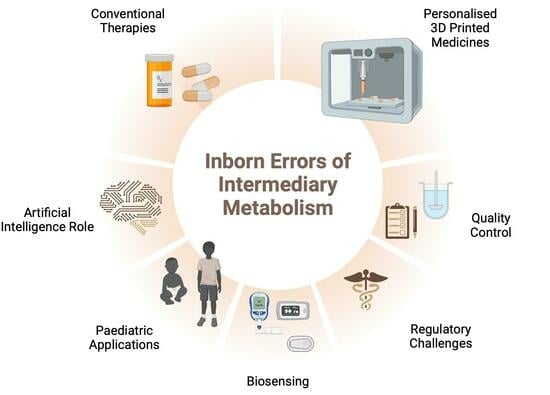
3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations
Abstract
1. Introduction
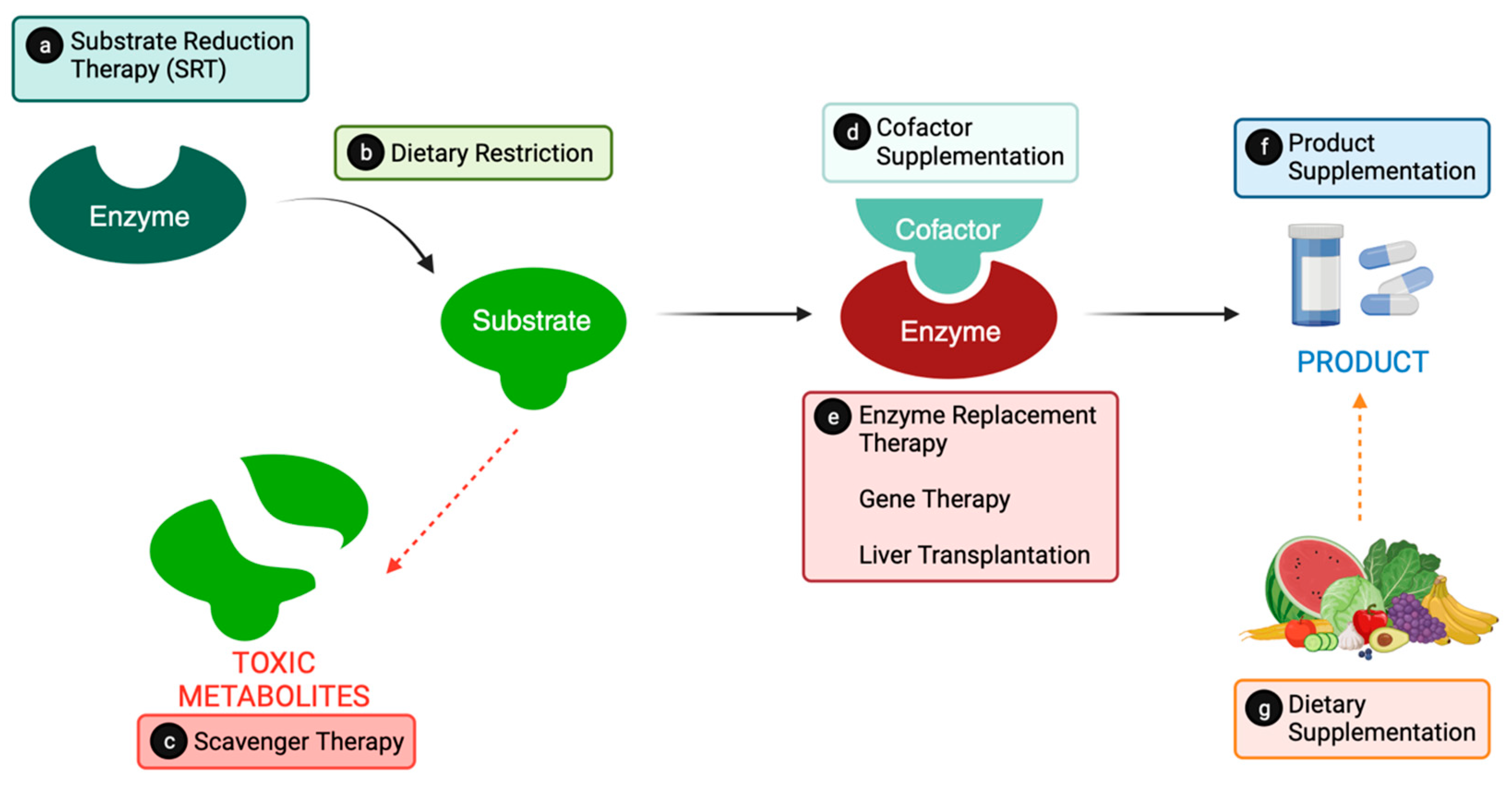
2. From Catalysts to Cures: Conventional Metabolic Therapies
2.1. Substrate Reduction
2.1.1. Substrate Reduction Therapy (SRT)
2.1.2. Substrate Dietary Restrictions
2.1.3. Scavenger Therapy
2.2. Providing the Product
2.2.1. Cofactor Supplementation
2.2.2. Enzyme Replacement
2.2.3. Dietary Supplementation
2.3. Liver Transplantation
2.4. Gene Therapy Research
3. Navigating Metabolic Mazes: Pioneering Precision Medicine in IEiM Management
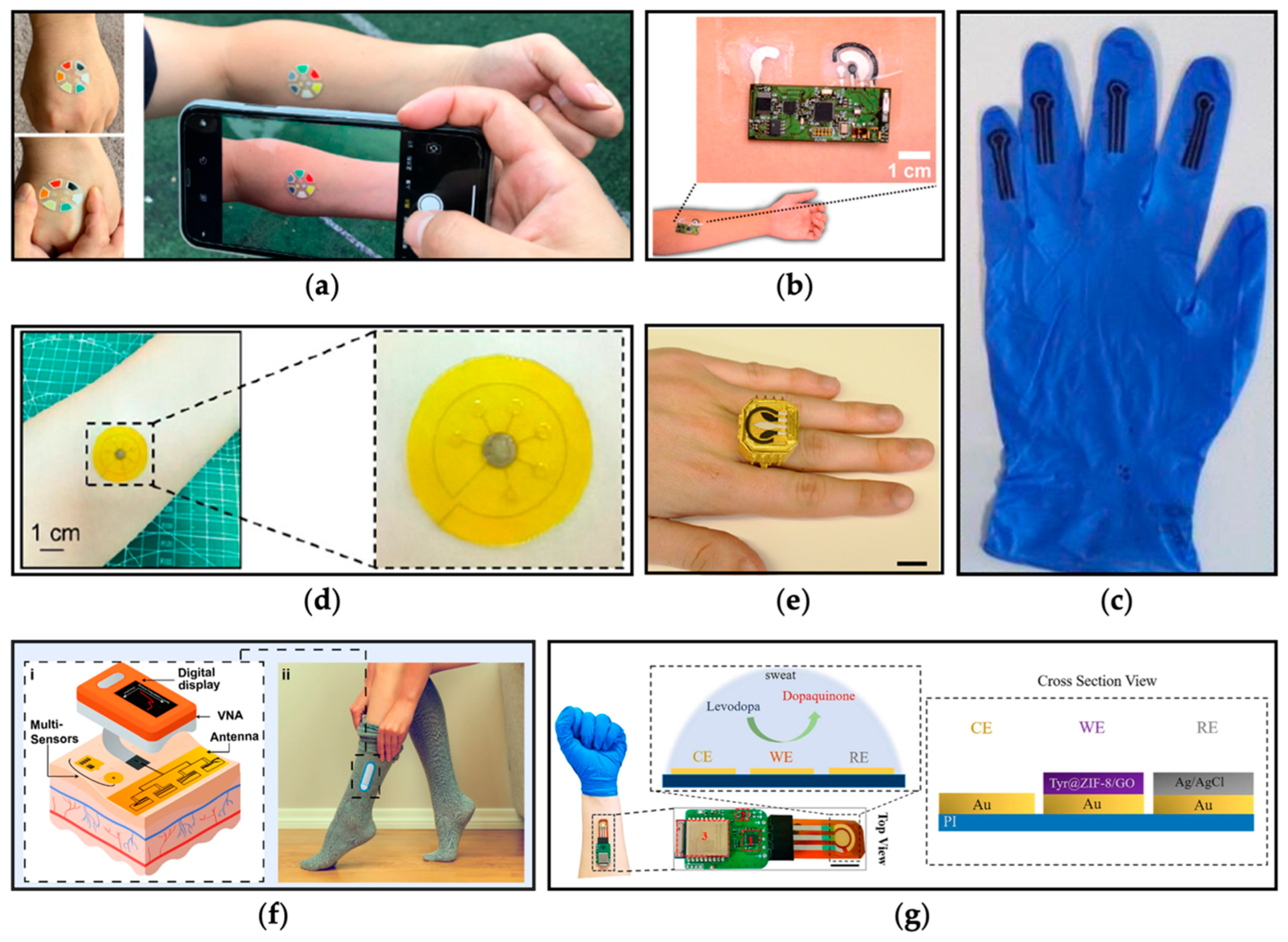
3.1. Detection and Monitoring
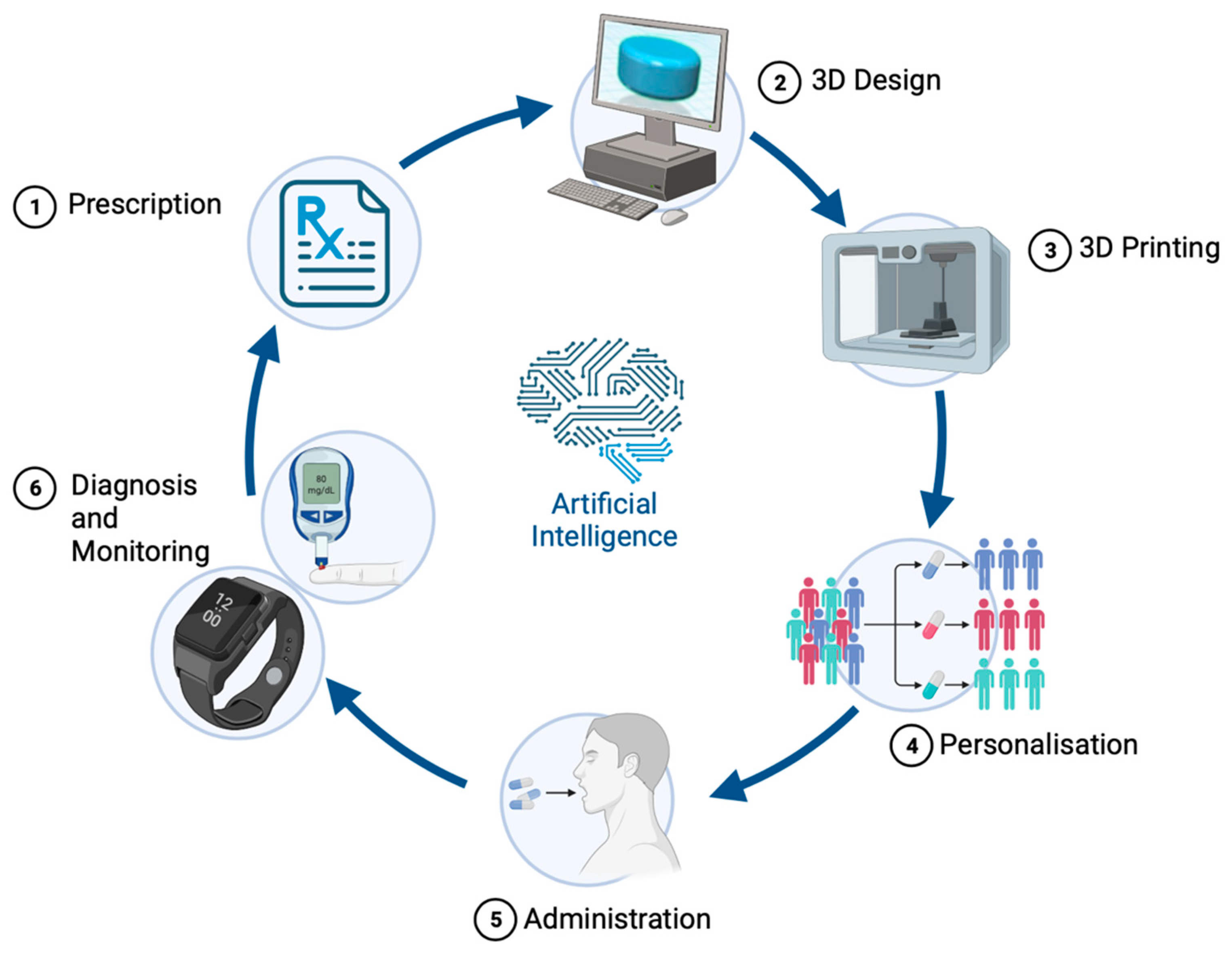
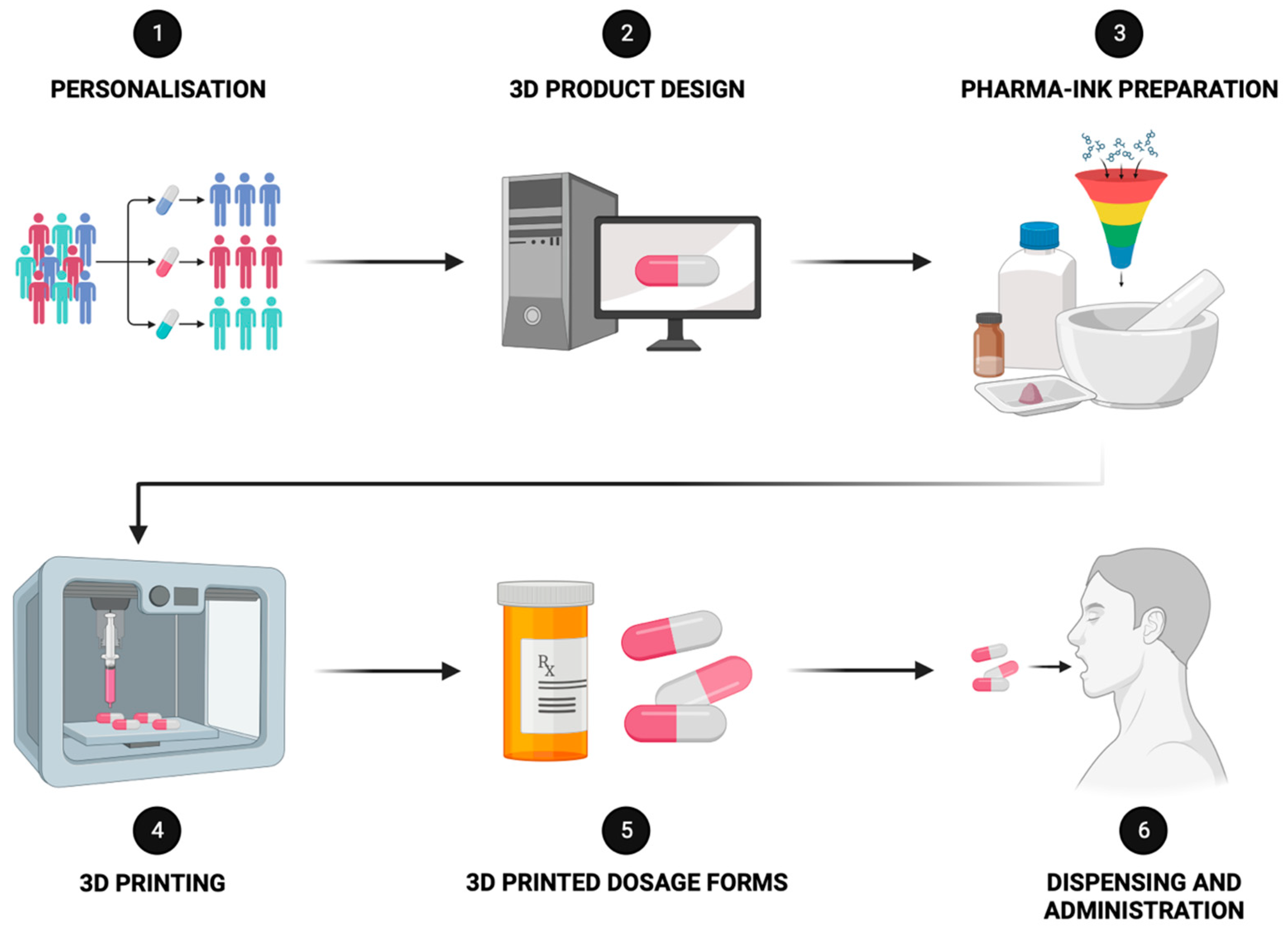
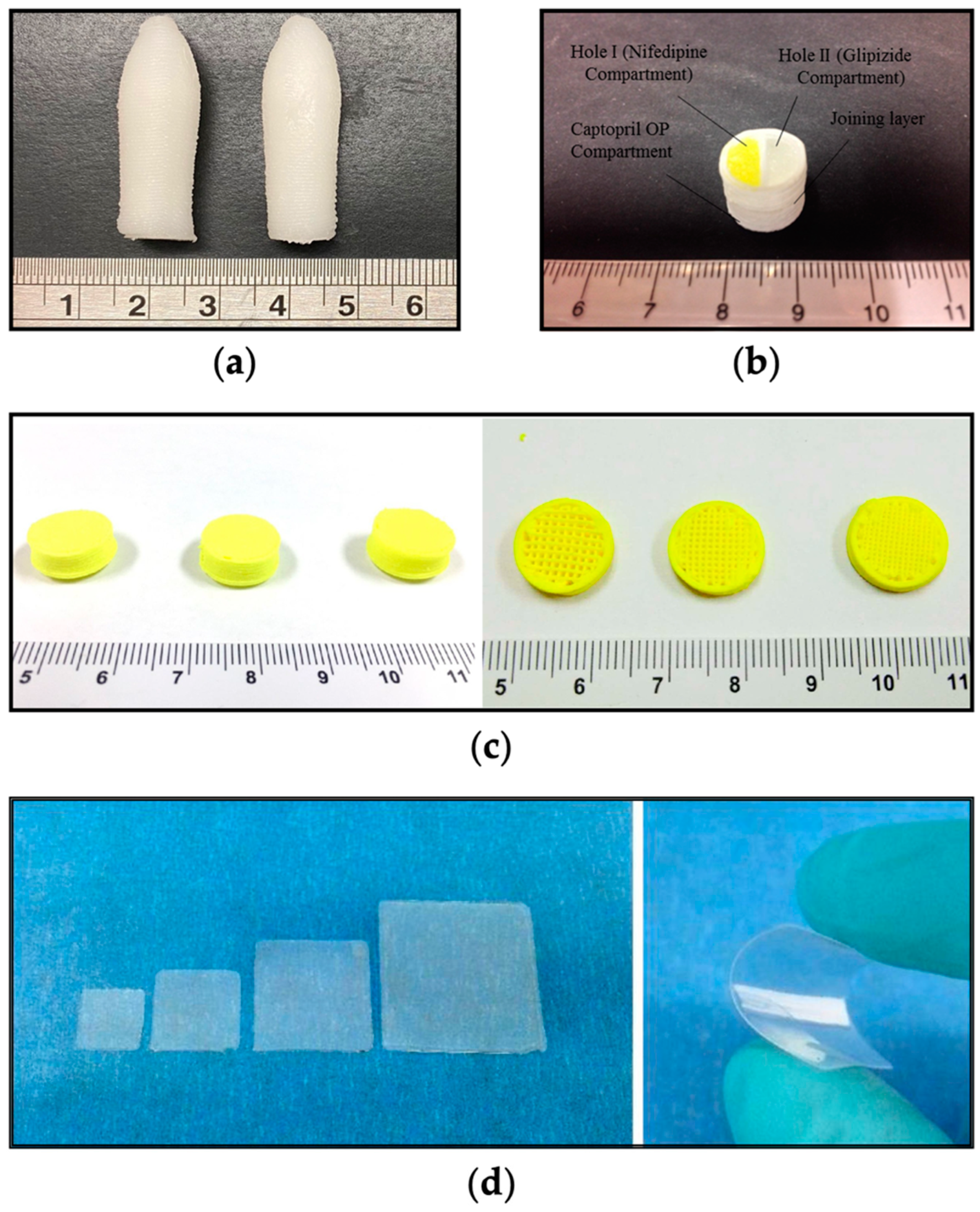
3.2. Advanced Therapies through 3D Printing Technology
Precision Medicine for Children: SSE 3D Printing’s Tailored Solutions
3.3. Artificial Intelligence (AI) in Therapeutics
4. Regulatory and Financial Challenges
5. Quality Control Assays
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, C.; Rahman, S.; Keller, M.; Zschocke, J. An International Classification of Inherited Metabolic Disorders (ICIMD). J. Inherit. Metab. Dis. 2021, 44, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Morava, E.; Rahman, S.; Peters, V.; Baumgartner, M.R.; Patterson, M.; Zschocke, J. Quo vadis: The re-definition of “inborn metabolic diseases”. J. Inherit. Metab. Dis. 2015, 38, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.; Adeloye, D.; Woolham, D.; Wastnedge, E.; Patel, S.; Rudan, I. Global birth prevalence and mortality from inborn errors of metabolism: A systematic analysis of the evidence. J. Glob. Health 2018, 8, 021102. [Google Scholar] [CrossRef] [PubMed]
- Saudubray, J.-M.; Mochel, F.; Lamari, F.; Garcia-Cazorla, A. Proposal for a simplified classification of IMD based on a pathophysiological approach: A practical guide for clinicians. J. Inherit. Metab. Dis. 2019, 42, 706–727. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.; Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E. Neonatal screening in Europe revisited: An ISNS perspective on the current state and developments since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, V.R.; Mei, J.V.; Cordovado, S.K.; Cuthbert, C.D. The newborn screening quality assurance program at the centers for disease control and prevention: Thirty-five year experience assuring newborn screening laboratory quality. Int. J. Neonatal Screen. 2015, 1, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari, D.; Van Karnebeek, C.; Münchau, A. Movement Disorders in Treatable Inborn Errors of Metabolism. Mov. Disord. 2019, 34, 598–613. [Google Scholar] [CrossRef]
- Saudubray, J.-M.; Garcia-Cazorla, À. Inborn errors of metabolism overview: Pathophysiology, manifestations, evaluation, and management. Pediat Clin. 2018, 65, 179–208. [Google Scholar]
- Gambello, M.J.; Li, H. Current strategies for the treatment of inborn errors of metabolism. J. Genet. Genom. 2018, 45, 61–70. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Santos, J.I.; Alves, S. Less is more: Substrate reduction therapy for lysosomal storage disorders. Int. J. Mol. Sci. 2016, 17, 1065. [Google Scholar] [CrossRef] [PubMed]
- Vara, R.; Rahman, Y. Inherited Metabolic Diseases. In Liver Disease in Adolescence; Hadžić, N., Samyn, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 107–127. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Couce, M.L.; Das, A.M.; de Laet, C.; Dionisi-Vici, C.; Lund, A.M.; Schiff, M.; Spada, M.; Sparve, E.; Szamosi, J. Long-term safety and outcomes in hereditary tyrosinaemia type 1 with nitisinone treatment: A 15-year non-interventional, multicentre study. Lancet Diabetes Endocrinol. 2021, 9, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.N.; Demirbas, D.; Mangel, M.; Gozalbo, M.E.R.; Levy, H.L.; Berry, G.T. The treatment of biochemical genetic diseases: From substrate reduction to nucleic acid therapies. Mol. Genet. Metab. 2023, 140, 107693. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Eminoğlu, F.T.; Öncül, Ü.; Kahveci, F.; Okulu, E.; Kraja, E.; Köse, E.; Kendirli, T. Characteristics of continuous venovenous hemodiafiltration in the acute treatment of inherited metabolic disorders. Pediatr. Nephrol. 2022, 37, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Häberle, J.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef] [PubMed]
- Driesen, K.; Witters, P. Understanding inborn errors of metabolism through metabolomics. Metabolites 2022, 12, 398. [Google Scholar] [CrossRef]
- Breilyn, M.S.; Wasserstein, M.P. Established and Emerging Treatments for Patients with Inborn Errors of Metabolism. NeoReviews 2020, 21, e699–e707. [Google Scholar] [CrossRef]
- Chuang, D.T.; Ku, L.S.; Cox, R.P. Thiamin-responsive maple-syrup-urine disease: Decreased affinity of the mutant branched-chain alpha-keto acid dehydrogenase for alpha-ketoisovalerate and thiamin pyrophosphate. Proc. Natl. Acad. Sci. USA 1982, 79, 3300–3304. [Google Scholar] [CrossRef]
- Blau, N. Sapropterin dihydrochloride for the treatment of hyperphenylalaninemias. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 1207–1218. [Google Scholar] [CrossRef]
- Hon, Y.Y.; Wang, J.; Abodakpi, H.; Balakrishnan, A.; Pacanowski, M.; Chakder, S.; Smpokou, P.; Donohue, K.; Wang, Y.-M.C. Dose selection for biological enzyme replacement therapy indicated for inborn errors of metabolism. Clin. Transl. Sci. 2023, 16, 2438–2457. [Google Scholar] [CrossRef] [PubMed]
- Camp, K.M.; Lloyd-Puryear, M.A.; Huntington, K.L. Nutritional treatment for inborn errors of metabolism: Indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol. Genet. Metab. 2012, 107, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, N.N.; Bosch, A.M.; Bouwhuis, N.; Häberle, J.; van Karnebeek, C.; van Spronsen, F.J.; Langeveld, M.; Hollak, C.E.M. Food or medicine? A European regulatory perspective on nutritional therapy products to treat inborn errors of metabolism. J. Inherit. Metab. Dis. 2023, 46, 1017–1028. [Google Scholar] [CrossRef]
- De Castro, M.-J.; Sánchez-Pintos, P.; Abdelaziz-Salem, N.; Leis, R.; Couce, M.L. Evaluation of Body Composition, Physical Activity, and Food Intake in Patients with Inborn Errors of Intermediary Metabolism. Nutrients 2021, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, I.; Lamperti, C.; Tiranti, V. Ethylmalonic Encephalopathy; University of Washington: Seattle, WA, USA, 2017. [Google Scholar]
- Wajner, M. Neurological manifestations of organic acidurias. Nat. Rev. Neurol. 2019, 15, 253–271. [Google Scholar] [CrossRef] [PubMed]
- González-Lamuño, D.; Sánchez-Pintos, P.; Andrade, F.; Couce, M.; Aldamiz-Echevarria, L. Treatment adherence in tyrosinemia type 1 patients. Orphanet J. Rare Dis. 2021, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Shchelochkov, O.A.; Dickinson, K.; Scharschmidt, B.F.; Lee, B.; Marino, M.; Le Mons, C. Barriers to drug adherence in the treatment of urea cycle disorders: Assessment of patient, caregiver and provider perspectives. Mol. Genet. Metab. Rep. 2016, 8, 43–47. [Google Scholar] [CrossRef]
- Merritt, J.L.; MacLeod, E.; Jurecka, A.; Hainline, B. Clinical manifestations and management of fatty acid oxidation disorders. Rev. Endocr. Metab. Disord. 2020, 21, 479–493. [Google Scholar] [CrossRef]
- Vimalesvaran, S.; Dhawan, A. Liver transplantation for pediatric inherited metabolic liver diseases. World J. Hepatol. 2021, 13, 1351. [Google Scholar] [CrossRef]
- Oishi, K.; Arnon, R.; Wasserstein, M.; Diaz, G. Liver transplantation for pediatric inherited metabolic disorders: Considerations for indications, complications, and perioperative management. Pediatr. Transplant. 2016, 20, 756–769. [Google Scholar] [CrossRef]
- Turner, A.; Glinton, K.E.; Sutton, V.R. Advancements in therapeutics for inborn errors of metabolism. Curr. Opin. Pediatr. 2022, 34, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.; Venditti, C. Gene Therapy for Methylmalonic Acidemia: Past, Present, and Future. Hum. Gene Ther. 2019, 30, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Pontoizeau, C.; Simon-Sola, M.; Gaborit, C.; Nguyen, V.; Rotaru, I.; Tual, N.; Colella, P.; Girard, M.; Biferi, M.-G.; Arnoux, J.-B.; et al. Neonatal gene therapy achieves sustained disease rescue of maple syrup urine disease in mice. Nat. Commun. 2022, 13, 3278. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kubara, K.; Ishii, S.; Kondo, K.; Suzuki, Y.; Miyazaki, T.; Mitsuhashi, K.; Ito, M.; Tsukahara, K. Lipid nanoparticle-targeted mRNA formulation as a treatment for ornithine-transcarbamylase deficiency model mice. Mol. Ther. Nucleic Acids 2023, 33, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bell, P.; Morizono, H.; He, Z.; Pumbo, E.; Yu, H.; White, J.; Batshaw, M.L.; Wilson, J.M. AAV gene therapy corrects OTC deficiency and prevents liver fibrosis in aged OTC-knock out heterozygous mice. Mol. Genet. Metab. Rep. 2017, 120, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Seker Yilmaz, B.; Gurung, S.; Perocheau, D.; Counsell, J.; Baruteau, J. Gene Therapy for Inherited Metabolic Diseases. J. Mother. Child. Health 2020, 24, 53–64. [Google Scholar] [CrossRef]
- Leal, A.F.; Fnu, N.; Benincore-Flórez, E.; Herreño-Pachón, A.M.; Echeverri-Peña, O.Y.; Alméciga-Díaz, C.J.; Tomatsu, S. The landscape of CRISPR/Cas9 for inborn errors of metabolism. Mol. Genet. Metab. 2023, 138, 106968. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R. The burden of rare diseases. Am. J. Med. Genet. Part A 2019, 179, 885–892. [Google Scholar] [CrossRef]
- Pirmohamed, M. Pharmacogenomics: Current status and future perspectives. Nat. Rev. Genet. 2023, 24, 350–362. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Jie Ong, J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected Healthcare: Improving Patient Care using Digital Health Technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.J.; den Burger, J.C.G.; Swart, E.L. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin. Pharmacokinet. 2014, 53, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Patel, D.R.; Neelakantan, M.; Ryali, B. Newborn screening and changing face of inborn errors of metabolism in the United States. Ann. Transl. Med. 2018, 6, 468. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Y.; Yang, Y.; Wang, J.; Cao, Y.; Luo, F.; Lu, W.; Peng, Y.; Yao, H.; Qiu, P. The screening of inborn errors of metabolism in sick Chinese infants by tandem mass spectrometry and gas chromatography/mass spectrometry. Clin. Chim. Acta 2011, 412, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Castiñeras, D.E.; Couce, M.-L.; Marín, J.L.; González-Lamuño, D.; Rocha, H. Newborn screening for metabolic disorders in Spain and worldwide. An. Pediatr. 2019, 91, 128e.1–128e.4. [Google Scholar] [CrossRef]
- Edelbroek, P.M.; van der Heijden, J.; Stolk, L.M.L. Dried Blood Spot Methods in Therapeutic Drug Monitoring: Methods, Assays, and Pitfalls. Ther. Drug Monit. 2009, 31, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Delaby, C.; Vialaret, J.; Ducos, J.; Hirtz, C. Current and future use of “dried blood spot” analyses in clinical chemistry. Clin. Chem. Lab. Med. 2013, 51, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Gecse, K.B. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology 2022, 162, 1512–1524. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef]
- Stone, W.L.; Basit, H.; Adil, A. Glycogen Storage Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Herbert, M.; Pendyal, S.; Rairikar, M.; Halaby, C.; Benjamin, R.W.; Kishnani, P.S. Role of continuous glucose monitoring in the management of glycogen storage disorders. J. Inherit. Metab. Dis. 2018, 41, 917–927. [Google Scholar] [CrossRef]
- Kasapkara, Ç.S.; Cinasal Demir, G.; Hasanoğlu, A.; Tümer, L. Continuous glucose monitoring in children with glycogen storage disease type I. Eur. J. Clin. Nutr. 2014, 68, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-M.; Teymourian, H.; De la Paz, E.; Sempionatto, J.R.; Mahato, K.; Sonsa-ard, T.; Huang, N.; Longardner, K.; Litvan, I.; Wang, J. Non-Invasive Sweat-Based Tracking of L-Dopa Pharmacokinetic Profiles Following an Oral Tablet Administration. Angew. Chem. Int. Ed. 2021, 60, 19074–19078. [Google Scholar] [CrossRef] [PubMed]
- Dauphin-Ducharme, P.; Yang, K.; Arroyo-Currás, N.; Ploense, K.L.; Zhang, Y.; Gerson, J.; Kurnik, M.; Kippin, T.E.; Stojanovic, M.N.; Plaxco, K.W. Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS Sens. 2019, 4, 2832–2837. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, W.; Wang, B.; Zhao, Y.; En, K.; Zhu, J.; Cheng, X.; Zhou, C.; Lin, H.; Wang, Z.; et al. Noninvasive wearable electroactive pharmaceutical monitoring for personalized therapeutics. Proc. Natl. Acad. Sci. USA 2020, 117, 19017–19025. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous Opioid Monitoring along with Nerve Agents on a Wearable Microneedle Sensor Array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef]
- Tai, L.-C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sempionatto, J.R.; Li, Z.; Brown, C.; Galdino, N.M.; Shah, R.; Liu, S.; Hubble, L.J.; Bagot, K.; Tapert, S.; et al. Simultaneous detection of salivary Δ9-tetrahydrocannabinol and alcohol using a Wearable Electrochemical Ring Sensor. Talanta 2020, 211, 120757. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical biosensors: A nexus for precision medicine. Drug Discov. Today 2020, 26, 69–79. [Google Scholar] [CrossRef]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical biosensors—Illuminating the path to personalized drug dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Bteich, M.; Tawk, Y.; Ramadan, A.H.; Dia, B.; Asadallah, F.A.; Eid, A.; Kanj, R.; Costantine, J.; Eid, A.A. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020, 6, eaba5320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, J.; Wang, F.; Kong, D. Stretchable and Superwettable Colorimetric Sensing Patch for Epidermal Collection and Analysis of Sweat. ACS Sens. 2021, 6, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.; Bandodkar, A.; Cinti, S.; Mercier, P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Luo, Y.; Xu, T.; Zhang, X. Wearable Plasmonic Sweat Biosensor for Acetaminophen Drug Monitoring. ACS Sens. 2023, 8, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Tawk, Y.; Azar, S.; Ramadan, A.; Dia, B.; Shamieh, E.; Zoghbi, S.; Kanj, R.; Costantine, J.; Eid, A. Wearable flexible body matched electromagnetic sensors for personalized non-invasive glucose monitoring. Sci. Rep. 2022, 12, 14885. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fan, C.; Xu, T.; Su, L.; Zhang, X. An electrochemical wearable sensor for levodopa quantification in sweat based on a metal–Organic framework/graphene oxide composite with integrated enzymes. Sens. Actuators B Chem. 2022, 359, 131586. [Google Scholar] [CrossRef]
- Andersson, H.C. Dietary guidelines for inborn errors of metabolism. J. Pediatr. 2017, 188, 1–2. [Google Scholar] [CrossRef][Green Version]
- Levatte, M.; Hassanzadeh Keshteli, A.; Zarei, P.; Wishart, D. Applications of Metabolomics to Precision Nutrition. Lifestyle Genom. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Frazier, D.M.; Allgeier, C.; Homer, C.; Marriage, B.J.; Ogata, B.; Rohr, F.; Splett, P.L.; Stembridge, A.; Singh, R.H. Nutrition management guideline for maple syrup urine disease: An evidence- and consensus-based approach. Mol. Genet. Metab. 2014, 112, 210–217. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Nahata, M.C.; Allen Jr, L.V. Extemporaneous drug formulations. Clin. Ther. 2008, 30, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, A.; Resseguier, N.; Cano, A.; De Lonlay, P.; Arnoux, J.-B.; Brassier, A.; Schiff, M.; Pichard, S.; Fabre, A.; Hoebeke, C. Determinants of quality of life in children with inborn errors of metabolism receiving a restricted diet. J. Pediatr. 2022, 242, 192–200.e193. [Google Scholar] [CrossRef]
- Yeowell, G.; Burns, D.S.; Fatoye, F. The burden of pharmacological treatment on health-related quality of life in people with a urea cycle disorder: A qualitative study. J. Patient-Rep. Outcomes 2021, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Ueda, K.; Houben, R.F.A.; Joa, J.; Giezen, A.; Cheng, B.; van Karnebeek, C.D.M. Metabolic Diet App Suite for inborn errors of amino acid metabolism. Mol. Genet. Metab. 2016, 117, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Alrige, M.; Banjar, H.; Shuaib, T.; Ahmed, A.; Gharbawi, R. Knowledge-Based Dietary Intake Recommendations of Nutrients for Pediatric Patients with Maple Syrup Urine Disease. Healthcare 2023, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.A.; Brown, C.S.; Greene, C.; Camp, K.M.; McDonough, S.; Bocchini, J.A., Jr. Medical Foods for Inborn Errors of Metabolism: History, Current Status, and Critical Need. Pediatrics 2020, 145, e20192261. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C.; Sheikh, A.A. 3D printing in aerospace and its long-term sustainability. Virtual Phys. Prototyp. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Englezos, K.; Wang, L.; Tan, E.C.K.; Kang, L. 3D printing for personalised medicines: Implications for policy and practice. Int. J. Pharm. 2023, 635, 122785. [Google Scholar] [CrossRef]
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Goyanes, A.; Basit, A.W.; Zidan, A.S.; Xu, C.; Li, W.; Narayan, R.J.; Chen, R.K. A Review of State-of-the-Art on Enabling Additive Manufacturing Processes for Precision Medicine. J. Manuf. Sci. Eng. 2023, 145, 010802. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The application of 3D printing in the formulation of multilayered fast dissolving oral films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Carou-Senra, P.; Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Inkjet Printing of Pharmaceuticals. Adv. Mater. 2023, 2309164. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Cho, H.W.; Baek, S.H.; Lee, B.J.; Jin, H.E. Orodispersible Polymer Films with the Poorly Water-Soluble Drug, Olanzapine: Hot-Melt Pneumatic Extrusion for Single-Process 3D Printing. Pharmaceutics 2020, 12, 692. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Yan, T.-T.; Lv, Z.-F.; Tian, P.; Lin, M.-M.; Lin, W.; Huang, S.-Y.; Chen, Y.-Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, D.-T.; Ana, S.-E. A micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int. J. Pharm. 2021, 605, 120854. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. Int. J. Pharm. 2021, 598, 120360. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, L.; Zhu, X.; Chen, F.; Han, X. Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering 2023, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Hollis, E.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis. Int. J. Pharm. X 2023, 5, 100165. [Google Scholar] [CrossRef]
- Awad, A.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed infliximab suppositories for rectal biologic delivery. Int. J. Pharm. X 2023, 5, 100176. [Google Scholar] [CrossRef] [PubMed]
- Domsta, V.; Krause, J.; Weitschies, W.; Seidlitz, A. 3D Printing of Paracetamol Suppositories: An Automated Manufacturing Technique for Individualized Therapy. Pharmaceutics 2022, 14, 2676. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D.; Bouropoulos, N.; Barmpalexis, P.; Eleftheriadis, G.K.; Fatouros, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 102292. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683. [Google Scholar] [CrossRef]
- Cui, M.; Li, Y.; Wang, S.; Chai, Y.; Lou, J.; Chen, F.; Li, Q.; Pan, W.; Ding, P. Exploration and Preparation of a Dose-Flexible Regulation System for Levetiracetam Tablets via Novel Semi-Solid Extrusion Three-Dimensional Printing. J. Pharm. Sci. 2019, 108, 977–986. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef]
- Rahman-Yildir, J.; Fischer, B.; Breitkreutz, J. Development of sustained-release drug-loaded intravesical inserts via semi-solid micro-extrusion 3D-printing for bladder targeting. Int. J. Pharm. 2022, 622, 121849. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Goto, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. Lyophilized ophthalmologic patches as novel corneal drug formulations using a semi-solid extrusion 3D printer. Int. J. Pharm. 2022, 617, 121448. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef]
- Van Riet-Nales, D.A.; Kozarewicz, P.; Aylward, B.; de Vries, R.; Egberts, T.C.G.; Rademaker, C.M.A.; Schobben, A.F.A.M. Paediatric Drug Development and Formulation Design—A European Perspective. AAPS PharmSciTech 2017, 18, 241–249. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef]
- Suárez-González, J.; Magariños-Triviño, M.; Díaz-Torres, E.; Cáceres-Pérez, A.R.; Santoveña-Estévez, A.; Fariña, J.B. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: A comparative study for hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H.E. Formulation and Characterisation of Carbamazepine Orodispersible 3D-Printed Mini-Tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, U.; Jorvekar, S.B.; Suryanarayana Murty, U.; Borkar, R.M.; Banerjee, S. Extrusion 3D printing of minicaplets for evaluating in vitro & in vivo praziquantel delivery capability. Int. J. Pharm. 2023, 630, 122445. [Google Scholar] [CrossRef] [PubMed]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert. Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Nyamweya, N.; Kimani, S. Chewable tablets: A review of formulation considerations. Pharm. Technol. 2020, 44, 38–44. [Google Scholar]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of developing hospital preparation by semisolid extrusion 3D printing: Personalized amlodipine besylate chewable tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- El-Gazayerly, O.N.; Rakkanka, V.; Ayres, J.W. Novel Chewable Sustained-Release Tablet Containing Verapamil Hydrochloride. Pharm. Dev. Technol. 2004, 9, 181–188. [Google Scholar] [CrossRef]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suñé-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef] [PubMed]
- Chatzitaki, A.-T.; Mystiridou, E.; Bouropoulos, N.; Ritzoulis, C.; Karavasili, C.; Fatouros, D.G. Semi-solid extrusion 3D printing of starch-based soft dosage forms for the treatment of paediatric latent tuberculosis infection. J. Pharm. Pharmacol. 2021, 74, 1498–1506. [Google Scholar] [CrossRef]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Siamidi, A.; Protopapa, C.; Sotiropoulou, I. A review on the colours, flavours and shapes used in paediatric 3D printed oral solid dosage forms. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad009. [Google Scholar] [CrossRef]
- Karavasili, C.; Zgouro, P.; Manousi, N.; Lazaridou, A.; Zacharis, C.K.; Bouropoulos, N.; Moschakis, T.; Fatouros, D.G. Cereal-Based 3D Printed Dosage Forms for Drug Administration During Breakfast in Pediatric Patients within a Hospital Setting. J. Pharm. Sci. 2022, 111, 2562–2570. [Google Scholar] [CrossRef]
- Secinaro, S.; Calandra, D.; Secinaro, A.; Muthurangu, V.; Biancone, P. The role of artificial intelligence in healthcare: A structured literature review. BMC Med. Inform. Decis. Mak. 2021, 21, 125. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, S.N. Application of artificial intelligence-based technologies in the healthcare industry: Opportunities and challenges. Int. J. Environ. Res. Public. Health 2021, 18, 271. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; McCoubrey, L.E.; Elbadawi, M.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advancing pharmacy and healthcare with virtual digital technologies. Adv. Drug Deliv. Rev. 2022, 182, 114098. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chakraborty, D.; Law, A. Artificial intelligence in Internet of things. CAAI Trans. Intell. Technol. 2018, 3, 208–218. [Google Scholar] [CrossRef]
- Jiménez, F.; Pérez-Sánchez, H.; Palma, J.; Sánchez, G.; Martínez, C. A methodology for evaluating multi-objective evolutionary feature selection for classification in the context of virtual screening. Soft Comput. 2019, 23, 8775–8800. [Google Scholar] [CrossRef]
- Pérez-Sánchez, H.; Cano, G.; García-Rodríguez, J. Improving drug discovery using hybrid softcomputing methods. Appl. Soft Comput. 2014, 20, 119–126. [Google Scholar] [CrossRef]
- Gunčar, G.; Kukar, M.; Notar, M.; Brvar, M.; Černelč, P.; Notar, M.; Notar, M. An application of machine learning to haematological diagnosis. Sci. Rep. 2018, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W.; Chen, Y.; Guo, Y.; Li, G.-Z.; Zhu, X. A novel multi-target regression framework for time-series prediction of drug efficacy. Sci. Rep. 2017, 7, 40652. [Google Scholar] [CrossRef] [PubMed]
- Maltarollo, V.G.; Gertrudes, J.C.; Oliveira, P.R.; Honorio, K.M. Applying machine learning techniques for ADME-Tox prediction: A review. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv. Drug Deliv. Rev. 2021, 175, 113805. [Google Scholar] [CrossRef]
- Obeid, S.; Madžarević, M.; Krkobabić, M.; Ibrić, S. Predicting drug release from diazepam FDM printed tablets using deep learning approach: Influence of process parameters and tablet surface/volume ratio. Int. J. Pharm. 2021, 601, 120507. [Google Scholar] [CrossRef]
- Ong, J.J.; Castro, B.M.; Gaisford, S.; Cabalar, P.; Basit, A.W.; Pérez, G.; Goyanes, A. Accelerating 3D printing of pharmaceutical products using machine learning. Int. J. Pharm. X 2022, 4, 100120. [Google Scholar] [CrossRef]
- Tagami, T.; Morimura, C.; Ozeki, T. Effective and simple prediction model of drug release from “ghost tablets” fabricated using a digital light projection-type 3D printer. Int. J. Pharm. 2021, 604, 120721. [Google Scholar] [CrossRef] [PubMed]
- Westphal, E.; Seitz, H. A machine learning method for defect detection and visualization in selective laser sintering based on convolutional neural networks. Addit. Manuf. 2021, 41, 101965. [Google Scholar] [CrossRef]
- Elbadawi, M.; Muñiz Castro, B.; Gavins, F.K.H.; Jie Ong, J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, Á. M3DISEEN: A Novel Machine Learning Approach for Predicting the 3D Printability of Medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef] [PubMed]
- Carou-Senra, P.; Ong, J.J.; Castro, B.M.; Seoane-Viano, I.; Rodríguez-Pombo, L.; Cabalar, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Pérez, G.; Goyanes, A. Predicting pharmaceutical inkjet printing outcomes using machine learning. Int. J. Pharm. X 2023, 5, 100181. [Google Scholar] [CrossRef] [PubMed]
- Rezapour Sarabi, M.; Alseed, M.M.; Karagoz, A.A.; Tasoglu, S. Machine Learning-Enabled Prediction of 3D-Printed Microneedle Features. Biosensors 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Lyousoufi, M.; Lafeber, I.; Kweekel, D.; de Winter, B.C.M.; Swen, J.J.; Le Brun, P.P.H.; Bijleveld-Olierook, E.C.M.; van Gelder, T.; Guchelaar, H.-J.; Moes, D.J.A.R.; et al. Development and Bioequivalence of 3D-Printed Medication at the Point-of-Care: Bridging the Gap Toward Personalized Medicine. Clin. Pharmacol. Ther. 2023, 113, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, K.; Hong, S.; Wang, Z.; Mo, M.; Li, S.; Yu, Y.; Chen, J.; Chen, J.; Zeng, W.; et al. Improving the quality and clinical efficacy of subdivided levothyroxine sodium tablets by 3D printing technology. J. Drug Deliv. Sci. Technol. 2023, 89, 105008. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Pérez-Ramos, T.; Liu, J.; Januskaite, P.; Guerra-Baamonde, E.; González-Ramírez, J.; Vázquez-Caruncho, M.; Basit, A.W.; Goyanes, A. Visualizing disintegration of 3D printed tablets in humans using MRI and comparison with in vitro data. J. Control Release 2024, 365, 348–357. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Technical Considerations for Additive Manufactured Medical Devices: Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/media/97633/download?attachment (accessed on 20 October 2023).
- Aprecia. Spritam. Available online: https://www.aprecia.com/zipdose-platform/zipdose-technology.php (accessed on 12 October 2023).
- Medicines and Healthcare products Regulatory Agency. Consultation on Point of Care Manufacturing. Available online: https://www.gov.uk/government/consultations/point-of-care-consultation/consultation-on-point-of-care-manufacturing (accessed on 12 October 2023).
- United States Food and Drug Administration. Distributed Manufacturing and Point-of-Care Manufacturing of Drugs. Available online: https://www.fda.gov/media/162157/download (accessed on 18 November 2023).
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Xu, X.; Ong, J.J.; Teyeb, A.; Gaisford, S.; Campos-Álvarez, A.; Stulz, A.; Marcuta, C.; Kraschew, L.; Mohr, W.; et al. A case study on decentralized manufacturing of 3D printed medicines. Int. J. Pharm. X 2023, 5, 100184. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Quality Attribute Considerations for Chewable Tablets Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Quality-Attribute-Considerations-for-Chewable-Tablets-Guidance-for-Industry.pdf (accessed on 26 July 2022).
- Jørgensen, A.K.; Ong, J.J.; Parhizkar, M.; Goyanes, A.; Basit, A.W. Advancing non-destructive analysis of 3D printed medicines. Trends Pharmacol. Sci. 2023, 44, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Seoane-Viaño, I.; Ong, J.J.; Januskaite, P.; Awwad, S.; Orlu, M.; Bande, M.F.; Basit, A.W.; Goyanes, A. Inkjet drug printing onto contact lenses: Deposition optimisation and non-destructive dose verification. Int. J. Pharm. X 2023, 5, 100150. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Szewc, J.; Zhong, L.; Leonova, A.; Giebułtowicz, J.; Habashy, R.; Isreb, A.; Alhnan, M.A. The use of near-infrared as process analytical technology (PAT) during 3D printing tablets at the point-of-care. Int. J. Pharm. 2023, 642, 123073. [Google Scholar] [CrossRef] [PubMed]
- Stranzinger, S.; Wolfgang, M.; Klotz, E.; Scheibelhofer, O.; Ghiotti, P.; Khinast, J.G.; Hsiao, W.-K.; Paudel, A. Near-infrared hyperspectral imaging as a monitoring tool for on-demand manufacturing of inkjet-printed formulations. AAPS PharmSciTech 2021, 22, 211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carou-Senra, P.; Rodríguez-Pombo, L.; Monteagudo-Vilavedra, E.; Awad, A.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; Couce, M.L. 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations. Nutrients 2024, 16, 61. https://doi.org/10.3390/nu16010061
Carou-Senra P, Rodríguez-Pombo L, Monteagudo-Vilavedra E, Awad A, Alvarez-Lorenzo C, Basit AW, Goyanes A, Couce ML. 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations. Nutrients. 2024; 16(1):61. https://doi.org/10.3390/nu16010061
Chicago/Turabian StyleCarou-Senra, Paola, Lucía Rodríguez-Pombo, Einés Monteagudo-Vilavedra, Atheer Awad, Carmen Alvarez-Lorenzo, Abdul W. Basit, Alvaro Goyanes, and María L. Couce. 2024. "3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations" Nutrients 16, no. 1: 61. https://doi.org/10.3390/nu16010061
APA StyleCarou-Senra, P., Rodríguez-Pombo, L., Monteagudo-Vilavedra, E., Awad, A., Alvarez-Lorenzo, C., Basit, A. W., Goyanes, A., & Couce, M. L. (2024). 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations. Nutrients, 16(1), 61. https://doi.org/10.3390/nu16010061