A Systematic Review of Dietary Interventions for Cancer Survivors and Their Families or Caregivers
Abstract
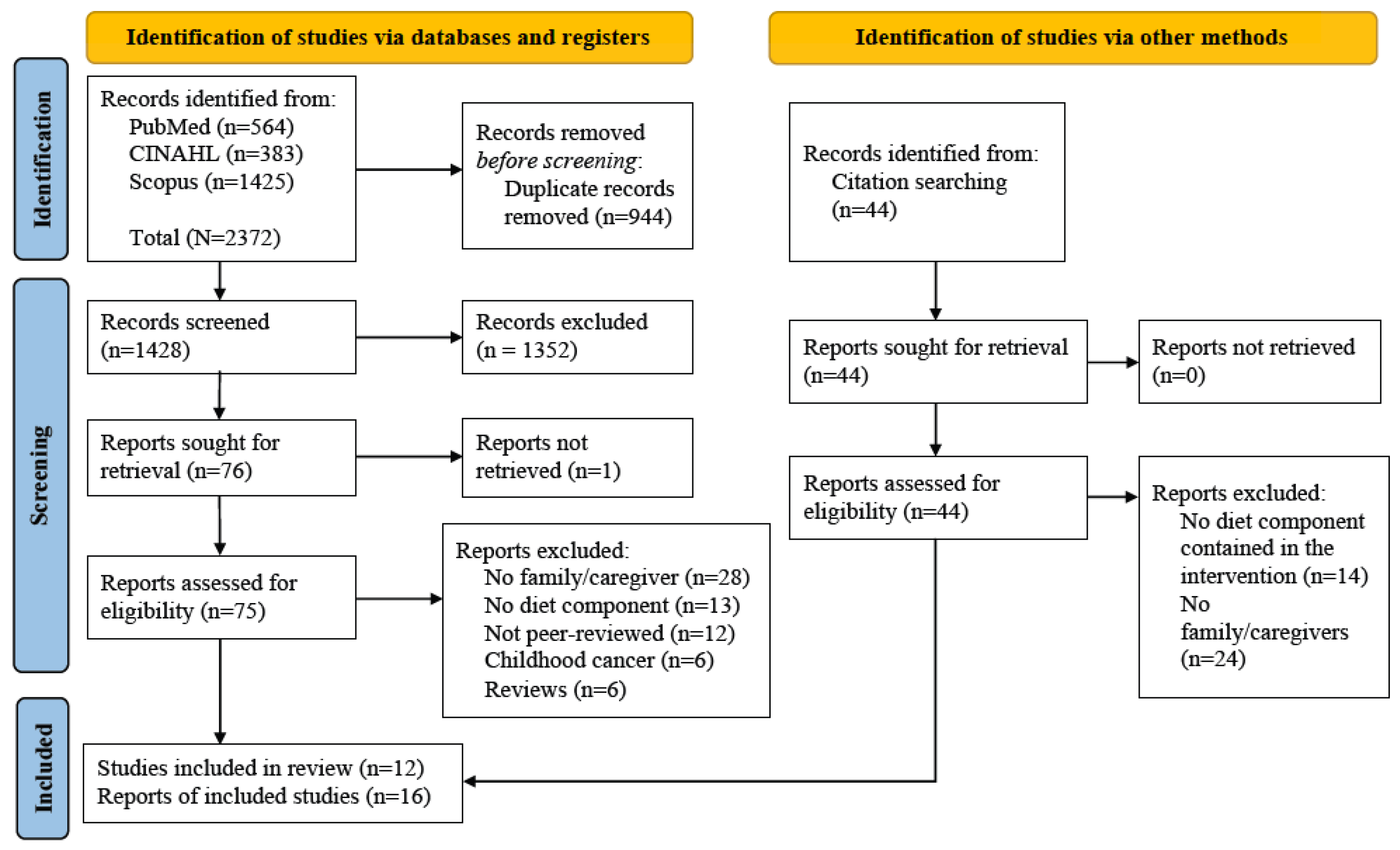
:1. Introduction
2. Materials and Methods
2.1. Search
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Quality Appraisal
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Intervention Characteristics
3.4. Behavior Change Techniques
3.5. Dietary Outcomes
3.5.1. Dietary Assessments
3.5.2. Overall Diet Quality/Patterns
3.5.3. Nutrients
3.5.4. Foods and Drinks
3.6. Health-Related Outcomes
3.6.1. Adiposity
3.6.2. Physical Performance
3.6.3. Physical Symptoms
3.6.4. Mental Conditions
3.6.5. Quality of Life
3.7. Psychosocial Constructs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chan, R.; Nekhlyudov, L. Overview of Cancer Survivorship Care for Primary Care and Oncology Providers. Available online: https://www.uptodate.com/contents/overview-of-cancer-survivorship-care-for-primary-care-and-oncology-providers/print#:~:text=There%20are%20more%20than%2018,million%20survivors%20worldwide%20%5B3%5D (accessed on 1 September 2023).
- Milajerdi, A.; Namazi, N.; Larijani, B.; Azadbakht, L. The Association of Dietary Quality Indices and Cancer Mortality: A Systematic Review and Meta-analysis of Cohort Studies. Nutr. Cancer 2018, 70, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The Facts about Food after Cancer Diagnosis: A Systematic Review of Prospective Cohort Studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef] [PubMed]
- Durazo, A.; Cameron, L.D. Representations of cancer recurrence risk, recurrence worry, and health-protective behaviours: An elaborated, systematic review. Health Psychol. Rev. 2019, 13, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Smethers, A.D.; Rolls, B.J. Dietary management of obesity: Cornerstones of healthy eating patterns. Med. Clin. N. Am. 2018, 102, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef]
- Patnode, C.D.; Redmond, N.; Iacocca, M.O.; Henninger, M. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: Updated evidence report and systematic review for the US preventive services task force. JAMA 2022, 328, 375–388. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Pallazola, V.A.; Davis, D.M.; Whelton, S.P.; Cardoso, R.; Latina, J.M.; Michos, E.D.; Sarkar, S.; Blumenthal, R.S.; Arnett, D.K.; Stone, N.J.; et al. A clinician’s guide to healthy eating for cardiovascular disease prevention. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 251–267. [Google Scholar] [CrossRef]
- Baguley, B.J.; Skinner, T.L.; Wright, O.R.L. Nutrition therapy for the management of cancer-related fatigue and quality of life: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The world cancer research fund/american institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lope, V.; Guerrero-Zotano, A.; Ruiz-Moreno, E.; Bermejo, B.; Antolín, S.; Montaño, Á.; Baena-Cañada, J.M.; Ramos Vázquez, M.; Fernández de Larrea-Baz, N.; Chacón, J.I.; et al. Clinical and Sociodemographic Determinants of Adherence to World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Recommendations in Breast Cancer Survivors-Health-EpiGEICAM Study. Cancers 2022, 14, 4705. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, R.; Nyrop, K.A.; Mayer, D.K. Healthy behaviors: Prevalence of uptake among cancer survivors. Clin. J. Oncol. Nurs. 2020, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to multiple health behaviours in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Tjon-A-Joe, S.; Pannekoek, S.; Kampman, E.; Hoedjes, M. Adherence to Diet and Body Weight Recommendations among Cancer Survivors after Completion of Initial Cancer Treatment: A Systematic Review of the Literature. Nutr. Cancer 2019, 71, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.; Sremanakova, J.; Jones, D.; Todd, C. Dietary interventions for cancer survivors. Proc. Nutr. Soc. 2019, 78, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Cheng, H.-L.; Tse, M.Y.M. A systematic review of nurse-led dietary interventions for cancer patients and survivors. Asia-Pac. J. Oncol. Nurs. 2022, 9, 81–87. [Google Scholar] [CrossRef]
- Keaver, L.; Douglas, P.; O’Callaghan, N. Perceived Barriers and Facilitators to a Healthy Diet among Cancer Survivors: A Qualitative Exploration Using the TDF and COM-B. Dietetics 2023, 2, 10. [Google Scholar] [CrossRef]
- Ryu, S.W.; Son, Y.G.; Lee, M.K. Motivators and barriers to adoption of a healthy diet by survivors of stomach cancer: A cross-sectional study. Eur. J. Oncol. Nurs. 2020, 44, 101703. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Jones, L.W.; Snyder, D.C.; Sloane, R.J.; Kimmick, G.G.; Hughes, D.C.; Badr, H.J.; Miller, P.E.; Burke, L.E.; Lipkus, I.M. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 2014, 120, 2522–2534. [Google Scholar] [CrossRef]
- Carmack, C.L.; Parker, N.H.; Demark-Wahnefried, W.; Shely, L.; Baum, G.; Yuan, Y.; Giordano, S.H.; Rodriguez-Bigas, M.; Pettaway, C.; Basen-Engquist, K. Healthy moves to improve lifestyle behaviors of cancer survivors and their spouses: Feasibility and preliminary results of intervention efficacy. Nutrients 2021, 13, 4460. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.M.; Partridge, J.A.; Morrissy, M.J. Cancer caregivers’ perceptions of an exercise and nutrition program. Support. Care Cancer 2013, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.R.; Raji, D.; Olaniran, M.; Alick, C.; Nichols, D.; Allicock, M. A systematic scoping review of post-treatment lifestyle interventions for adult cancer survivors and family members. J. Cancer Surviv. 2022, 16, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gong, J.; Li, Q. The application of eHealth in cancer survivorship care: A review of web-based dyadic interventions for post-treatment cancer survivors and caregivers. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100109. [Google Scholar] [CrossRef]
- Veritas Health Innovation Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2022.
- Drisko, J.; Maschi, T. Content Analysis; Oxford University Press: Oxford, UK, 2015; ISBN 9780190215491. [Google Scholar]
- UCL BCT Taxonomy Behavior Change Technique Taxonomy Online Training. Available online: http://www.bct-taxonomy.com/?n=1 (accessed on 6 September 2023).
- National Heart, Lung, and Blood Institute Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 September 2023).
- Bagias, C.; Sukumar, N.; Weldeselassie, Y.; Oyebode, O.; Saravanan, P. Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1897. [Google Scholar] [CrossRef]
- Crane, T.E.; Badger, T.A.; O’Connor, P.; Segrin, C.; Alvarez, A.; Freylersythe, S.J.; Penaloza, I.; Pace, T.W.W.; Sikorskii, A. Lifestyle intervention for Latina cancer survivors and caregivers: The Nuestra Salud randomized pilot trial. J. Cancer Surviv. 2021, 15, 607–619. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Oster, R.A.; Crane, T.E.; Rogers, L.Q.; Cole, W.W.; Kaur, H.; Farrell, D.; Parrish, K.B.; Badr, H.J.; Wolin, K.Y.; et al. Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners. Cancers 2023, 15, 1577. [Google Scholar] [CrossRef]
- Pekmezi, D.W.; Crane, T.E.; Oster, R.A.; Rogers, L.Q.; Hoenemeyer, T.; Farrell, D.; Cole, W.W.; Wolin, K.; Badr, H.; Demark-Wahnefried, W. Rationale and Methods for a Randomized Controlled Trial of a Dyadic, Web-Based, Weight Loss Intervention among Cancer Survivors and Partners: The DUET Study. Nutrients 2021, 13, 3472. [Google Scholar] [CrossRef] [PubMed]
- James, E.L.; Stacey, F.G.; Chapman, K.; Boyes, A.W.; Burrows, T.; Girgis, A.; Asprey, G.; Bisquera, A.; Lubans, D.R. Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: A pragmatic randomized controlled trial. BMC Cancer 2015, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- James, E.L.; Stacey, F.; Chapman, K.; Lubans, D.R.; Asprey, G.; Sundquist, K.; Boyes, A.; Girgis, A. Exercise and nutrition routine improving cancer health (ENRICH): The protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers. BMC Public Health 2011, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Stacey, F.G.; Lubans, D.R.; Chapman, K.; Bisquera, A.; James, E.L. Maintenance of Lifestyle Changes at 12-month Follow-up in a Nutrition and Physical Activity Trial for Cancer Survivors. Am. J. Health Behav. 2017, 41, 784–795. [Google Scholar] [CrossRef]
- Manne, S.L.; Kashy, D.A.; Zaider, T.; Kissane, D.; Lee, D.; Kim, I.Y.; Heckman, C.J.; Penedo, F.J.; Murphy, E.; Virtue, S.M. Couple-focused interventions for men with localized prostate cancer and their spouses: A randomized clinical trial. Br. J. Health Psychol. 2019, 24, 396–418. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.A.; Kahan, M.; Martinez, M.; Isaac, K.; Rossi, A.; Skyhart, R.; Wylie-Rosett, J.; Moadel-Robblee, A. Development and evaluation of the curriculum for BOLD (bronx oncology living daily) healthy living: A diabetes prevention and control program for underserved cancer survivors. J. Cancer Educ. 2015, 30, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, C.S.; Somers, T.J.; Shelby, R.A.; Winger, J.G.; Patel, M.L.; Kimmick, G.; Craighead, L.; Keefe, F.J. Development, feasibility, and acceptability of a behavioral weight and symptom management intervention for breast cancer survivors and intimate partners. J. Cancer Rehabil. 2022, 5, 7–16. [Google Scholar] [CrossRef]
- Knobf, M.T.; Erdos, D.; Jeon, S. Healthy Sisters: A Feasibility study of a health behavior intervention for women of color breast cancer survivors. J. Psychosoc. Oncol. 2018, 36, 597–608. [Google Scholar] [CrossRef]
- Krouse, R.S.; Grant, M.; McCorkle, R.; Wendel, C.S.; Cobb, M.D.; Tallman, N.J.; Ercolano, E.; Sun, V.; Hibbard, J.H.; Hornbrook, M.C. A chronic care ostomy self-management program for cancer survivors. Psychooncology 2016, 25, 574–581. [Google Scholar] [CrossRef]
- Grant, M.; McCorkle, R.; Hornbrook, M.C.; Wendel, C.S.; Krouse, R. Development of a chronic care ostomy self-management program. J. Cancer Educ. 2013, 28, 70–78. [Google Scholar] [CrossRef]
- Stoutenberg, M.; Sogor, A.; Arheart, K.; Cutrono, S.E.; Kornfeld, J. A Wellness Program for Cancer Survivors and Caregivers: Developing an Integrative Pilot Program with Exercise, Nutrition, and Complementary Medicine. J. Cancer Educ. 2016, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kelley, H.H.; Thibaut, T.W. Interpersonal Relations: A Theory of Interdependence; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Rentscher, K.E. Communal coping in couples with health problems. Front. Psychol. 2019, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Functional Fitness Normative Scores for Community-Residing Older Adults, Ages 60–94. J. Aging Phys. Act. 1999, 7, 162–181. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Yin, X.; Zou, C.; Li, H. Effects of behavioral change techniques on diet and physical activity in colorectal cancer patients: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 29. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Tolia, M.; Poultsidi, A.; Vasios, G.K.; Papandreou, D.; Theocharis, S.; Kavantzas, N.; Troumbis, A.Y.; Giaginis, C. Adherence to Mediterranean Diet and Nutritional Status in Women with Breast Cancer: What Is Their Impact on Disease Progression and Recurrence-Free Patients’ Survival? Curr. Oncol. 2022, 29, 589. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 dietary guidance to improve cardiovascular health: A scientific statement from the american heart association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Quattrocchi, A.; Degrassi, F.; Catalano, F.; Basile, G.; Agodi, A. The Effects of Diet and Dietary Interventions on the Quality of Life among Breast Cancer Survivors: A Cross-Sectional Analysis and a Systematic Review of Experimental Studies. Cancers 2020, 12, 322. [Google Scholar] [CrossRef]
- Bektas Akpinar, N.; Beduk, T.; Cay Senler, F. The effect of caregiver educational program on caregiver reactions and lifestyle behaviors for caregivers of colorectal cancer patients: A quasi-experimental study. Support. Care Cancer 2022, 30, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Romo, R.D.; Campbell, C.L. A systematic review of interventions for family caregivers who care for patients with advanced cancer at home. Patient Educ. Couns. 2020, 103, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Samad, S.; Ahmed, F.; Naher, S.; Kabir, M.A.; Das, A.; Amin, S.; Islam, S.M.S. Smartphone apps for tracking food consumption and recommendations: Evaluating artificial intelligence-based functionalities, features and quality of current apps. Intell. Syst. Appl. 2022, 15, 200103. [Google Scholar] [CrossRef]
- Rowland, S.A.; Schumacher, K.L.; Leinen, D.D.; Phillips, B.G.; Schulz, P.S.; Yates, B.C. Couples’ experiences with healthy lifestyle behaviors after cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2018, 38, 170–174. [Google Scholar] [CrossRef] [PubMed]
- August, K.J. Correlates of diet-related spousal involvement among both members of couples managing diabetes. Fam. Syst. Health 2021, 39, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.; Wang, Z.; Li, Q. Couple-Based Communication Interventions for Cancer Patient-Spousal Caregiver Dyads’ Psychosocial Adaptation to Cancer: A Systematic Review. Healthcare 2023, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.J.; Leavitt, C.E.; Hansen, D.; Gill, S. Diet-related relationship pressure and conflict: Generational differences in the moderating effects of mindfulness. Res. Hum. Dev. 2020, 17, 195–210. [Google Scholar] [CrossRef]
- Badr, H.; Bakhshaie, J.; Chhabria, K. Dyadic interventions for cancer survivors and caregivers: State of the science and new directions. Semin. Oncol. Nurs. 2019, 35, 337–341. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, S.Y.; Choi, G.-S. Facilitators and barriers to adoption of a healthy diet in survivors of colorectal cancer. J. Nurs. Scholarsh. 2019, 51, 509–517. [Google Scholar] [CrossRef]
- Yannitsos, D.; Murphy, R.A.; Pollock, P.; Di Sebastiano, K.M. Facilitators and barriers to participation in lifestyle modification for men with prostate cancer: A scoping review. Eur. J. Cancer Care 2020, 29, e13193. [Google Scholar] [CrossRef]
- Hoedjes, M.; Nijman, I.; Hinnen, C. Psychosocial Determinants of Lifestyle Change after a Cancer Diagnosis: A Systematic Review of the Literature. Cancers 2022, 14, 2026. [Google Scholar] [CrossRef] [PubMed]
- Dolan, H.R.; Alvarez, A.A.; Freylersythe, S.J.; Penaloza, I.; Grijalva, S.; Taylor-Piliae, R.; Crane, T.E. Barriers and facilitators for adopting a healthy lifestyle among Latina cancer survivors: A qualitative descriptive study. Support. Care Cancer 2022, 30, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef] [PubMed]
- Langlais, C.S.; Graff, R.E.; Van Blarigan, E.L.; Palmer, N.R.; Washington, S.L.; Chan, J.M.; Kenfield, S.A. Post-Diagnostic Dietary and Lifestyle Factors and Prostate Cancer Recurrence, Progression, and Mortality. Curr. Oncol. Rep. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Balhareth, A.; Aldossary, M.Y.; McNamara, D. Impact of physical activity and diet on colorectal cancer survivors’ quality of life: A systematic review. World J. Surg. Oncol. 2019, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, S.; Colatruglio, S.; La Vela, V.; Tagliabue, E.; Mariani, L.; Gavazzi, C. Nutritional intervention in head and neck cancer patients during chemo-radiotherapy. Nutrition 2018, 51–52, 95–97. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Gala, D.; Wright, H.H.; Zigori, B.; Marshall, S.; Crichton, M. Dietary strategies for chemotherapy-induced nausea and vomiting: A systematic review. Clin. Nutr. 2022, 41, 2147–2155. [Google Scholar] [CrossRef]
- Litzelman, K. Caregiver Well-being and the Quality of Cancer Care. Semin. Oncol. Nurs. 2019, 35, 348–353. [Google Scholar] [CrossRef]
- Jadalla, A.; Ginex, P.; Coleman, M.; Vrabel, M.; Bevans, M. Family Caregiver Strain and Burden: A Systematic Review of Evidence-Based Interventions When Caring for Patients With Cancer. Clin. J. Oncol. Nurs. 2020, 24, 31–50. [Google Scholar] [CrossRef]
- Frazelle, M.L.; Friend, P.J. Optimizing the teachable moment for health promotion for cancer survivors and their families. J. Adv. Pract. Oncol. 2016, 7, 422–433. [Google Scholar]
- Glenn, B.A.; Hamilton, A.S.; Nonzee, N.J.; Maxwell, A.E.; Crespi, C.M.; Ryerson, A.B.; Chang, L.C.; Deapen, D.; Bastani, R. Obesity, physical activity, and dietary behaviors in an ethnically-diverse sample of cancer survivors with early onset disease. J. Psychosoc. Oncol. 2018, 36, 418–436. [Google Scholar] [CrossRef]
- Dibble, K.E.; Connor, A.E. Evaluation of disparities in maintaining healthy lifestyle behaviors among female cancer survivors by race/ethnicity and US nativity. Cancer Epidemiol. 2022, 80, 102235. [Google Scholar] [CrossRef]
- Dhunna, S.; Tarasuk, V. Black-white racial disparities in household food insecurity from 2005 to 2014, Canada. Can. J. Public Health 2021, 112, 888–902. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and racial disparity in breast cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef]
- Minas, T.Z.; Kiely, M.; Ajao, A.; Ambs, S. An overview of cancer health disparities: New approaches and insights and why they matter. Carcinogenesis 2021, 42, 2–13. [Google Scholar] [CrossRef]
- Zhang, F.F.; Cudhea, F.; Shan, Z.; Michaud, D.S.; Imamura, F.; Eom, H.; Ruan, M.; Rehm, C.D.; Liu, J.; Du, M.; et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019, 3, pkz034. [Google Scholar] [CrossRef]
- Stolley, M.R.; Sharp, L.K.; Oh, A.; Schiffer, L. A weight loss intervention for African American breast cancer survivors, 2006. Prev. Chronic. Dis. 2009, 6, A22. [Google Scholar]
- Er, V.; Lane, J.A.; Martin, R.M.; Persad, R.; Chinegwundoh, F.; Njoku, V.; Sutton, E. Barriers and facilitators to healthy lifestyle and acceptability of a dietary and physical activity intervention among African Caribbean prostate cancer survivors in the UK: A qualitative study. BMJ Open 2017, 7, e017217. [Google Scholar] [CrossRef]
- Chlebowy, D.O.; Hood, S.; LaJoie, A.S. Facilitators and barriers to self-management of type 2 diabetes among urban African American adults: Focus group findings. Diabetes Educ. 2010, 36, 897–905. [Google Scholar] [CrossRef]
- Crookes, D.M.; Shelton, R.C.; Tehranifar, P.; Aycinena, C.; Gaffney, A.O.; Koch, P.; Contento, I.R.; Greenlee, H. Social networks and social support for healthy eating among Latina breast cancer survivors: Implications for social and behavioral interventions. J. Cancer Surviv. 2016, 10, 291–301. [Google Scholar] [CrossRef]

| Author (Year) | Country | n (SUR/FC) | Role of FC | Cancer | Time since Diagnosis (Years) | Treatment | Age (SUR/FC) | Gender (M/F) | Race/Ethnicity | Education Level |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized Controlled Trials | ||||||||||
| Carmack (2021) [22] | US | 22/22 | Spouse | Breast, Prostate, Colorectal | NR | Surgery = 17, Radiation = 11, Hormonal = 11, Chemo = 8, Other = 3 | 64.1/63.4 | 21/23 | NHW = 32, Hispanic = 7, NHB = 3, Other = 2 | High school diploma/GED = 2, Some college = 6, Bachelor’s = 8, Advanced degree = 6, |
| Crane (2021) [34] | US | 45/45 | Child = 11, Spouse/partner =10, Friend = 9, Sibling = 4, Parent = 2, Other = 1 | Breast, Head or Neck, Liver, Colon, Kidney, Lymphoma, Other | IG 9.02 CG 11.91 | Undergoing active cancer treatments = 6 | 61.16/52.19 | 0/90 | Latina only | <High school = 6, High school/GED = 5, Vocational/technical/community college = 5, 4-year college = 3, Post-graduate = 2 |
| Denmark-Wahnefried (2014) [21] | US | 68/68 | Daughter | Breast | 2 | NR | 61.3/32.9 | 0/136 | NHW=100, NHB=24, Hispanic White=10, Asian=2 | <High school = 1, High school graduate = 25, Some college/junior college/trade school = 49, College graduate = 60 |
| Denmark-Wahnefried (2023) [35] Pekmezi (2021) [36] | US | 56/56 | Spouse = 23, Friend = 17, Sibling = 7, Child = 6, Other = 3 | Breast, Other | 5.6 | NR | 58.4 | 26/86 | NHW = 70, NHB = 41, Other = 1 | ≤High school = 16, Some college/junior college/trade school = 34, College degree = 59, Unknown = 2 |
| James (2015) [37] James (2011) [38] Stacey (2017) [39] | Australia | 96/24 12 both | Spouse/partner = 23, Other relative or friend = 10 | Breast, Prostate, Bowel, Colorectal, Melanoma, Other | CG 3.8 IG 3.7 | Surgery = 100, Chemo = 73, Radiotherapy = 62, Hormonal = 50 | 56.6 | 30/103 | NR | Completed post-school qualifications = 95 |
| Manne (2021) [40] | US | 237/237 | Spouse | Prostate | 0.4 | Surgery only = 200, Radiation only = 15, Radiation and hormone Rx = 10, Radiation, surgery, and hormone Rx = 2, Surgery and hormone Rx = 8, Surgery and radiation = 2 | 60.6/57.1 | 239/235 | White = 355, Black = 93, Asian = 5, Hispanic = 11, Other = 7 | <High school = 62, Some college = 88, College degree = 114, Above college = 208 |
| Single-group pre-experimental studies | ||||||||||
| Anton (2013) [23] | US | 0/12 | Husband = 6, Wife = 3, Girlfriend = 1, Friend = 2 | Breast, Uterine, Prostate, Leukemia, Testicular | UN | NR | NA/61.2 | 6/6 | NR | NR |
| Conlon (2016) [41] | US | 66/17 | NR | Breast, Gynecological, Lung, Other | 5.1 | NR | 60.5 | 4/79 | NHB = 46, Hispanic/Latino = 22, Other = 15 | NR |
| Dorfman (2022) [42] | US | 12/12 | Married partner | Breast | 1.7 | Surgery = 13, Chemo = 8, Radiation = 8, hormonal = 11 | 58.9/62.7 | 12/12 | White = 20, Black = 4, Non-Hispanic = 23 | High school diploma/GED = 5, Some college = 6, Bachelor’s = 11, Master’s = 2 |
| Knobf (2018) [43] | US | 35/14 | Partner (not necessarily romantic) | Breast | NR | NR | 55.7 | 0/49 | Black = 35, White = 1, Hispanic = 4 | High school = 8, Technical school = 2, College = 23, Graduate school = 7 |
| Krouse (2016) [44] Grant (2013) [45] | US | 38/22 | Caregiver | Colorectal, Bladder, Prostate, Ovarian | NR | Colostomy/ileostomy = 23, Urostomy = 11, Unknown = 4 | 71.3/NA | 28/10 | NHW = 32, Hispanic White =1, Black = 1 | NR |
| Stoutenberg (2016) [46] | US | 16/4 | Caregiver | Breast, Multiple, Prostate, Gastric, Myeloma, Pancreatic | NR | NR | 62.5 | 5/15 | NR | Some college = 5, Bachelor’s or greater = 15 |
| Author (Year) | DB/ IB | Theory | Interventionist | Frequency | Duration in months | Assessments | Intervention Content | Comparand/Control |
|---|---|---|---|---|---|---|---|---|
| Randomized Controlled Trials | ||||||||
| Carmack (2021) [22] | DB | Social Cognitive Theory | Counselor | Sessions 1–3 weekly, Sessions 4–5 once every other week, Sessions 6–9 once per month | 6 | Baseline, 6-month | 9 web-based or telephone-based counseling sessions for a couple together; printed tailored workbook and 3 newsletters; guidance, tools, and logbooks to track diet behaviors (couple-based) | No reference to working with a spouse on behavior changes (survivor only) |
| Crane (2021) [34] | IB | Social Cognitive Theory | Trained bicultural health coach | Once per week | 3 | Baseline, week 13 | 20–30 min coaching calls in English or Spanish with dyads separately or together depending on their preferences; Fitbit; symptom management and survivorship handbook | A call from the research team for symptom assessment and change tracking every week |
| Denmark-Wahnefried (2014) [21] | DB | Social Cognitive Theory, the Transtheoretical Model of Behavior Change, Interdependence Theory, Theory of Communal Coping | NR | Bi-monthly | 12 | Baseline, 6-month, and 12-month; bi-monthly survey to track progress | Individual group: a personalized initial workbook, 6 tailored newsletters, supplies, and equipment for self-monitoring Dyad group: information and supplies identical to individual group and information to promote effective dyadic communications | Standardized diet and exercise materials |
| Denmark-Wahnefried (2023) [35] Pekmezi (2021) [36] | DB | Social Cognitive Theory, Interdependence Theory, Theory of Communal Coping | NR | Weekly | 6 | Baseline, 3-month, and 6-month | A website to provide 24 interactive sessions (15 min each) for feedback and guidance, prompt text messages per week, and daily tips for weight management, diet, and exercise; Portion Doctor tableware; Fitbits; Aria 2 digital scales; and instructions for MyFitnessPal | Waitlist control |
| James (2015) [37] James (2011) [38] Stacey (2017) [39] | IB | Social Cognitive Theory, Chronic Disease Self-Management Framework | Qualified exercise specialists and accredited practicing dietitian | Weekly and fortnightly | 2 | Baseline, post-treatment, and 3-month post-treatment (20 weeks) | Walking program, resistance training program, information about healthy eating and maintaining a healthy weight, information delivery and practical activities, workbook, pedometer, Gymstick | Waitlist control |
| Manne (2021) [40] | DB | Relationship Intimacy Model of Cancer Adaptation | Psychologists, social workers, certified nutritionists, personal trainers | Weekly, booster call 2–3 weeks after | 2 | Baseline, 5-week, 3-month, and 6-month | 5 sessions (90 min) and 1 booster call (30–45 min); Intimacy-Enhancing Therapy intervention arm: couples’ communication regarding cancer, mutual understanding and support, constructive discussion of cancer concerns, emotional intimacy; General Health and Wellness intervention arm: dietary assessment, setting goals, plant-based diet, relaxation | Standard care |
| Pre-experimental Trials | ||||||||
| Anton (2013) [23] | IB | NR | Class instructor, personal trainer | Class twice per week, exercise session twice per week | 3 | Post-intervention | Basic nutrition and exercise class, individual-tailored exercise session | NA |
| Conlon (2016) [41] | IB | Bloom’s Taxonomy of Cognitive Development, Social-ecological Framework | Registered dietitian nutritionist, exercise physiologist, or trained staff | Once per week | 3 or 1 | Pre- to post-program | Culturally and medically adapted nutrition education class (60–75 min) and group exercise class (60 min), a diabetes prevention and management toolkit related to goal setting, food and activity journaling, daily pedometer use, and a “buddy system” | NA |
| Dorfman (2022) [42] | DB | Interdependence Model of Communal Coping and Behavior Change | Clinical psychologist | Sessions 1–6 weekly, Sessions 7–12 bi-weekly | 4.5 | Baseline, post-treatment, and 3-month | 12 couple-based sessions of weight management, diet and physical activity guidance, appetite awareness training, symptom management protocols, and progress review; written patient manual, Fitbit, food diaries | NA |
| Knobf (2018) [43] | IB | NR | Exercise physiologist, dietician, oncology nurse practitioner students | Weekly | 1.5 | Baseline, post-program, 3-month, and 6-month | Face-to-face interactive sessions regarding symptom management, physical activity, healthy eating, bonding, and community sources; prayer at the end of each session | NA |
| Krouse (2016) [44] Grant (2013) [45] | IB | Chronic Care Model | Research staff, experienced ostomy nurses, peer ostomates | Sessions 1 and 2 on one day, Sessions 3, 4, and 5 one month later | 1 | Pre-intervention, post-intervention, and 6-month | 4 sessions and 1 phone call boost, self-management, social well-being and body image, caregiving, and healthy lifestyle for ostomy | NA |
| Stoutenberg (2016) [46] | IB | Social Cognitive Theory, the Health Belief Model | Trained facilitator | Weekly | 2.5 | Baseline, post-program | 10 lessons and interactive discussions about general lifestyle activity, resistance training, aerobic activity, general nutrition, cancer nutrition, healthy shopping, weight management, quality sleep, acupuncture and Chinese medicine, and mindfulness | NA |
| Group | Techniques | Number of Studies | Selected Quotes (Author, Year) |
|---|---|---|---|
| 1. Goal and Planning | 1.1 Goal setting (behavior) | 6 | “The behavioral goals were for participants to … consume a diet of ≥7 F and V servings/day for women or ≥9 F and V servings/day for men and ≤7% of total calories from saturated fat” (Carmack et al., 2021) [22] (p. 4) |
| 1.2 Problem solving | 11 | “the 3 major foods contributing the highest percentage of kilocalories to each participant’s diet were identified from the dietary recalls performed at baseline … participants were encouraged to … problem-solve on overcoming perceived barriers to healthy behaviors …” (Demark-Wahnefried et al., 2014) [21] (p. 2526) | |
| 1.3 Goal setting (outcome) | 6 | “[the intervention] promoting a (weight) loss of roughly 0.5 kg per week” (Demark-Wahnefried et al., 2023) [35] (p. 4) | |
| 1.4 Action planning | 4 | “... and developing an action plan” (Pekmezi et al., 2021) [36] (p. 5) | |
| 1.5 Review behavior goal(s) | 2 | “...participants were surveyed bimonthly on their progress and plans … The 6 subsequent newsletters provided tailored messages regarding progress toward goals” (Demark-Wahnefried et al., 2014) [21] (p. 2526) | |
| 1.6 Discrepancy between current behavior and goal | 4 | “...illustrations of current behaviors in relation to national guidelines…” (Carmack et al., 2021) [22] (p. 4) “Count calorie consumption and compare to recommended target.” (Manne et al., 2021) [40] (Table S1) | |
| 1.7 Review outcome goal(s) | 2 | “...on Fridays, a “call-to-action” inquired about progress towards incremental goals.” (Demark-Wahnefried et al., 2023) [35] (p. 4) | |
| 2. Feedback and Monitoring | 2.2 Feedback on behavior | 6 | “The 6 subsequent letters provide … feedback on portion control …” (Demark-Wahnefried et al., 2014) [21] (p. 2526) |
| 2.3 Self-monitoring of behavior | 9 | “... participants were encouraged to keep records of their food intake and physical activity (self-monitoring) …” (Demark-Wahnefried et al., 2014) [21] (p. 2526) | |
| 2.4 Self-monitoring of outcome(s) of behavior | 3 | “... self-monitoring (through the incorporation of new technologies, i.e., Fitbits and Aria Scales)... Upon randomization, one dyad member was mailed … two sets of instructions to connect to MyFitnessPal® to automate weight …” (Demark-Wahnefried et al., 2023) [35] (pp. 3–4) | |
| 2.7 Feedback on outcome(s) of behavior | 2 | “… participants were weighed and paper food diaries were reviewed” (Dorfman et al., 2022) [42] (p. 12) | |
| 3. Social Support | 3.1 Social support (unspecified) | 7 | “Discuss couple working together to be partners in health and ways to support one another in managing weight and symptoms.” (Dorfman et al., 2022) [42] (p. 11) |
| 3.2 Social support (practical) | 1 | “[participants] were encouraged to share any recipes they have tried with the group” (Knobf et al., 2018) [43] (p. 601) | |
| 3.3 Social support (emotional) | 5 | “Discuss social support and matching support needs with the appropriate support person for managing emotional triggers” (Dorfman et al., 2022) [42] (p. 11) | |
| 4. Shaping Knowledge | 4.1 Instruction on how to perform the behavior | 12 | “Each group-based session delivered simultaneous multiple health behavior content covering … information about healthy eating (the Australian Guide to Healthy Eating, fruit and vegetables, maintaining a healthy weight, fats, meat, salt, dietary supplements, alcohol, and food labels).” (James et al., 2015) [37] (p. 4) |
| 4.2 Information about antecedents | 3 | “[researchers] have participants identify individual and joint eating triggers.” (Dorfman et al., 2022) [42] (p. 11) | |
| 4.3 Re-attribution | 1 | “How to recognize hunger, and manage emotional or habitual eating” (Pekmezi et al., 2021) [36] (p. 6) | |
| 5. Natural Consequences | 5.1 Information about health consequences | 7 | “Nutrition therapy is recommended for all people with type 1 and type 2 diabetes as an effective component of the overall treatment plan … how eating right and moving more can prevent and control diabetes.” (Conlon et al., 2016) [41] (p. 537) |
| 5.3 Information about social and environmental consequences | 5 | “... designed to educate survivors and caregivers on the … importance of basic nutrition and exercise in the management of the many physical and psychosocial issues survivors and caregivers endure” (Anton et al., 2013) [23] (p. 804) | |
| 5.6 Information about emotional consequences | 1 | “... designed to educate survivors and caregivers on the … importance of basic nutrition and exercise in the management of the many physical and psychosocial issues survivors and caregivers endure” (Anton et al., 2013) [23] (p. 804) | |
| 6. Comparison of Behavior | 6.1 Demonstration of the behavior | 12 | “24 weekly interactive sessions averaging 15 min in length were created using Articulate Storyline software (Articulate Global, LLC, New York, NY, USA) to guide participants through topics such as portion control, grocery shopping and food preparation …” (Demark-Wahnefried et al., 2023) [35] (p. 4) |
| 6.2 Social comparison | 1 | “Mothers and daughters assigned to the team-based intervention received … information on their other team member” (Demark-Wahnefried et al., 2014) [21] (p. 2526) | |
| 8. Repetition and Substitution | 8.1 Behavioral practice/rehearsal | 12 | “... 12-week classes to educate survivors and caregivers on the techniques and importance of basic nutrition and exercise.” (Anton et al., 2013) [23] (p. 804) |
| 8.2 Behavior substitution | 2 | “Substituting low-glycemic foods for higher-glycemic load foods…” (Conlon et al., 2016) [41] (p. 537) | |
| 8.6 Generalization of target behavior | 2 | “After the educational portion of the session, participants were engaged in applied activities, such as … reading nutrition labels to understand calories, sugar, and protein content.” (Stoutenberg et al., 2016) [46] (p. 49) | |
| 8.7 Graded tasks | 4 | “... each dyad member was encouraged to set incremental goals that would eventually lead over the course of the 6-month intervention …” (Demark-Wahnefried et al., 2023) [35] (p. 4) | |
| 9. Comparison of Outcomes | 9.1 Credible source | 9 | “… a presentation on breast cancer among women of color by a Breast Medical Oncologist with a review of common symptoms and symptom management with advanced practice nurses” (Knobf et al., 2018) [43] (p. 601) |
| 9.2 Pros and cons | 2 | “Evaluating pros/cons” (Pekmezi et al., 2021) [36] (p. 5) | |
| 10. Reward and Threat | 10.2 Material reward (behavior) | 1 | “A variety of jewelry stones, beads, and jewelry making components were available for each participant to create her ‘own significant unique necklace’ as a memorabilia of the (behavioral intervention) experience” (Knobf et al., 2018) [43] (p. 601) |
| 10.4 Social reward | 4 | “[participants have] celebration with invited family and guests” (Conlon et al., 2016) [41] (p. 538) | |
| 11. Regulation | 11.2 Reducing negative emotions | 6 | “Recognizing how stress influences physical and emotional well-being and strategies to manage it” (Pekmezi et al., 2021) [36] (p. 6) |
| 11.3 Conserving mental resources | 2 | “To maintain the pleasure of eating by only limiting food choices when indicated by scientific evidence.” (Conlon et al., 2016) [41] (p. 538) | |
| 12. Antecedents | 12.1 Restructuring the physical environment | 1 | “Increase access to affordable, healthy foods in communities, places of work, and schools.” (Conlon et al., 2016) [41] (p. 538) |
| 12.2 Restructuring the social environment | 6 | “The survivors and caregivers were encouraged to engage each other in enacting the healthy lifestyle behaviors.” (Crane et al.) [34] (p. 610) | |
| 12.3 Avoidance/reducing exposure to cues for the behaviors | 2 | “Review the use of assertive communication for managing environmental triggers (specifically making requests and saying no)” (Dorfman et al., 2022) [42] (p. 11) | |
| 12.5 Adding objects to the environment | 3 | “They also received portion control tableware (Portion Doctor; Portion Health Products, St. Augustine Beach, Fla) … and shoe chips (Nike Inc, Beaverton, Ore) to monitor steps taken, minutes of physical activity, and kilocalories burned.” (Demark-Wahnefried et al., 2014) [21] (p. 2526) | |
| 13. Identity | 13.2 Framing/Reframing | 3 | “Learn to refocus and reframe unhelpful thoughts (for weight management goals and wellbeing).” (Dorfman et al., 2022) [42] (p. 11) |
| 15. Self-belief | 15.3 Focusing on past success | 2 | “…such strategies build upon small successes with lifestyle change and thereby enhance self-efficacy” (Pekmezi et al., 2021) [36] (p. 4) |
| Author (Year) | Dietary Outcomes | Health Outcomes | ||||
|---|---|---|---|---|---|---|
| Survivors | Families/Caregivers | Combined | Survivors | Families/Caregivers | Combined | |
| Randomized Controlled Trials | ||||||
| Carmack (2021) [22] | Positive within-group changes in fruit and vegetable and saturated fat intake in IG and CG | Positive within-group changes in fruit and vegetable, total fat, and saturated fat intake in IG | NA | Positive within-group changes in weight and physical performances in IG and CG | Positive within-group changes in weight in IG | NA |
| Crane (2021) [34] | Medium-to-large effect sizes for fruit and vegetable total, vegetable only, sugar; medium size for dietary fiber | Medium-to-large effects for total sugar and sugar from sugar-sweetened beverages; medium effect sizes for vegetable intake | NA | Medium-to-large effect sizes for summed symptom severity; small sizes for global symptom distress | NSR | NA |
| Denmark-Wahnefried (2014) [21] | NSR | NSR | NSR | Greater decreases in BMI and waist circumference in IG than CG | Greater decreases in waist circumference in IG than CG | Greater positive changes in VO2 peak and waist circumference in IG than CG |
| Denmark-Wahnefried (2023) [35] Pekmezi (2021) [36] | Positive within-group changes in calorie intake in IG and CG | Positive within-group changes in calorie intake in IG and CG | Positive within-group changes in calorie intake in IG and CG | Positive changes in weight, waist circumference, physical performance, and physical quality of life in IG and CG; greater improvements in the sit-and-reach test in IG than CG | Positive changes in weight, waist circumference, and physical performance in IG and CG | Positive changes in weight, waist circumference, and physical performance in IG and CG; greater weight loss and improvements in the sit-and-reach test in IG than CG |
| James (2015) [37] James (2011) [38] Stacey (2017) [39] | NA | NA | Positive within-group changes in the consumption of fruit, vegetables, dietary fiber, fat, and alcohol; between-group differences in changes in daily vegetable consumption between IG and CG; changes not maintained at 12-month follow-up | NA | NA | Positive changes in BMI and weight; between-group differences in weight loss and BMI reduction between IG and CG; changes not maintained in 12-month follow-up |
| Manne (2021) [40] | NA | NA | NA | Short relationship: positive within-group changes in psychological adjustment in the intimacy-enhancing IG; long relationship: positive within-group changes in intimacy-enhancing and general health and wellness IG | Short relationship: positive within-group changes in psychological adjustment in all groups; long relationship: positive within-group changes in psychological adjustment in the intimacy-enhancing IG and CG | NA |
| Pre-experimental Trials | ||||||
| Anton (2013) [23] | NA | NSR | NA | NA | Positive within-group improvements in physical performances and psychological outcomes | NA |
| Conlon (2016) [41] | NA | NA | NSR | NA | NA | Positive within-group changes in waist circumference (12-week) and perceived health (4-week and 12-week) |
| Dorfman (2022) [42] | Positive within-group changes in eating behaviors | Positive within-group changes in eating behaviors | NA | Positive within-group changes in weight, pain interference, fatigue, symptom self-efficacy, and psychological distress | Positive within-group changes in weight, weight and symptom self-efficacy, and psychological distress | NA |
| Knobf (2018) [43] | Positive within-group changes in nutrition | Positive within-group changes in nutrition | NA | Positive within-group changes in emotional well-being and stress management | NSR | NA |
| Krouse (2016) [44] Grant (2013) [45] | NA | NA | NA | Positive within-group changes in health-related quality of life, physical wellbeing, social wellbeing, and anxiety | NA | NA |
| Stoutenberg (2016) [46] | NA | NA | Positive within-group changes in dietary patterns and self-efficacy of eating habits (sticking to it) | NA | NA | NSR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Hoover, R.L.; Woodard, N.; Leeman, J.; Hirschey, R. A Systematic Review of Dietary Interventions for Cancer Survivors and Their Families or Caregivers. Nutrients 2024, 16, 56. https://doi.org/10.3390/nu16010056
Xu J, Hoover RL, Woodard N, Leeman J, Hirschey R. A Systematic Review of Dietary Interventions for Cancer Survivors and Their Families or Caregivers. Nutrients. 2024; 16(1):56. https://doi.org/10.3390/nu16010056
Chicago/Turabian StyleXu, Jingle, Rebecca L. Hoover, Nathaniel Woodard, Jennifer Leeman, and Rachel Hirschey. 2024. "A Systematic Review of Dietary Interventions for Cancer Survivors and Their Families or Caregivers" Nutrients 16, no. 1: 56. https://doi.org/10.3390/nu16010056
APA StyleXu, J., Hoover, R. L., Woodard, N., Leeman, J., & Hirschey, R. (2024). A Systematic Review of Dietary Interventions for Cancer Survivors and Their Families or Caregivers. Nutrients, 16(1), 56. https://doi.org/10.3390/nu16010056





