Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review
Abstract
:1. Introduction
2. The Role of Natural Products in Human Health
2.1. History of Rosmarinic Acid
2.2. Natural Occurrences of Rosmarinic Acid
2.3. Biosynthesis of Rosmarinic Acid
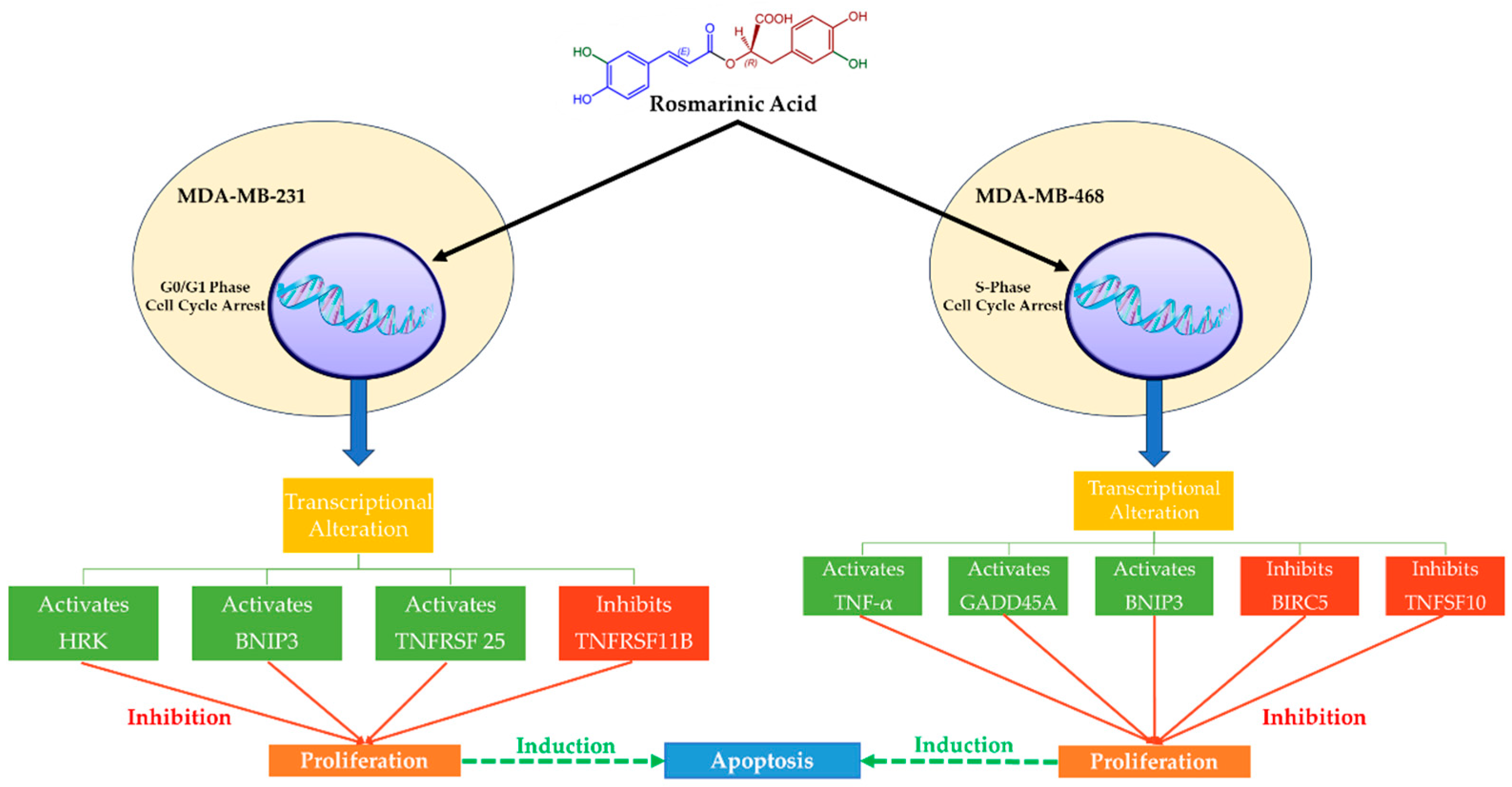
2.4. Effects of Rosmarinic Acid in MDA-MB-231 and MDA-MB-468 TNBC Cells
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Michaels, E.; Worthington, R.O.; Rusiecki, J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. N. Am. 2023, 107, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Howell, S.; Evans, D.G. Polygenic risk scores and breast cancer risk prediction. Breast 2023, 67, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Dimitrova, L.; Dimitrova, M.; Denev, P.; Teneva, D.; Georgieva, A.; Petkova-Kirova, P.; Lazarova, M.; Tasheva, K. Antitumor and Antioxidant Activities of In Vitro Cultivated and Wild-Growing Clinopodium vulgare L. Plants. Plants 2023, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.E.F.; Souza, M.V.F.; Carobeli, L.R.; Morelli, F.; Mari, N.L.; Damke, E.; Shinobu Mesquita, C.S.; Teixeira, J.J.V.; Consolaro, M.E.L.; Silva, V. Combination of Conventional Drugs with Biocompounds Derived from Cinnamic Acid: A Promising Option for Breast Cancer Therapy. Biomedicines 2023, 11, 275. [Google Scholar] [CrossRef]
- González-Palacios Torres, C.; Barrios-Rodríguez, R.; Muñoz-Bravo, C.; Toledo, E.; Dierssen, T.; Jiménez-Moleón, J.J. Mediterranean diet and risk of breast cancer: An umbrella review. Clin. Nutr. 2023, 42, 600–608. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Pirie, K.; Reeves, G.; Green, J.; Beral, V. Combined and progestagen-only hormonal contraceptives and breast cancer risk: A UK nested case-control study and meta-analysis. PLoS Med. 2023, 20, e1004188. [Google Scholar] [CrossRef]
- Cyr, A.E.; Kennard, K. Individualizing Breast Cancer Risk Assessment in Clinical Practice. Surg. Oncol. Clin. N. Am. 2023, 32, 647–661. [Google Scholar] [CrossRef]
- Kim, M.Y. Breast Cancer Metastasis. Adv. Exp. Med. Biol. 2021, 1187, 183–204. [Google Scholar] [CrossRef]
- Luo, P.; Lu, G.; Fan, L.L.; Zhong, X.; Yang, H.; Xie, R.; Lv, Z.; Lv, Q.Z.; Fu, D.; Yang, L.X.; et al. Dysregulation of TMPRSS3 and TNFRSF11B correlates with tumorigenesis and poor prognosis in patients with breast cancer. Oncol. Rep. 2017, 37, 2057–2062. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Kösters, J.P.; Gøtzsche, P.C. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst. Rev. 2003, 2003, Cd003373. [Google Scholar] [CrossRef] [PubMed]
- Berdzuli, N. Breast cancer: From awareness to access. BMJ (Clin. Res. Ed.) 2023, 380, 290. [Google Scholar] [CrossRef] [PubMed]
- Acciavatti, R.J.; Lee, S.H.; Reig, B.; Moy, L.; Conant, E.F.; Kontos, D.; Moon, W.K. Beyond Breast Density: Risk Measures for Breast Cancer in Multiple Imaging Modalities. Radiology 2023, 306, e222575. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Suh, N. Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Biol. 2016, 40–41, 170–191. [Google Scholar] [CrossRef]
- Donovan, M.; Fabian, M.; Chukwuemeka, N.; Cameil, W.-C.; Melisa, A.; Lennox, A.-J.; Lowen, W. Micronutrient Antioxidants in the Chemoprevention of Breast Cancer and Effect on Breast Cancer Outcomes. In Antioxidants; Viduranga, W., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–23. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Tarantino, P.; Criscitiello, C.; Curigliano, G. HER2-Low Breast Cancer: A New Subtype? Curr. Treat. Options Oncol. 2023, 24, 468–478. [Google Scholar] [CrossRef]
- Bartels, S.A.L.; Donker, M.; Poncet, C.; Sauvé, N.; Straver, M.E.; van de Velde, C.J.H.; Mansel, R.E.; Blanken, C.; Orzalesi, L.; Klinkenbijl, J.H.G.; et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2159–2165. [Google Scholar] [CrossRef]
- Whelan, T.J.; Smith, S.; Parpia, S.; Fyles, A.W.; Bane, A.; Liu, F.F.; Rakovitch, E.; Chang, L.; Stevens, C.; Bowen, J.; et al. Omitting Radiotherapy after Breast-Conserving Surgery in Luminal A Breast Cancer. N. Engl. J. Med. 2023, 389, 612–619. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis Int. J. Program. Cell Death 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef]
- Faria, S.S.; Costantini, S.; de Lima, V.C.C.; de Andrade, V.P.; Rialland, M.; Cedric, R.; Budillon, A.; Magalhães, K.G. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021, 28, 26. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, W.; Liu, S.; Chen, C. Targeting Breast Cancer Stem Cells. Int. J. Biol. Sci. 2023, 19, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Ho, Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.A.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A.; et al. Garlic (Allium sativum L.): Its Chemistry, Nutritional Composition, Toxicity, and Anticancer Properties. Curr. Top. Med. Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Han, C.; Li, Q.; Li, F.; Zhang, J.; Jiang, Q.; Zhao, F.; Guo, C.; Chen, J.; Jiang, T.; et al. IGF2BP3-EGFR-AKT axis promotes breast cancer MDA-MB-231 cell growth. Biochim. Et Biophys. Acta. Mol. Cell Res. 2023, 1870, 119542. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.; Shen, Y.; Zhu, L.; Yao, L.; Wang, X.; Zhang, A.; Li, J.; Wu, J.; Qin, L. Rosmarinic acid, the active component of Rubi Fructus, induces apoptosis of SGC-7901 and HepG2 cells through mitochondrial pathway and exerts anti-tumor effect. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y.; et al. Rosmarinic Acid Decreases the Malignancy of Pancreatic Cancer Through Inhibiting Gli1 Signaling. Phytomed. Int. J. Phytother. Phytopharm. 2022, 95, 153861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in Breast Cancer Treatment: A Review of Its Phytochemistry, Derivatives, Nanoparticles, and Potential Mechanisms. Front. Pharmacol. 2022, 13, 872085. [Google Scholar] [CrossRef]
- Xu, L.; Cao, M.; Wang, Q.; Xu, J.; Liu, C.; Ullah, N.; Li, J.; Hou, Z.; Liang, Z.; Zhou, W.; et al. Insights into the plateau adaptation of Salvia castanea by comparative genomic and WGCNA analyses. J. Adv. Res. 2022, 42, 221–235. [Google Scholar] [CrossRef]
- Singla, R.K.; Wang, X.; Gundamaraju, R.; Joon, S.; Tsagkaris, C.; Behzad, S.; Khan, J.; Gautam, R.; Goyal, R.; Rakmai, J.; et al. Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: A comprehensive review of preclinical and clinical studies. Crit. Rev. Food Sci. Nutr. 2022, 15, 1–45. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Eisvand, F.; Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. In Vivo and In Vitro Protective Effects of Rosmarinic Acid against Doxorubicin-Induced Cardiotoxicity. Nutr. Cancer 2022, 74, 747–760. [Google Scholar] [CrossRef]
- Karatoprak, G.; Göger, F.; Çelik, İ.; Budak, Ü.; Akkol, E.K.; Aschner, M. Phytochemical profile, antioxidant, antiproliferative, and enzyme inhibition-docking analyses of Salvia ekimiana Celep & Doğan. S. Afr. J. Bot. 2022, 146, 36–47. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Gaspar, M.M.; Coelho, J.M.P.; Marques, V.; Viana, A.S.; Ascensão, L.; Carvalho, L.; Rodrigues, C.M.P.; Ferreira, H.A.; Ferreira, D.; et al. The Role of Rosmarinic Acid on the Bioproduction of Gold Nanoparticles as Part of a Photothermal Approach for Breast Cancer Treatment. Biomolecules 2022, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Heleno, S.A.; Prieto, M.A. Natural Products as Health Promoters. Curr. Pharm. Des. 2023, 29, 803. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. PTR 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M. Applications of Natural Products in Food. Foods 2021, 10, 300. [Google Scholar] [CrossRef]
- Stephen, J.; Manoharan, D.; Radhakrishnan, M. Immune boosting functional components of natural foods and its health benefits. Food Prod. Process. Nutr. 2023, 5, 61. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Valsamidou, E.; Kleftaki, S.-A.; Tzavara, C.; Kalaitzopoulou, A.; Stergiou, I.; Smyrnioudis, I.; Kaliora, A.C. Chios mastiha essential oil exhibits antihypertensive, hypolipidemic and anti-obesity effects in metabolically unhealthy adults—A randomized controlled trial. Pharmacol. Res. 2023, 194, 106821. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.M.; Szopa, A. Biological Activities of Natural Products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Kogiannou, D.A.A.; Kalogeropoulos, N.; Kaliora, A.C. Phenolic Compounds: Bioavailability and Health Effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 339–345. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Brugge, J.S. Cancer: The enemy of my enemy is my friend. Nature 2015, 527, 170–171. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Andrijauskaite, K.; Wargovich, M.J. Role of natural products in breast cancer related symptomology: Targeting chronic inflammation. Semin. Cancer Biol. 2022, 80, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Bonofiglio, D.; Giordano, C.; De Amicis, F.; Lanzino, M.; Andò, S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016, 16, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Medica 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Trócsányi, E.; György, Z.; Zámboriné-Németh, É. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Ellis, B.E.; Towers, G.H. Biogenesis of rosmarinic acid in Mentha. Biochem. J. 1970, 118, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, A.; Ellis, B.E. Rosmarinic Acid Production in Coleus Cell Cultures. Planta 1977, 137, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Levsh, O.; Pluskal, T.; Carballo, V.; Mitchell, A.J.; Weng, J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019, 294, 15193–15205. [Google Scholar] [CrossRef] [PubMed]
- Hossan, S.; Rahman, S.; Jahan, R.; Al-Nahain, A.; Rahmatullah, M. Rosmarinic Acid: A Review of its anticancer action. World J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M. Cytochrome P450-dependent hydroxylation in the biosynthesis of rosmarinic acid in Coleus. Phytochemistry 1997, 45, 1165–1172. [Google Scholar] [CrossRef]
- Yuan, H.; Dan, L.; Sun, Q.Y.; Han, W. Total Synthesis of (±)-Rosmarinic Acid. Acta Chim. Sin. 2011, 69, 945–948. [Google Scholar]
- Sun, Y.; Fan, P.; Zhao, X.; Liu, D.; Ru, Y.; Bai, Y.; Bai, Y.; Zheng, X. A Practical, Tandem and Protecting-Group-Free Synthesis of (+)-Rosmarinic Acid and its Analogues. Tetrahedron Lett. 2023, 154879. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2011, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhuang, H.L.; Lin, J.J.; Zhang, Y.F.; Huang, H.; Luo, T.; Yu, W.T.; Ni, F. Effect of rosmarinic acid from Sarcandra glabra in inhibiting proliferation and migration and inducing apoptosis of MDA-MB-231 cells via regulation of expressions of Bcl-2 and Bax. China J. Chin. Mater. Medica 2018, 43, 3335–3340. [Google Scholar] [CrossRef]
- Hoff, E.R.; Tubbs, R.R.; Myles, J.L.; Procop, G.W. HER2/neu amplification in breast cancer: Stratification by tumor type and grade. Am. J. Clin. Pathol. 2002, 117, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Havstad, S.; Zarbo, R.J.; Divine, G.; Mackowiak, P.; Velanovich, V. The Association of HER-2/neu Amplification With Breast Cancer Recurrence. Arch. Surg. 2000, 135, 1469–1474. [Google Scholar] [CrossRef]
- Azimian, H.; Dayyani, M.; Toossi, M.T.B.; Mahmoudi, M. Bax/Bcl-2 expression ratio in prediction of response to breast cancer radiotherapy. Iran. J. Basic Med. Sci. 2018, 21, 325–332. [Google Scholar] [CrossRef]
- Altieri, D.C. Survivin and IAP proteins in cell-death mechanisms. Biochem. J. 2010, 430, 199–205. [Google Scholar] [CrossRef]
- Sturm, I.; Papadopoulos, S.; Hillebrand, T.; Benter, T.; Lück, H.J.; Wolff, G.; Dörken, B.; Daniel, P.T. Impaired BAX protein expression in breast cancer: Mutational analysis of the BAX and the p53 gene. Int. J. Cancer 2000, 87, 517–521. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khanzadeh, T.; Zadi Heydarabad, M.; Khorrami, A.; Jahanban Esfahlan, A.; Ghavipanjeh, S.; Gholipour Belverdi, M.; Darvishani Fikouhi, S.; Darbin, A.; Najafpour, M.; et al. Evaluation of BAX and BCL-2 Gene Expression and Apoptosis Induction in Acute Lymphoblastic Leukemia Cell Line CCRFCEM after High- Dose Prednisolone Treatment. Asian Pac. J. Cancer Prev. 2018, 19, 2319–2323. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Chen, H.-H.; Huang, E.; Zhuang, H.-L.; Li, D.; Ni, F. Rosmarinic acid inhibits stem-like breast cancer through hedgehog and Bcl-2/Bax signaling pathways. Pharmacogn. Mag. 2019, 15, 600–606. [Google Scholar] [CrossRef]
- Wang, W.; Nag, S.A.; Zhang, R. Targeting the NFκB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015, 22, 264–289. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Labbozzetta, M.; D’Alessandro, N.; Notarbartolo, M. NF-κB Is a Potential Molecular Drug Target in Triple-Negative Breast Cancers. Omics J. Integr. Biol. 2017, 21, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Jana, D.; Patil, P.S.; Chaudhari, K.S.; Chattopadhyay, B.K.; Chikkala, B.R.; Mandal, S.; Chowdhary, P. Role of NF-κB as a Prognostic Marker in Breast Cancer: A Pilot Study in Indian Patients. Indian J. Surg. Oncol. 2013, 4, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Miraghazadeh, B.; Cook, M.C. Nuclear Factor-kappaB in Autoimmunity: Man and Mouse. Front. Immunol. 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Osteoprotegerin (OPG) Ratio Is Increased in Severe Osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Naz, F.; Anjum, F.; Islam, A.; Ahmad, F.; Hassan, M.I. Microtubule Affinity-Regulating Kinase 4: Structure, Function, and Regulation. Cell Biochem. Biophys. 2013, 67, 485–499. [Google Scholar] [CrossRef]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Heidary Arash, E.; Shiban, A.; Song, S.; Attisano, L. MARK4 inhibits Hippo signaling to promote proliferation and migration of breast cancer cells. EMBO Rep. 2017, 18, 420–436. [Google Scholar] [CrossRef]
- Saiko, P.; Steinmann, M.T.; Schuster, H.; Graser, G.; Bressler, S.; Giessrigl, B.; Lackner, A.; Grusch, M.; Krupitza, G.; Bago-Horvath, Z.; et al. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Peng, S.; Zhang, Y.; Qiao, Y. Rosmarinic Acid as a Candidate in a Phenotypic Profiling Cardio-/Cytotoxicity Cell Model Induced by Doxorubicin. Molecules 2020, 25, 836. [Google Scholar] [CrossRef] [PubMed]
- Jafaripour, L.; Naserzadeh, R.; Alizamani, E.; Javad Mashhadi, S.M.; Moghadam, E.R.; Nouryazdan, N.; Ahmadvand, H. Effects of Rosmarinic Acid on Methotrexate-induced Nephrotoxicity and Hepatotoxicity in Wistar Rats. Indian J. Nephrol. 2021, 31, 218–224. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, D.; Zhai, X.; Zhang, L.; Luo, X.; Fu, X. Oral Administration of Prunella vulgaris L. Improves the Effect of Taxane on Preventing the Progression of Breast Cancer and Reduces Its Side Effects. Front. Pharmacol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed]


| Lamiaceae taxa | |||
| Ajuga | Agastache | Calamintha | Cedronella |
| Coleus | Collimsonia | Dracocephalum | Elsholtzia |
| Glechoma | Hornium | Lavandula | Lycopus |
| Melissa | Mentha | Micromeria | Monarda |
| Origanum | Perilla | Perovskia | Plectranthus |
| Salvia | Satureja | Thymus | |
| Boraginaceae taxa | |||
| Cerinthe | Echium | Heliotropium | Lindefolia |
| Lithospermum | Nonea | Symphytum | Hydrophyllum |
| Nemophila | Phacelia | ||
| Salvia officinalis | Rosmarinus officinalis | Mentha piperita |
| Salvia limbata | Lavandula angustifolia | Mentha pulegium |
| Salvia virgata | Thymus daenensis | Mentha longifolia |
| Salvia hypoleuca | Thymus citriodorous | Mentha spicata |
| Salvia macrosiphon | Thymus pubescens | Mentha aquatica |
| Salvia choloroleuca | Thymus vulgaris | Mentha crispa |
| Melissa officinalis | Zataria multiflora | Zhumeria majdae |
| Origanum vulgare | Ocimum sanctum | Perovskia artemisoides |
| Satureja khuzistanica | Satureja atropatana | Satureja macrantha |
| Satureja bachtiarica | Satureja mutica | Satureja hortensis |
| Forsythia koreana | Hyptis pectinata |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, E.K.; Panagiotopoulos, A.A.; Argyri, K.; Panoutsopoulos, G.I.; Dimitriou, M.; Gioxari, A. Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review. Nutrients 2024, 16, 2. https://doi.org/10.3390/nu16010002
Konstantinou EK, Panagiotopoulos AA, Argyri K, Panoutsopoulos GI, Dimitriou M, Gioxari A. Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review. Nutrients. 2024; 16(1):2. https://doi.org/10.3390/nu16010002
Chicago/Turabian StyleKonstantinou, Evangelia K., Athanasios A. Panagiotopoulos, Konstantina Argyri, George I. Panoutsopoulos, Maria Dimitriou, and Aristea Gioxari. 2024. "Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review" Nutrients 16, no. 1: 2. https://doi.org/10.3390/nu16010002
APA StyleKonstantinou, E. K., Panagiotopoulos, A. A., Argyri, K., Panoutsopoulos, G. I., Dimitriou, M., & Gioxari, A. (2024). Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review. Nutrients, 16(1), 2. https://doi.org/10.3390/nu16010002