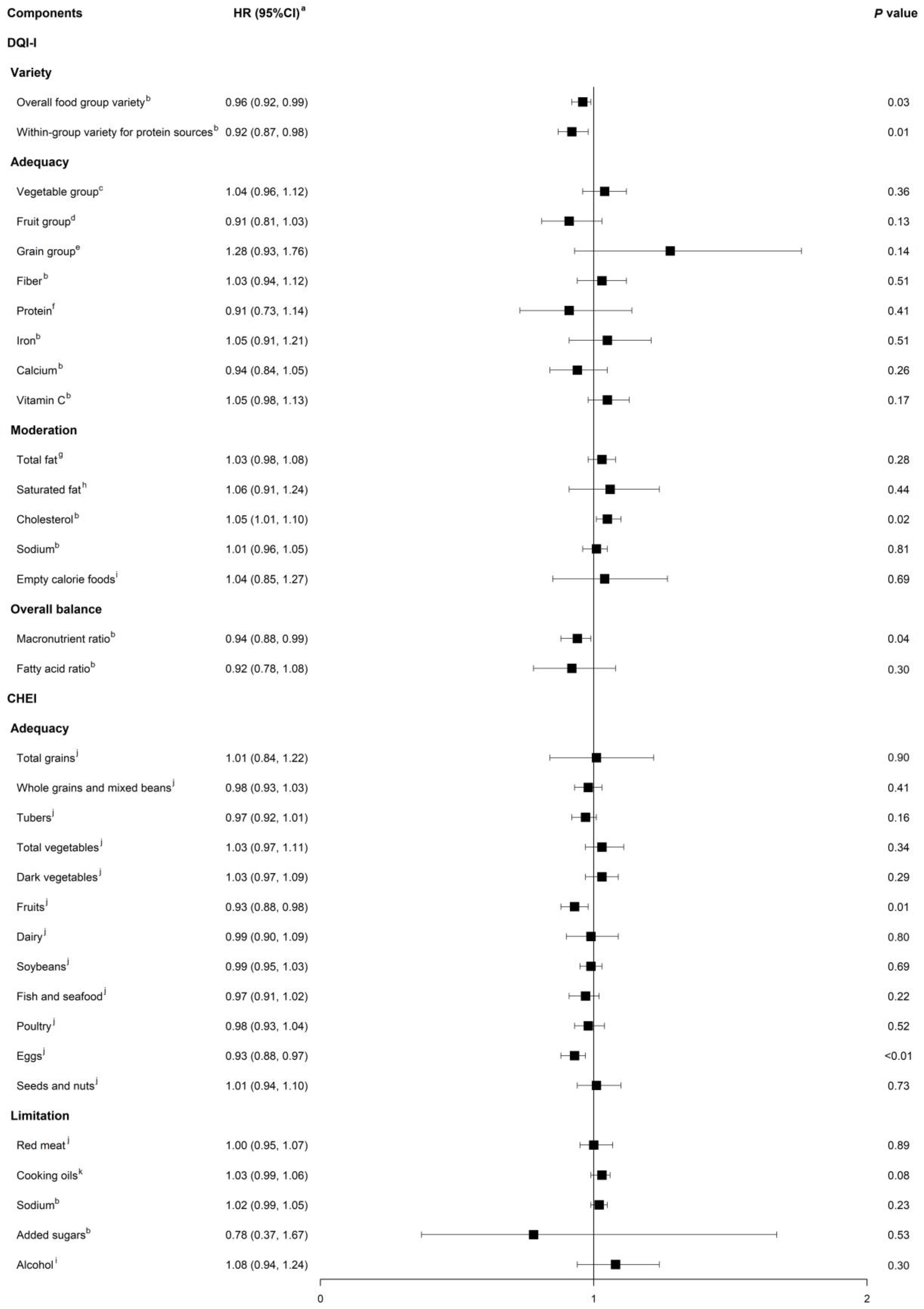
3.2. Associations between Component Scores of Dietary Indices and All-Cause Mortality
3.3. Population Attributable Fraction (PAF) of All-Cause Mortality Owing to the Lowest Diet Quality
3.4. Stratified Associations between Dietary Indices and All-Cause Mortality
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.K.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary patterns: Challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e1915. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Dietary Inflammatory Index and its Association with the Risk of Cardiovascular Diseases, Metabolic Syndrome, and Mortality: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2018, 50, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.D.; Calle, E.E.; Flagg, E.W.; Coates, R.J.; Ford, E.S.; Thun, M.J. Diet Quality Index as a predictor of short-term mortality in the American Cancer Society Cancer Prevention Study II Nutrition Cohort. Am. J. Epidemiol. 2003, 157, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Arablou, T.; Jayedi, A.; Salehi-Abargouei, A. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. J. 2020, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin. Nutr. 2018, 37, 542–550. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Yuan, L.; Li, T.; Hu, H.; Li, X.; Huang, H.; et al. Dose-Response Association of Dietary Inflammatory Potential with All-Cause and Cause-Specific Mortality. Adv. Nutr. 2022, 13, 1834–1845. [Google Scholar] [CrossRef]
- Brlek, A.; Gregorič, M. Diet quality indices and their associations with all-cause mortality, CVD and type 2 diabetes mellitus: An umbrella review. Br. J. Nutr. 2023, 130, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kang, M.; Shvetsov, Y.B.; Setiawan, V.W.; Boushey, C.J.; Haiman, C.A.; Wilkens, L.R.; Le Marchand, L. Diet quality and all-cause and cancer-specific mortality in cancer survivors and non-cancer individuals: The Multiethnic Cohort Study. Eur. J. Nutr. 2022, 61, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.S.M.; Yu, B.W.M.; Leung, J.; Lee, J.S.W.; Auyeung, T.W.; Kwok, T.; Woo, J. How Dietary Patterns are Related to Inflammaging and Mortality in Community-Dwelling Older Chinese Adults in Hong Kong—A Prospective Analysis. J. Nutr. Health Aging 2019, 23, 181–194. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Qiao, Y.; Xiong, S.; Liu, S.; Ke, C.; Shen, Y. Association between Dietary Quality and Prediabetes based on the Diet Balance Index. Sci. Rep. 2020, 10, 3190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Li, F.; Dong, R.H.; Chen, J.S.; He, G.S.; Li, S.G.; Chen, B. The Development of a Chinese Healthy Eating Index and Its Application in the General Population. Nutrients 2017, 9, 977. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Koh, W.P.; Yuan, J.M.; Gross, M.D.; Pereira, M.A. Dietary patterns and mortality in a Chinese population. Am. J. Clin. Nutr. 2014, 100, 877–883. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Adherence to dietary guidelines and mortality: A report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am. J. Clin. Nutr. 2014, 100, 693–700. [Google Scholar] [CrossRef]
- Li, F.; Hou, L.N.; Chen, W.; Chen, P.L.; Lei, C.Y.; Wei, Q.; Tan, W.L.; Zheng, S.B. Associations of dietary patterns with the risk of all-cause, CVD and stroke mortality: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2015, 113, 16–24. [Google Scholar] [CrossRef]
- Shi, Z.; Zhen, S.; Zimmet, P.Z.; Zhou, Y.; Zhou, Y.; Magliano, D.J.; Taylor, A.W. Association of impaired fasting glucose, diabetes and dietary patterns with mortality: A 10-year follow-up cohort in Eastern China. Acta Diabetol. 2016, 53, 799–806. [Google Scholar] [CrossRef]
- Neelakantan, N.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. Diet-Quality Indexes Are Associated with a Lower Risk of Cardiovascular, Respiratory, and All-Cause Mortality among Chinese Adults. J. Nutr. 2018, 148, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.W.; McNaughton, S.A.; He, Q.Q.; Leech, R. Longitudinal trajectories of diet quality and subsequent mortality among Chinese adults: Results from the China health and nutrition survey 1997–2015. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, J.; Xuan, J.; Zhu, A.; Ji, J.S.; Liu, X.; Cao, Y.; Zong, G.; Zeng, Y.; Wang, X.; et al. Plant-based dietary patterns in relation to mortality among older adults in China. Nat. Aging 2022, 2, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, X.R.; Li, Z.H.; Zhang, Y.J.; Lv, Y.B.; Wang, Z.H.; Shen, D.; Chen, P.L.; Zhong, W.F.; Huang, Q.M.; et al. Association of dietary diversity changes and mortality among older people: A prospective cohort study. Clin. Nutr. 2021, 40, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, H.; Djalalinia, S.; Asayesh, H.; Mansourian, M.; Esmaeili Abdar, Z.; Mahdavi Gorabi, A.; Ansari, H.; Noroozi, M.; Qorbani, M. A Higher Dietary Inflammatory Index Score is Associated with a Higher Risk of Incidence and Mortality of Cancer: A Comprehensive Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Hong, X.; Chen, M.; Chen, T.; Wang, J.; Zhang, N. Dietary inflammatory index and cardiovascular risk and mortality: A meta-analysis of cohort studies. Medicine 2020, 99, e20303. [Google Scholar] [CrossRef] [PubMed]
- Farazi, M.; Jayedi, A.; Shab-Bidar, S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: An umbrella review of meta-analyses of observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 57–66. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. 1), 2–7. [Google Scholar] [CrossRef]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Dietary Pattern Is Associated with Obesity in Older People in China: Data from China Health and Nutrition Survey (CHNS). Nutrients 2015, 7, 8170–8188. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef]
- Su, C.; Zhao, J.; Wu, Y.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B. Temporal Trends in Dietary Macronutrient Intakes among Adults in Rural China from 1991 to 2011: Findings from the CHNS. Nutrients 2017, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Fogli-Cawley, J.J.; Dwyer, J.T.; Saltzman, E.; McCullough, M.L.; Troy, L.M.; Jacques, P.F. The 2005 Dietary Guidelines for Americans Adherence Index: Development and application. J. Nutr. 2006, 136, 2908–2915. [Google Scholar] [CrossRef]
- Millen, A.E.; Midthune, D.; Thompson, F.E.; Kipnis, V.; Subar, A.F. The National Cancer Institute diet history questionnaire: Validation of pyramid food servings. Am. J. Epidemiol. 2006, 163, 279–288. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, J.H.; Chon, D.; Lee, K.E. Diet quality and osteosarcopenic obesity in community-dwelling adults 50 years and older. Maturitas 2017, 104, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Li, F.; Wu, H.; Wang, Y.C.; Chen, J.S.; He, G.S.; Li, S.G.; Chen, B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients 2018, 10, 114. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Jiang, H.; Liang, X.; Wang, Y.; Lu, W. Associations of BMI and Waist Circumference with All-Cause Mortality: A 22-Year Cohort Study. Obesity 2019, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika 1980, 67, 145–153. [Google Scholar] [CrossRef]
- Spiegelman, D.; Hertzmark, E.; Wand, H.C. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control 2007, 18, 571–579. [Google Scholar] [CrossRef]
- Zapata-Diomedi, B.; Barendregt, J.J.; Veerman, J.L. Population attributable fraction: Names, types and issues with incorrect interpretation of relative risks. Br. J. Sports Med. 2018, 52, 212–213. [Google Scholar] [CrossRef]
- Pan, J.; Han, W.; Jiang, Y.; Wu, J.; Zhou, X. Association of dietary cholesterol and dyslipidemia in Chinese health examinees. J. Health Popul. Nutr. 2022, 41, 15. [Google Scholar] [CrossRef]
- Qu, X.; Na, X.; Yang, J.; Yu, H.; Chen, A.; Zhao, A. Reaching and maintaining higher dietary diversity is associated with decreased risk of all-cause mortality: A longitudinal study from the China Health and Nutrition Survey. Front. Nutr. 2022, 9, 947290. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, S.; Zhang, Y.; Wu, Q.; Ye, Z.; Liu, M.; He, P.; Zhang, Y.; Li, R.; Liu, C.; et al. Relations of Variety and Quantity of Dietary Proteins Intake from Different Sources with Mortality Risk: A Nationwide Population-Based Cohort. J. Nutr. Health Aging 2022, 26, 1078–1086. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Liaset, B.; Kristiansen, K. Links between Dietary Protein Sources, the Gut Microbiota, and Obesity. Front. Physiol. 2017, 8, 1047. [Google Scholar] [CrossRef]
- Bernstein, M.A.; Tucker, K.L.; Ryan, N.D.; O’Neill, E.F.; Clements, K.M.; Nelson, M.E.; Evans, W.J.; Fiatarone Singh, M.A. Higher dietary variety is associated with better nutritional status in frail elderly people. J. Am. Diet. Assoc. 2002, 102, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D. Putting the balance back in diet. Cell 2015, 161, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.D. Perspective: The Saturated Fat-Unsaturated Oil Dilemma: Relations of Dietary Fatty Acids and Serum Cholesterol, Atherosclerosis, Inflammation, Cancer, and All-Cause Mortality. Adv. Nutr. 2021, 12, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.S.C.; Chan, W.; Fielding, R. The Associations of Fruit and Vegetable Intakes with Burden of Diseases: A Systematic Review of Meta-Analyses. J. Acad. Nutr. Diet. 2019, 119, 464–481. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies1,2. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef]
- Kazemi, A.; Soltani, S.; Mokhtari, Z.; Khan, T.; Golzarand, M.; Hosseini, E.; Jayedi, A.; Ebrahimpour-Koujan, S.; Akhlaghi, M. The relationship between major food sources of fructose and cardiovascular disease, cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 4274–4287. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ Br. Med. J. 2014, 349, g4490. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Jeemon, P.; Mini, G.; Thankappan, K.; Sylaja, P. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Zhuang, P.; Jiao, J.; Wu, F.; Mao, L.; Zhang, Y. Egg and egg-sourced cholesterol consumption in relation to mortality: Findings from population-based nationwide cohort. Clin. Nutr. 2020, 39, 3520–3527. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-F.; Yang, J.-J.; Lipworth, L.P.; Shu, X.-O.; Cai, H.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Yu, D. Cholesterol and Egg Intakes with Cardiometabolic and All-Cause Mortality among Chinese and Low-Income Black and White Americans. Nutrients 2021, 13, 2094. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Zargarzadeh, N.; Rigi, S.; Persad, E.; Pizarro, A.B.; Hasani-Ranjbar, S.; Larijani, B.; Willett, W.C.; Esmaillzadeh, A. Egg consumption and risk of all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2022, 13, 1762–1773. [Google Scholar] [CrossRef]
- Darooghegi Mofrad, M.; Naghshi, S.; Lotfi, K.; Beyene, J.; Hypponen, E.; Pirouzi, A.; Sadeghi, O. Egg and Dietary Cholesterol Intake and Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 878979. [Google Scholar] [CrossRef]
- Jessri, M.; Hennessey, D.; Bader Eddeen, A.; Bennett, C.; Sanmartin, C.; Manuel, D. Mortality and Life Expectancy Lost in Canada Attributable to Dietary Patterns: Evidence From Canadian National Nutrition Survey Linked to Routinely Collected Health Administrative Databases. Am. J. Epidemiol. 2023, 192, 377–396. [Google Scholar] [CrossRef]

| Characteristics | DQI-I | CHEI | E-DII | |||
|---|---|---|---|---|---|---|
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| n | 3229 | 3229 | 3229 | 3228 | 3228 | 3229 |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Age (years) | 48.72 (0.28) | 45.17 (0.25) | 48.56 (0.27) | 46.09 (0.27) | 48.38 (0.27) | 46.04 (0.27) |
| Dietary factors | ||||||
| Total energy (kcal/d) | 1841.94 (14.88) | 2296.04 (12.71) | 2096.02 (15.84) | 2048.55 (11.84) | 1934.42 (13.10) | 2228.86 (13.57) |
| Carbohydrate (g/d) | 234.32 (1.71) | 357.88 (2.36) | 282.05 (2.12) | 295.75 (2.15) | 273.33 (2.10) | 316.52 (2.34) |
| Protein (g/d) | 51.83 (0.40) | 75.70 (0.53) | 54.46 (0.38) | 73.66 (0.45) | 68.26 (0.51) | 59.71 (0.38) |
| Fat (g/d) | 77.96 (1.21) | 65.17 (0.50) | 82.47 (1.24) | 68.34 (0.50) | 63.83 (0.72) | 82.29 (0.83) |
| Dietary fiber (g/d) | 7.37 (0.08) | 14.31 (0.18) | 8.86 (0.13) | 12.72 (0.14) | 14.06 (0.20) | 8.03 (0.07) |
| Grain group (g/d) | 321.92 (2.46) | 440.61 (3.49) | 382.22 (2.95) | 367.08 (3.07) | 352.21 (2.90) | 415.62 (3.26) |
| Vegetable group (g/d) | 31.42 (0.40) | 44.88 (0.66) | 38.54 (0.50) | 39.00 (0.54) | 44.65 (0.71) | 34.15 (0.41) |
| Fruit group (g/d) | 8.84 (0.37) | 29.68 (0.74) | 5.47 (0.32) | 35.96 (0.66) | 24.83 (0.66) | 10.13 (0.41) |
| Dairy group (g/d) | 10.08 (0.52) | 14.06 (0.55) | 3.45 (0.27) | 24.52 (0.65) | 14.85 (0.55) | 7.51 (0.42) |
| Soybean group (g/d) | 15.84 (0.45) | 28.34 (0.55) | 14.02 (0.43) | 29.93 (0.54) | 26.98 (0.60) | 16.41 (0.44) |
| Fish and seafood group (g/d) | 12.19 (0.41) | 23.12 (0.54) | 9.96 (0.38) | 27.00 (0.56) | 21.50 (0.53) | 12.82 (0.41) |
| Red meat group (g/d) | 17.60 (0.33) | 20.68 (0.34) | 19.45 (0.36) | 19.61 (0.31) | 18.42 (0.32) | 20.97 (0.35) |
| Poultry group (g/d) | 8.20 (0.37) | 11.83 (0.42) | 3.69 (0.28) | 17.49 (0.45) | 11.66 (0.40) | 7.90 (0.35) |
| Egg group (g/d) | 14.91 (0.28) | 12.72 (0.25) | 10.02 (0.24) | 18.21 (0.36) | 14.12 (0.32) | 13.01 (0.25) |
| Seeds and nuts group (g/d) | 2.52 (0.21) | 2.93 (0.16) | 0.75 (0.09) | 5.90 (0.24) | 4.32 (0.22) | 0.95 (0.09) |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Baseline year | ||||||
| 2004 | 1720 (53.27) | 1801 (55.78) | 2169 (67.17) | 1256 (38.91) | 1592 (49.32) | 2018 (62.50) |
| 2006 | 239 (7.40) | 290 (8.98) | 265 (8.21) | 249 (7.43) | 233 (7.22) | 298 (9.23) |
| 2009 | 313 (9.69) | 484 (14.99) | 261 (8.08) | 506 (15.68) | 379 (11.74) | 376 (11.64) |
| 2011 | 957 (29.64) | 654 (20.25) | 534 (16.54) | 1226 (37.98) | 1024 (31.72) | 537 (16.63) |
| Sex | ||||||
| Male | 1899 (58.81) | 1537 (47.60) | 1810 (56.05) | 1713 (53.07) | 1785 (55.30) | 1672 (51.78) |
| Female | 1330 (41.19) | 1692 (52.40) | 1419 (43.95) | 1515 (46.93) | 1443 (44.70) | 1557 (48.22) |
| Educational level | ||||||
| Primary school and lower | 1257 (38.93) | 1001 (31.00) | 1462 (45.28) | 691 (21.41) | 1084 (33.58) | 1217 (37.69) |
| Junior and senior middle school | 1707 (52.74) | 1890 (58.53) | 1614 (49.98) | 2004 (62.08) | 1777 (55.05) | 1801 (55.78) |
| College and higher | 258 (7.99) | 324 (10.03) | 135 (4.18) | 523 (16.20) | 347 (10.75) | 195 (6.04) |
| Missing | 11 (0.34) | 14 (0.43) | 18 (0.56) | 10 (0.31) | 20 (0.62) | 16 (0.50) |
| Marital status | ||||||
| Married | 2619 (81.11) | 2763 (85.57) | 2636 (81.64) | 2742 (84.94) | 2681 (83.05) | 2660 (82.38) |
| Not in a marriage a | 590 (18.27) | 444 (13.75) | 568 (17.59) | 466 (14.44) | 517 (16.02) | 546 (16.91) |
| Missing | 20 (0.62) | 22 (0.68) | 25 (0.77) | 20 (0.62) | 30 (0.93) | 23 (0.71) |
| Household income per year b | ||||||
| Low, CNY < 16,962 | 1070 (33.14) | 901 (27.90) | 1358 (42.06) | 574 (17.78) | 915 (28.35) | 901 (27.90) |
| Medium, CNY 16,962–39,590 | 1124 (34.81) | 1096 (33.94) | 1136 (35.18) | 979 (30.33) | 993 (30.76) | 1096 (33.94) |
| High, CNY ≥39,590 | 1002 (31.03) | 1211 (37.50) | 685 (21.21) | 1658 (51.36) | 1291 (39.99) | 1211 (37.50) |
| Missing | 33 (1.02) | 21 (0.65) | 50 (1.55) | 17 (0.53) | 29 (0.90) | 34 (1.05) |
| Self-reported health status | ||||||
| Good and above | 1137 (35.21) | 1310 (40.57) | 1377 (42.64) | 950 (29.43) | 1066 (33.02) | 1415 (43.82) |
| Fair | 655 (20.28) | 658 (20.38) | 840 (26.01) | 473 (14.65) | 624 (19.33) | 737 (22.82) |
| Poor and below | 157 (4.76) | 106 (3.28) | 198 (6.13) | 69 (2.14) | 119 (3.69) | 144 (4.46) |
| Missing | 1280 (39.64) | 1155 (35.77) | 814 (25.21) | 1736 (53.78) | 1419 (43.96) | 933 (28.89) |
| Smoking status | ||||||
| Never | 2307 (71.45) | 2132 (66.03) | 2214 (68.57) | 2293 (71.03) | 2242 (69.45) | 2198 (68.07) |
| Former | 108 (3.34) | 131 (4.06) | 94 (2.91) | 134 (4.15) | 138 (4.28) | 103 (3.19) |
| Current | 802 (24.84) | 951 (29.45) | 909 (28.15) | 792 (24.54) | 833 (25.81) | 909 (28.15) |
| Missing | 12 (0.37) | 15 (0.46) | 12 (0.37) | 9 (0.28) | 15 (0.46) | 19 (0.59) |
| Drinking status | ||||||
| Never | 2242 (69.43) | 1995 (61.78) | 2192 (67.88) | 2091 (64.78) | 2079 (64.41) | 2171 (67.23) |
| Ever | 974 (30.16) | 1223 (37.88) | 1027 (31.81) | 1131 (35.04) | 1139 (35.29) | 1039 (32.18) |
| Missing | 13 (0.40) | 11 (0.34) | 10 (0.31) | 6 (0.19) | 10 (0.31) | 19 (0.59) |
| BMI status | ||||||
| Underweight (<18.5 kg/m2) | 164 (5.08) | 168 (5.20) | 186 (5.76) | 151 (4.68) | 159 (4.93) | 167 (5.17) |
| Normal (18.5–24.0 kg/m2) | 1580 (48.93) | 1688 (52.28) | 1652 (51.16) | 1592 (49.32) | 1661 (51.46) | 1685 (52.18) |
| Overweight (24.0–28.0 kg/m2) | 963 (29.82) | 918 (28.43) | 873 (27.04) | 1043 (32.31) | 954 (29.55) | 887 (27.47) |
| Obese (≥28.0 kg/m2) | 342 (10.59) | 266 (8.24) | 305 (9.45) | 323 (10.01) | 296 (9.17) | 291 (9.01) |
| Missing | 180 (5.57) | 189 (5.85) | 213 (6.60) | 119 (3.69) | 158 (4.89) | 199 (6.16) |
| Physical activity level c | ||||||
| Low, <52.6 (MET h/wk) | 1134 (35.12) | 841 (26.05) | 1130 (35.00) | 835 (25.87) | 951 (29.46) | 994 (30.78) |
| Medium, 52.6–146.6 (MET h/wk) | 918 (21.43) | 1079 (33.42) | 762 (23.60) | 1319 (40.86) | 1136 (35.19) | 879 (27.22) |
| High, ≥146.6 (MET h/wk) | 801 (24.81) | 1046 (32.39) | 947 (29.33) | 857 (26.55) | 848 (26.27) | 999 (30.94) |
| Missing | 376 (11.64) | 263 (8.14) | 390 (12.08) | 217 (6.72) | 293 (9.08) | 357 (11.06) |
| History of comorbidities d | ||||||
| No | 2709 (83.90) | 2820 (87.33) | 2809 (86.99) | 2684 (83.15) | 2655 (82.25) | 2850 (88.26) |
| Yes | 489 (15.14) | 381 (11.80) | 378 (11.71) | 517 (16.02) | 533 (16.51) | 343 (10.62) |
| Missing | 31 (0.96) | 28 (0.87) | 42 (1.30) | 27 (0.84) | 40 (1.24) | 36 (1.11) |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trenda | HRcontinuous (95%CI) b | |
|---|---|---|---|---|---|---|
| DQI-I | ||||||
| Total score, mean (range) | 44.01 (24.18–49.09) | 51.72 (49.10–54.06) | 56.42 (54.07–58.87) | 63.06 (58.88–82.82) | ||
| Events/person-years | 116/21,111 | 131/22,781 | 130/23,744 | 84/22,966 | ||
| Model 1, HR (95%CI) c | Ref. | 1.27 (0.98, 1.63) | 1.25 (0.97, 1.62) | 0.94 (0.70, 1.24) | 0.32 | 0.99 (0.98, 1.01) |
| Model 2, HR (95%CI) d | Ref. | 1.29 (1.00, 1.68) | 1.31 (1.01, 1.70) | 1.03 (0.77, 1.39) | 0.99 | 1.00 (0.99, 1.01) |
| Variety | ||||||
| Total score, mean (range) | 6.89 (3.00–9.00) | 10.10 (10.00–11.00) | 12.77 (12.00–14.00) | 16.68 (15.00–20.00) | ||
| Model 1, HR (95%CI) c | Ref. | 1.04 (0.81, 1.34) | 0.83 (0.65, 1.07) | 0.56 (0.43, 0.74) | <0.01 | 0.94 (0.92, 0.96) |
| Model 2, HR (95%CI) d | Ref. | 1.03 (0.80, 1.33) | 0.89 (0.69, 1.15) | 0.69 (0.52, 0.92) | <0.01 | 0.96 (0.94, 0.99) |
| Adequacy | ||||||
| Total score, mean (range) | 20.99 (9.43–23.93) | 25.58 (23.94–27.07) | 28.40 (27.08–29.80) | 32.14 (29.81–40.00) | ||
| Model 1, HR (95%CI) c | Ref. | 0.84 (0.65, 1.07) | 0.88 (0.68, 1.13) | 0.84 (0.65, 1.09) | 0.18 | 0.99 (0.97, 1.01) |
| Model 2, HR (95%CI) d | Ref. | 0.94 (0.73, 1.21) | 0.96 (0.74, 1.25) | 1.07 (0.80, 1.43) | 0.58 | 1.01 (0.98, 1.03) |
| Moderation | ||||||
| Total score, mean (range) | 7.37 (0–9.00) | 12.00 (12.00–12.00) | 15.00 (15.00–15.00) | 19.43 (18.00–27.00) | ||
| Model 1, HR (95%CI) c | Ref. | 1.02 (0.76, 1.37) | 1.22 (0.92, 1.63) | 1.70 (1.31, 2.20) | <0.01 | 1.05 (1.03, 1.07) |
| Model 2, HR (95%CI) d | Ref. | 0.92 (0.68, 1.24) | 1.00 (0.75, 1.34) | 1.35 (1.03, 1.77) | 0.01 | 1.03 (1.01, 1.05) |
| Overall balance e | ||||||
| Total score, mean (range) | 0 | 3.11 (2.00–10.00) | ||||
| Model 1, HR (95%CI) c | Ref. | 0.84 (0.69, 1.03) | 0.09 | 0.95 (0.90, 1.01) | ||
| Model 2, HR (95%CI) d | Ref. | 0.81 (0.66, 0.99) | 0.02 | 0.93 (0.88, 0.99) | ||
| CHEI | ||||||
| Total score, mean (range) | 36.82 (17.23–42.12) | 45.22 (42.13–48.18) | 51.14 (48.19–54.45) | 60.97 (54.46–88.51) | ||
| Events/person-years | 154/24,122 | 146/23,881 | 102/23,186 | 59/19,144 | ||
| Model 1, HR (95%CI) c | Ref. | 1.14 (0.91, 1.43) | 0.88 (0.68, 1.13) | 0.63 (0.47, 0.85) | <0.01 | 0.98 (0.97, 0.99) |
| Model 2, HR (95%CI) d | Ref. | 1.16 (0.92, 1.45) | 0.98 (0.76, 1.26) | 0.90 (0.66, 1.23) | 0.30 | 0.99 (0.98, 1.01) |
| Adequacy | ||||||
| Total score, mean (range) | 13.41 (2.80–16.82) | 19.06 (16.83–21.23) | 23.89 (21.24–26.96) | 33.31 (26.97–55.86) | ||
| Model 1, HR (95%CI) c | Ref. | 0.94 (0.74, 1.17) | 0.75 (0.59, 0.96) | 0.40 (0.29, 0.55) | <0.01 | 0.96 (0.95, 0.97) |
| Model 2, HR (95%CI) d | Ref. | 0.96 (0.76, 1.20) | 0.87 (0.67, 1.11) | 0.60 (0.43, 0.84) | <0.01 | 0.98 (0.96, 0.99) |
| Limitation | ||||||
| Total score, mean (range) | 18.91 (7.40–22.81) | 24.88 (22.82–26.82) | 28.55 (26.83–30.03) | 32.13 (30.04–35.00) | ||
| Model 1, HR (95%CI) c | Ref. | 1.27 (0.98, 1.64) | 1.35 (1.04, 1.75) | 1.28 (0.97, 1.67) | 0.03 | 1.02 (1.00, 1.04) |
| Model 2, HR (95%CI) d | Ref. | 1.25 (0.96, 1.62) | 1.34 (1.04, 1.75) | 1.29 (0.98, 1.70) | 0.03 | 1.02 (1.00, 1.04) |
| E-DII | ||||||
| Mean (range) | –0.45 (−4.28–0.47) | 0.91 (0.48–1.31) | 1.66 (1.32–2.02) | 2.50 (2.03–4.21) | ||
| Events/person-years | 107/21,023 | 124/22,561 | 111/23,578 | 119/23,440 | ||
| Model 1, HR (95%CI) c | Ref. | 1.17 (0.90, 1.51) | 1.04 (0.80, 1.35) | 1.06 (0.82, 1.38) | 0.52 | 1.03 (0.95, 1.11) |
| Model 2, HR (95%CI) d | Ref. | 1.03 (0.79, 1.33) | 0.89 (0.68, 1.16) | 0.85 (0.65, 1.11) | 0.21 | 0.95 (0.88, 1.03) |
| Exposures a | Partial PAF (95%CI) b |
|---|---|
| DQI-I total score | 5.3% (−10.9%, 21.2%) |
| Variety score | 20.1% (9.3%, 30.5%) |
| Adequacy score | 3.7% (−12.7%, 19.9%) |
| Moderation score | NA d |
| Overall balance score c | 13.9% (1.8%, 25.7%) |
| CHEI total score | 13.0% (−5.4%, 30.6%) |
| Adequacy score | 31.3% (14.6%, 46.3%) |
| Limitation score | NA d |
| E-DII score | NA d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhu, T.; Li, F.; Wu, H.; Jiang, S.; Shivappa, N.; Hébert, J.R.; Li, X.; Li, Y.; Wang, H. Diet Quality and Mortality among Chinese Adults: Findings from the China Health and Nutrition Survey. Nutrients 2024, 16, 94. https://doi.org/10.3390/nu16010094
Zheng J, Zhu T, Li F, Wu H, Jiang S, Shivappa N, Hébert JR, Li X, Li Y, Wang H. Diet Quality and Mortality among Chinese Adults: Findings from the China Health and Nutrition Survey. Nutrients. 2024; 16(1):94. https://doi.org/10.3390/nu16010094
Chicago/Turabian StyleZheng, Jiali, Tianren Zhu, Fangyu Li, Han Wu, Shuo Jiang, Nitin Shivappa, James R. Hébert, Xiaoguang Li, Yan Li, and Hui Wang. 2024. "Diet Quality and Mortality among Chinese Adults: Findings from the China Health and Nutrition Survey" Nutrients 16, no. 1: 94. https://doi.org/10.3390/nu16010094
APA StyleZheng, J., Zhu, T., Li, F., Wu, H., Jiang, S., Shivappa, N., Hébert, J. R., Li, X., Li, Y., & Wang, H. (2024). Diet Quality and Mortality among Chinese Adults: Findings from the China Health and Nutrition Survey. Nutrients, 16(1), 94. https://doi.org/10.3390/nu16010094







