Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database
Abstract
:1. Introduction
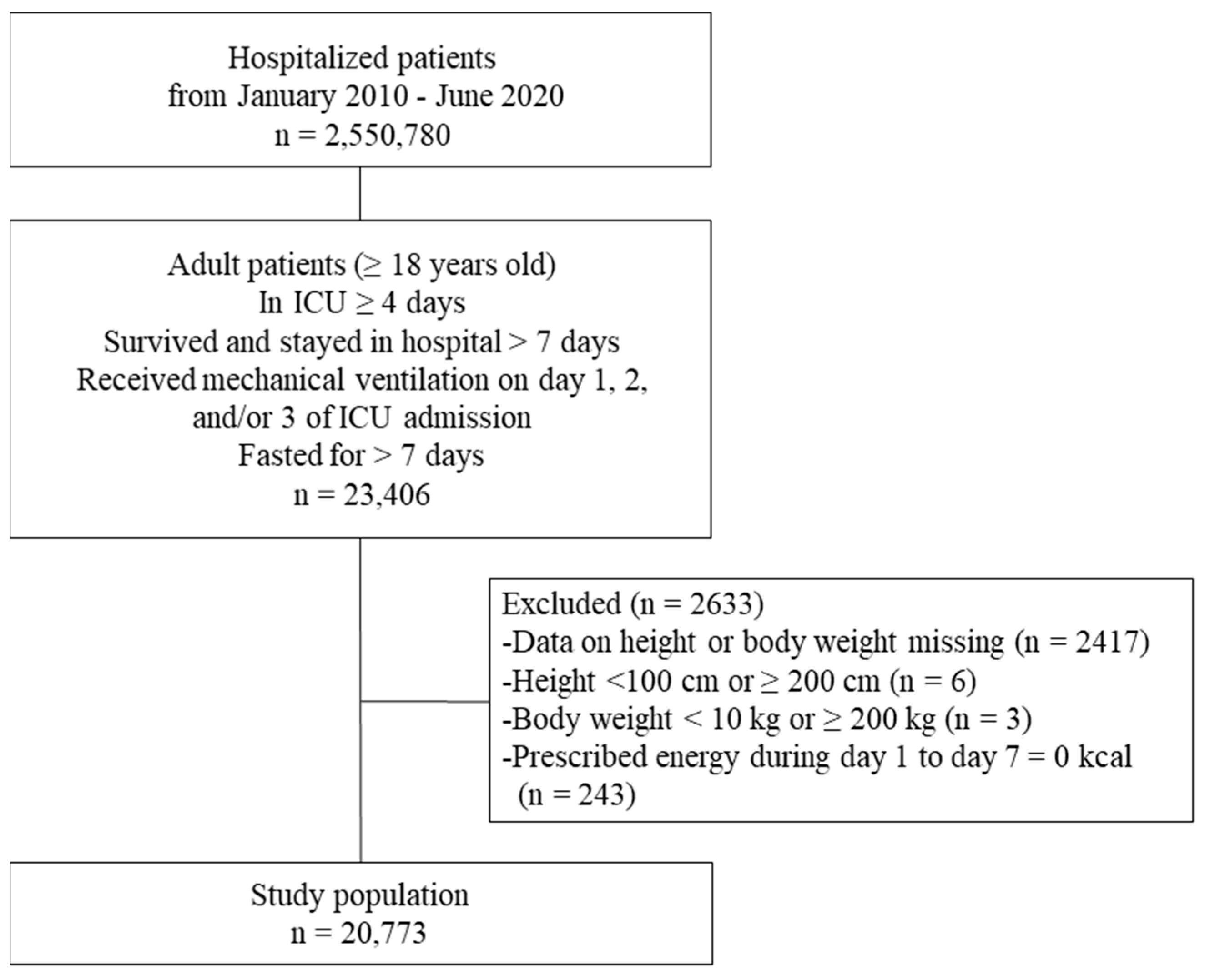
2. Materials and Methods
2.1. Data Source
2.2. Patient Population
2.3. Comparisons
2.3.1. Comparison 1: Parenteral Energy Dose
2.3.2. Comparison 2: Parenteral Amino Acid Dose
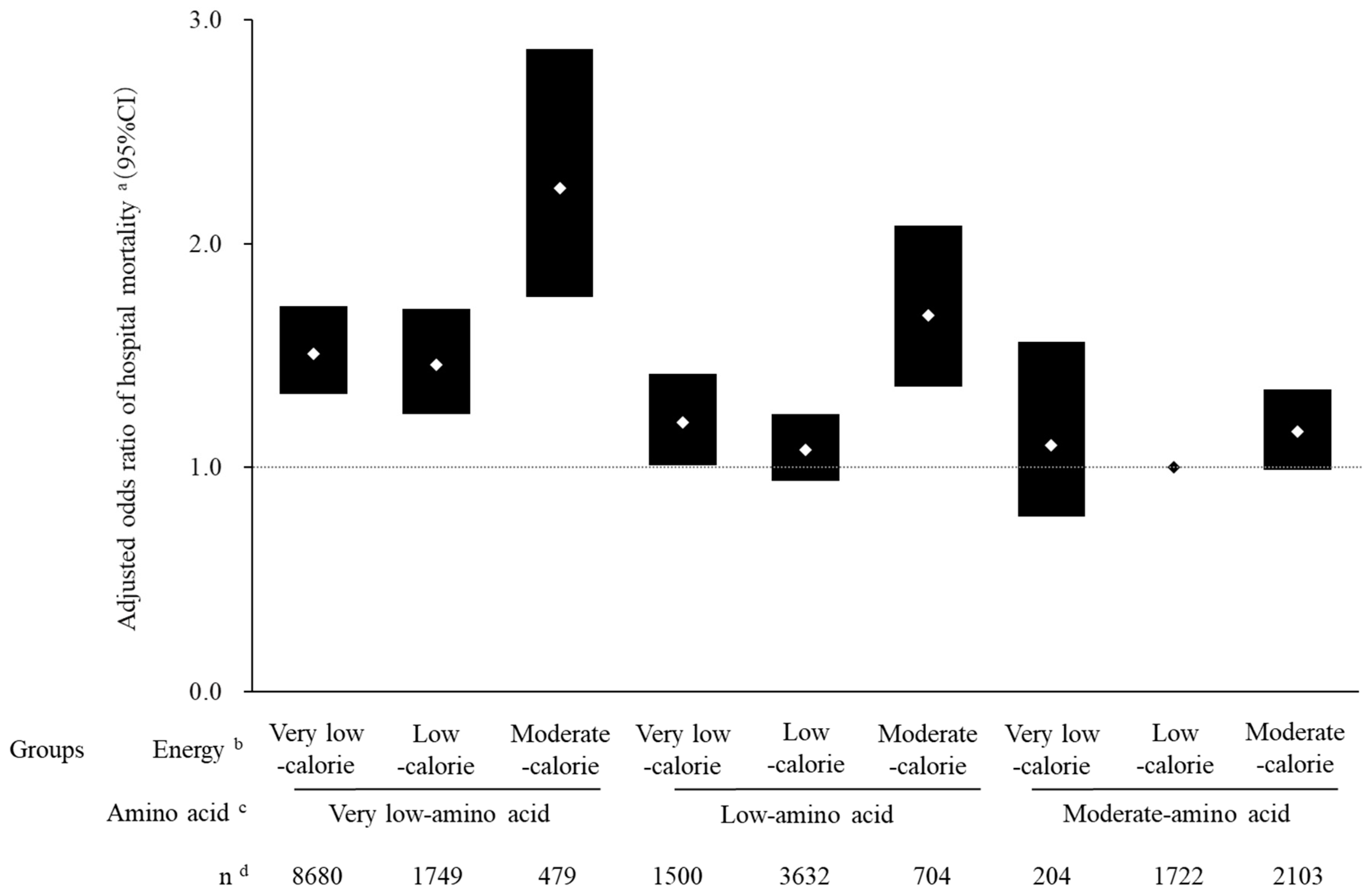
2.3.3. Comparison 3: Parenteral Energy and Amino Acid Combinations
2.4. Endpoints
2.5. Variables
2.6. Statistical Methods
3. Results
3.1. Comparison 1: Parenteral Energy Dose
3.2. Comparison 2: Parenteral Amino Acid Dose
3.3. Comparison 3: Parenteral Energy and Amino Acid Combinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Stapel, S.N.; de Groot, S.D.; Driessen, R.H.; de Jong, E.; Girbes, A.R.; Strack, R.J.; Beishuizen, A. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: A prospective observational cohort study. JPEN J. Parenter. Enter. Nutr. 2012, 36, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y.; Gonzalez, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- The Committee on Japanese Guideline for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients, Japanese Society of Intensive Care Medicine. Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients. J. Jpn. Soc. Intensive Care Med. 2016, 23, 185–281. [Google Scholar] [CrossRef]
- Yasuda, H.; Horikoshi, Y.; Kamoshita, S.; Kuroda, A.; Moriya, T. Nutritional management of ICU patients receiving mechanical ventilation: A retrospective cohort study using a medical claims database. Clin. Nutr. Open Sci. 2022, 42, 84–98. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Ridley, E.J.; Peake, S.L.; Jarvis, M.; Deane, A.M.; Lange, K.; Davies, A.R.; Chapman, M.; Heyland, D. Nutrition Therapy in Australia and New Zealand Intensive Care Units: An International Comparison Study. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1349–1357. [Google Scholar] [CrossRef]
- Yatabe, T. Strategies for optimal calorie administration in critically ill patients. J. Intensive Care 2019, 7, 15. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Shigematsu, K.; Nakano, H.; Watanabe, Y. The eye response test alone is sufficient to predict stroke outcome—Reintroduction of Japan Coma Scale: A cohort study. BMJ Open 2013, 3, e002736. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report from the Global Clinical Nutrition Community. JPEN J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Jo, T.; Yamana, H.; Matsui, H.; Fushimi, K.; Yasunaga, H. Early enteral nutrition for cardiogenic or obstructive shock requiring venoarterial extracorporeal membrane oxygenation: A nationwide inpatient database study. Intensive Care Med. 2018, 44, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Yasunaga, H.; Noiri, E.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Nangaku, M.; Doi, K. Choice of renal replacement therapy modality in intensive care units: Data from a Japanese Nationwide Administrative Claim Database. J. Crit. Care 2015, 30, 381–385. [Google Scholar] [CrossRef]
- Tagami, T.; Matsui, H.; Moroe, Y.; Fukuda, R.; Shibata, A.; Tanaka, C. Antithrombin use and 28-day in-hospital mortality among severe-burn patients: An observational nationwide study. Ann. Intensive Care 2017, 7, 18. [Google Scholar] [CrossRef]
- Meseguer, J.I.H.; Lopez-Delgado, J.C.; García, M.P.M. Recommendations for specialized nutritional-metabolic management of the critical patient: Indications, timing and access routes. Metabolism and Nutrition Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiv. 2020, 44 (Suppl. S1), 33–38. [Google Scholar] [CrossRef]
- Lopez-Delgado, J.C.; Grau-Carmona, T.; Mor-Marco, E.; Bordeje-Laguna, M.L.; Portugal-Rodriguez, E.; Lorencio-Cardenas, C.; Vera-Artazcoz, P.; Macaya-Redin, L.; Llorente-Ruiz, B.; Iglesias-Rodriguez, R.; et al. The Enpic Study Group. Parenteral Nutrition: Current Use, Complications, and Nutrition Delivery in Critically Ill Patients. Nutrients 2023, 15, 4665. [Google Scholar] [CrossRef]
- Servia-Goixart, L.; Lopez-Delgado, J.C.; Grau-Carmona, T.; Trujillano-Cabello, J.; Bordeje-Laguna, M.L.; Mor-Marco, E.; Portugal-Rodriguez, E.; Lorencio-Cardenas, C.; Montejo-Gonzalez, J.C.; Vera-Artazcoz, P. Evaluation of Nutritional Practices in the Critical Care patient (The ENPIC study): Does nutrition really affect ICU mortality? Clin. Nutr. ESPEN 2022, 47, 325–332. [Google Scholar] [CrossRef] [PubMed]
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012, 307, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, I.; Zusman, O.; Kagan, I.; Theilla, M.; Cohen, J.; Singer, P. Early Administration of Protein in Critically Ill Patients: A Retrospective Cohort Study. Nutrients 2019, 11, 106. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. PermiT Trial Group: Permissive underfeeding or standard enteral feeding in critically ill adults. N. Engl. J. Med. 2015, 372, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Twisk, J.W.; Oudemans-van Straaten, H.M.; Weijs, P.J. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef]
- Fukatsu, K.; Fukushima, R. Surgeons’ awareness of albumin and current status of postoperative nutrition management in Japan: Results of a web-based questionnaire. Jpn. J. Surg. Metabol. Nutr. 2021, 55, 141–150. [Google Scholar] [CrossRef]
- Moick, S.; Hiesmayr, H.; Mouhieddine, M.; Kiss, N.; Bauer, P.; Sulz, I.; Singer, P.; Simon, J. Reducing the knowledge to action gap in hospital nutrition care-Developing and implementing Nutrition Day 2.0. Clin. Nutr. 2021, 40, 936–945. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study; working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Higashibeppu, N.; Sanui, M.; Sobue, K.; Sato, T.; Shiotsuka, J.; Mizuno, A.; Yasuda, H.; Miyake, K.; Tokuhira, N.; Izawa, J.; et al. Current nutritional therapy in Japanese intensive care units: What did we learn from the International Nutritional Survey? J. Jpn. Soc. Intensive Care Med. 2014, 21, 243–252. [Google Scholar] [CrossRef]
- Boot, R.; Koekkoek, K.W.A.C.; van Zanten, A.R.H. Refeeding syndrome: Relevance for the critically ill patient. Curr. Opin. Crit. Care 2018, 24, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Chittams, J.; Sammarco, T.; Higashibeppu, N.; Higashiguchi, T.; Heyland, D.K. Greater Nutrient Intake Is Associated with Lower Mortality in Western and Eastern Critically Ill Patients with Low BMI: A Multicenter, Multinational Observational Study. JPEN J. Parenter. Enter. Nutr. 2018, 43, 63–69. [Google Scholar] [CrossRef]

| Characteristics | Patient Groups Based on Energy Dose a | Patient Groups Based on Amino Acid Dose b | |||||
|---|---|---|---|---|---|---|---|
| Very Low Calories | Low Calories | Moderate Calories | Very low Amino Acids | Low Amino Acids | Moderate Amino Acids | ||
| n = 10,384 | n = 7103 | n = 3286 | n = 10,908 | n = 5836 | n = 4029 | ||
| Age, years, n (%) | <60 | 1926 (18.5) | 1211 (17.0) | 500 (15.2) | 2056 (18.8) | 954 (16.3) | 627 (15.6) |
| 60–69 | 2000 (19.3) | 1396 (19.7) | 653 (19.9) | 2148 (19.7) | 1129 (19.3) | 772 (19.2) | |
| 70–79 | 2959 (28.5) | 2211 (31.1) | 1083 (33.0) | 3194 (29.3) | 1780 (30.5) | 1279 (31.7) | |
| 80–89 | 2898 (27.9) | 1945 (27.4) | 899 (27.4) | 2948 (27.0) | 1650 (28.3) | 1144 (28.4) | |
| ≥90 | 601 (5.8) | 340 (4.8) | 151 (4.6) | 562 (5.2) | 323 (5.5) | 207 (5.1) | |
| Sex, n (%) | Male | 6537 (63.0) | 4686 (66.0) | 1929 (58.7) | 6999 (64.2) | 3854 (66.0) | 2299 (57.1) |
| Female | 3847 (37.0) | 2417 (34.0) | 1357 (41.3) | 3909 (35.8) | 1982 (34.0) | 1730 (42.9) | |
| Body mass index c, kg/m2, n (%) | <16 | 653 (6.3) | 456 (6.4) | 210 (6.4) | 619 (5.7) | 388 (6.6) | 312 (7.7) |
| 16–<18.5 | 1432 (13.8) | 979 (13.8) | 482 (14.7) | 1400 (12.8) | 876 (15.0) | 617 (15.3) | |
| 18.5–<22.5 | 3615 (34.8) | 2641 (37.2) | 1222 (37.2) | 3797 (34.8) | 2138 (36.6) | 1543 (38.3) | |
| 22.5–<25 | 2149 (20.7) | 1474 (20.8) | 658 (20.0) | 2301 (21.1) | 1221 (20.9) | 759 (18.8) | |
| ≥25 | 2535 (24.4) | 1553 (21.9) | 714 (21.7) | 2791 (25.6) | 1213 (20.8) | 798 (19.8) | |
| Primary diagnosis d, n (%) | Sepsis | 451 (4.3) | 529 (7.4) | 271 (8.2) | 605 (5.5) | 412 (7.1) | 234 (5.8) |
| Neoplasm | 645 (6.2) | 988 (13.9) | 639 (19.4) | 727 (6.7) | 700 (12.0) | 845 (21.0) | |
| Diseases of the nervous system | 517 (5.0) | 194(2.7) | 59 (1.8) | 468 (4.3) | 189 (3.2) | 113 (2.8) | |
| Ischemic heart disease | 738 (7.1) | 441(6.2) | 172 (5.2) | 866 (7.9) | 397 (6.8) | 88 (2.2) | |
| Heart failure | 666 (6.4) | 330 (4.6) | 144 (4.4) | 719 (6.6) | 320 (5.5) | 101 (2.5) | |
| Cerebrovascular disorders | 1617 (15.6) | 570 (8.0) | 224 (6.8) | 1449 (13.3) | 584 (10.0) | 378 (9.4) | |
| Other circulatory system diseases | 1837 (17.7) | 1028 (14.5) | 402 (12.2) | 2148 (19.7) | 733 (12.6) | 386 (9.6) | |
| Pneumonia | 474 (4.6) | 247 (3.5) | 85 (2.6) | 448 (4.1) | 243 (4.2) | 115 (2.9) | |
| Interstitial respiratory diseases | 299 (2.9) | 227 (3.2) | 72 (2.2) | 289 (2.6) | 209 (3.6) | 100 (2.5) | |
| Other respiratory diseases | 766 (7.4) | 406 (5.7) | 137 (4.2) | 638 (5.8) | 422 (7.2) | 249 (6.2) | |
| Diseases of the digestive system | 1158 (11.2) | 1289 (18.1) | 654 (19.9) | 1248 (11.4) | 938 (16.1) | 915 (22.7) | |
| Kidney diseases | 113 (1.1) | 85 (1.2) | 73 (2.2) | 187 (1.7) | 57 (1.0) | 27 (0.7) | |
| Injury, poisoning, other consequences external causes | 557 (5.4) | 298 (4.2) | 91 (2.8) | 519 (4.8) | 254 (4.4) | 173 (4.3) | |
| Other | 546 (5.3) | 471 (6.6) | 263 (8.0) | 597 (5.5) | 378 (6.5) | 305 (7.6) | |
| Charlson Comorbidity Index, n (%) | 0 | 6075 (58.5) | 3454 (48.6) | 1456 (44.3) | 6070 (55.6) | 2936 (50.3) | 1979 (49.1) |
| 1–2 | 3205 (30.9) | 2572 (36.2) | 1257 (38.3) | 3496 (32.0) | 2090 (35.8) | 1448 (35.9) | |
| ≥3 | 1104 (10.6) | 1077 (15.2) | 573(17.4) | 1342 (12.3) | 810 (13.9) | 602 (14.9) | |
| Barthel Index, n (%) | 100 | 1582 (15.2) | 1652 (23.3) | 989 (30.1) | 1877 (17.2) | 1236 (21.2) | 1110 (27.6) |
| 65–95 | 268 (2.6) | 271 (3.8) | 149 (4.5) | 321 (2.9) | 203 (3.5) | 164 (4.1) | |
| 45–60 | 278 (2.7) | 252 (3.5) | 119 (3.6) | 285 (2.6) | 221 (3.8) | 143 (3.5) | |
| 25–40 | 182 (1.8) | 145 (2.0) | 88 (2.7) | 195 (1.8) | 117 (2.0) | 103 (2.6) | |
| 5–20 | 422 (4.1) | 334 (4.7) | 150 (4.6) | 420 (3.9) | 282 (4.8) | 204 (5.1) | |
| 0 | 6166 (59.4) | 3408 (48.0) | 1308 (39.8) | 6261 (57.4) | 2912 (49.9) | 1709 (42.4) | |
| NA | 1486 (14.3) | 1041 (14.7) | 483 (14.7) | 1549 (14.2) | 865 (14.8) | 596 (14.8) | |
| Malnutrition e, n (%) | <70 years and BMI < 18.5 | 538 (5.2) | 458 (6.4) | 207 (6.3) | 568 (5.2) | 368 (6.3) | 267 (6.6) |
| ≥70 years and BMI < 20 | 2400 (23.1) | 1622 (22.8) | 763 (23.2) | 2349 (21.5) | 1403 (24.0) | 1033 (25.6) | |
| Prescription/Treatment f, n (%) | Catecholamines | 6971 (67.1) | 5470 (77.0) | 2608 (79.4) | 7845 (71.9) | 4252 (72.9) | 2952 (73.3) |
| Transfusions g | 4275 (41.2) | 4010 (56.5) | 2101 (63.9) | 5206 (47.7) | 2975 (51.0) | 2205 (54.7) | |
| Albumin | 3708 (35.7) | 4110 (57.9) | 2175 (66.2) | 4612 (42.3) | 3016 (51.7) | 2365 (58.7) | |
| Renal replacement therapy | 1800 (17.3) | 1756 (24.7) | 988 (30.1) | 2704 (24.8) | 1211 (20.8) | 629 (15.6) | |
| Intra-aortic balloon pump | 876 (8.4) | 556 (7.8) | 234 (7.1) | 1082 (9.9) | 474 (8.1) | 110 (2.7) | |
| Plasmapheresis | 39 (0.4) | 31 (0.4) | 36 (1.1) | 56 (0.5) | 28 (0.5) | 22 (0.5) | |
| ECMO | 489 (4.7) | 302 (4.3) | 107 (3.3) | 639 (5.9) | 203 (3.5) | 56 (1.4) | |
| Nutritional support team | 128 (1.2) | 184 (2.6) | 94 (2.9) | 152 (1.4) | 143 (2.5) | 111 (2.8) | |
| Rehabilitation h | 4040 (38.9) | 3328 (46.9) | 1545 (47.0) | 4014 (36.8) | 2790 (47.8) | 2109 (52.3) | |
| Energy i, kcal/kg/d, median [Q1, Q3] | 4.8 [3.1, 6.5] | 11.6 [9.8, 13.9] | 19.7 [17.1, 23.1] | 5.1 [3.2, 7.9] | 10.7 [8.1, 13.9] | 16.3 [12.6, 20.2] | |
| Amino acids i, g/kg/d, median [Q1, Q3] | 0.00 [0.00, 0.15] | 0.32 [0.20, 0.46] | 0.52 [0.35, 0.69] | 0.00 [0.00, 0.11] | 0.32 [0.25, 0.41] | 0.59 [0.48, 0.73] | |
| Lipids i, g/kg/d, median [Q1, Q3] | 0.00 [0.00, 0.06] | 0.04 [0.00, 0.13] | 0.08 [0.01, 0.22] | 0.01 [0.00, 0.09] | 0.03 [0.00, 0.12] | 0.04 [0.00, 0.15] | |
| Carbohydrates, g/kg/d, median [Q1, Q3] | 1.00 [0.67, 1.35] | 2.37 [1.94, 2.89] | 4.09 [3.49, 4.84] | 1.09 [0.69, 1.68] | 2.18 [1.59, 2.88] | 3.28 [2.40, 4.10] | |
| PN j initiation day, median [Q1, Q3] | 6 [3, 9] | 3 [2, 5] | 2 [1, 3] | 6 [3, 9] | 3 [2, 4] | 2 [1, 3] | |
| PN j duration, days, median [Q1, Q3] | 6 [0, 17] | 17 [10, 31] | 20 [12, 37] | 5 [0, 17] | 17 [10, 31] | 20 [12, 36] | |
| Fasting duration k, days, median [Q1, Q3] | 12 [9, 18] | 13 [9, 21] | 14 [9, 23] | 12 [9, 19] | 13 [9, 21] | 13 [9, 22] | |
| Patient Groups Based on Energy Dose a | Patient Groups Based on Amino Acid Dose b | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Outcomes | Very Low Calories | Low Calories | Moderate Calories | p-Value | Very Low Amino Acids | Low Amino Acids | Moderate Amino Acids | p-Value |
| n = 10,384 | n = 7103 | n = 3286 | n = 10,908 | n = 5836 | n = 4029 | |||
| In-hospital mortality, n (%) | 4724 (45.5) | 2713 (38.2) | 1384 (42.1) | p < 0.001 e | 5159 (47.3) | 2272 (38.9) | 1390 (34.5) | p < 0.001 e |
| Hospital readmission cd n (%) | 240 (4.2) | 187 (4.3) | 84 (4.4) | 0.95 e | 258 (4.5) | 151 (4.2) | 102 (3.9) | 0.42 e |
| Length of hospital stay d, days, median [Q1, Q3] | 46 [30, 70] | 47 [31, 73] | 49 [32, 77] | p < 0.001 e | 47 [30, 72] | 47 [31, 74] | 45 [29, 70] | p = 0.003 e |
| AOR/regression coefficient fg (95%CI) | AOR/regression coefficient fg (95%CI) | |||||||
| In-hospital mortality | Reference | 0.85 (0.78–0.92) | 1.09 (0.96–1.24) | Reference | 0.76 (0.70–0.82) | 0.69 (0.63–0.76) | ||
| Hospital readmission cd | Reference | 1.11 (0.85–1.44) | 1.18 (0.80–1.74) | Reference | 0.96 (0.76–1.21) | 0.81 (0.61–1.07) | ||
| Length of hospital stay d | Reference | 1.83 (−0.89–4.55) | 0.76 (−3.03–4.55) | Reference | 1.13 (−1.66–3.93) | −0.98 (−4.27–2.30) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, H.; Horikoshi, Y.; Kamoshita, S.; Kuroda, A.; Moriya, T. Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database. Nutrients 2024, 16, 57. https://doi.org/10.3390/nu16010057
Yasuda H, Horikoshi Y, Kamoshita S, Kuroda A, Moriya T. Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database. Nutrients. 2024; 16(1):57. https://doi.org/10.3390/nu16010057
Chicago/Turabian StyleYasuda, Hideto, Yuri Horikoshi, Satoru Kamoshita, Akiyoshi Kuroda, and Takashi Moriya. 2024. "Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database" Nutrients 16, no. 1: 57. https://doi.org/10.3390/nu16010057
APA StyleYasuda, H., Horikoshi, Y., Kamoshita, S., Kuroda, A., & Moriya, T. (2024). Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database. Nutrients, 16(1), 57. https://doi.org/10.3390/nu16010057






