The Influence of APOE Genotype, DHA, and Flavanol Intervention on Brain DHA and Lipidomics Profile in Aged Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Animals Experimental Design and Dietary Treatments
2.3. Lipid Extraction and Fatty Acid Analysis in Red Blood Cells and Feeds
2.4. Lipid Extraction and Fatty Acid Analysis in Plasma
2.5. Brain Lipidomics Profile
2.6. Statistical Analysis
3. Results
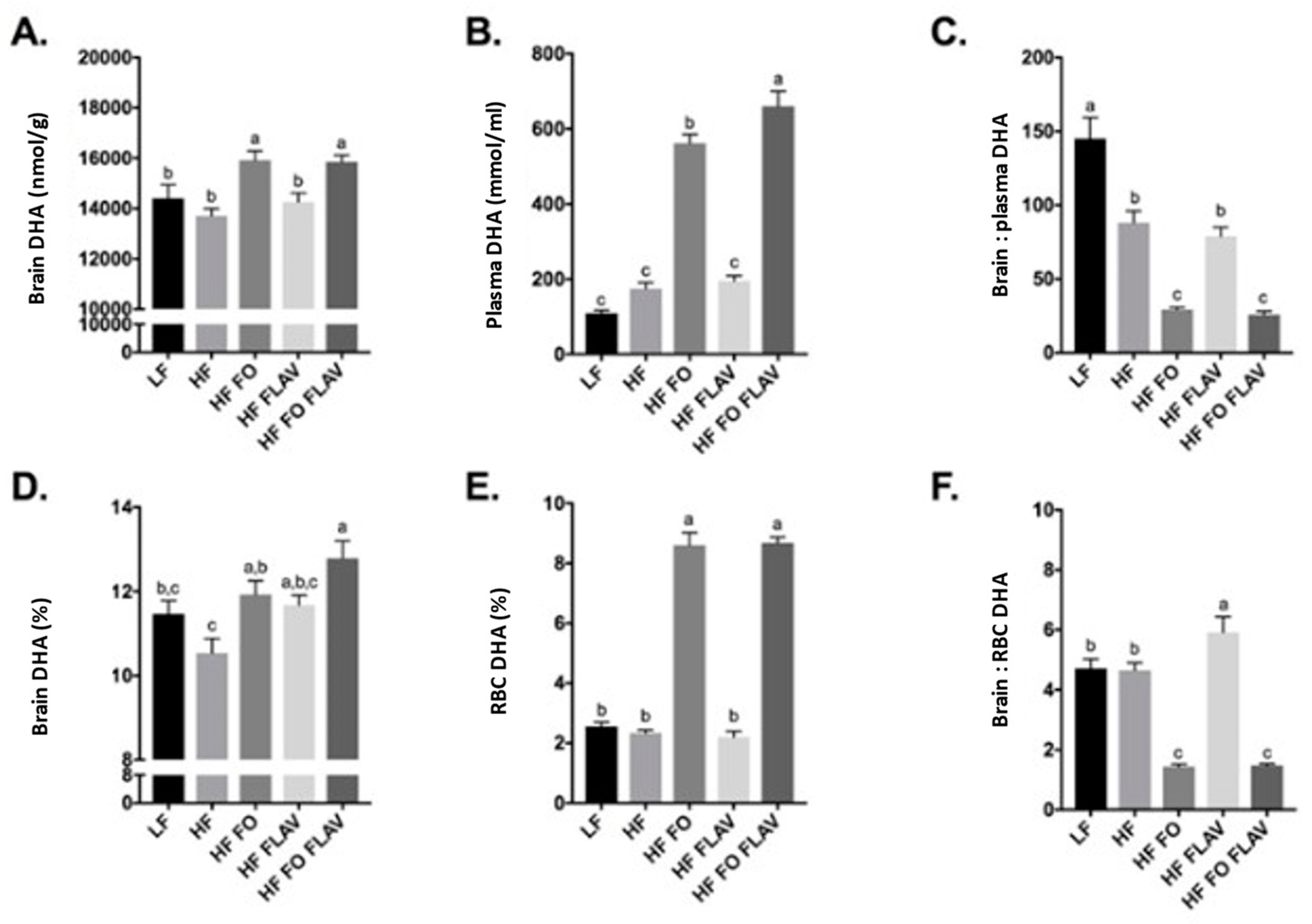
3.1. Effect of Diet on Brain, Plasma, and Red Blood Cell DHA Content
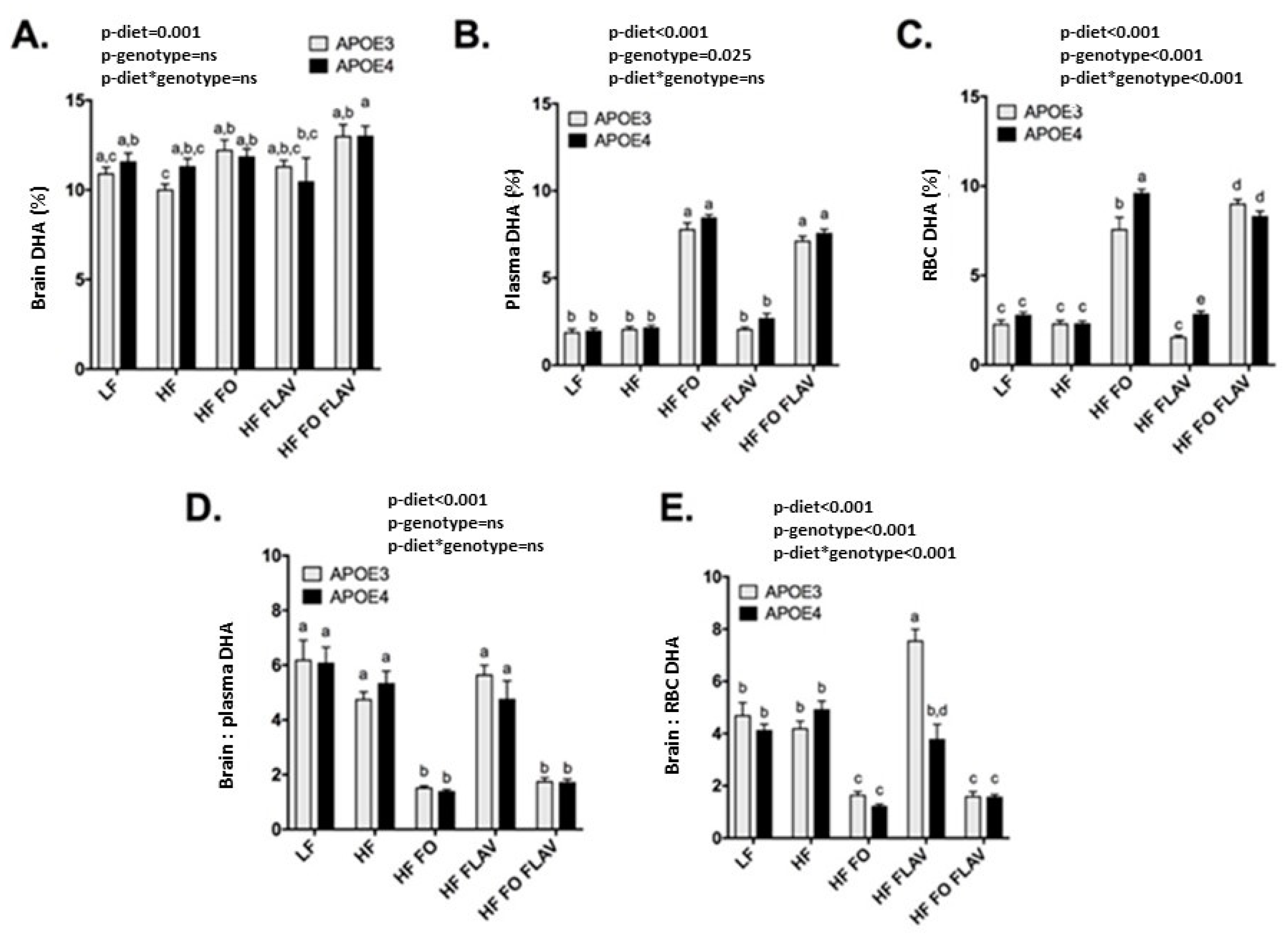
3.2. Effect of APOE Genotype and Diet on Brain, Plasma, and RBC DHA Levels
3.3. Impact of Diet and Genotype on DHA: AA Is More Evident in APOE4 Mice
3.4. Impact of Diet and APOE Genotype on Brain Phospholipid Subclasses’ DHA Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidson, Y.; Gibbons, L.; Pritchard, A.; Hardicre, J.; Wren, J.; Stopford, C.; Julien, C.; Thompson, J.; Payton, A.; Pickering-Brown, S.M.; et al. Apolipoprotein E ε4 Allele Frequency and Age at Onset of Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2006, 23, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.L.; Chidgey, C.; Peng, P.; Masters, C.L.; Roberts, B.R. The Neurobiology and Age-Related Prevalence of the epsilon4 Allele of Apolipoprotein E in Alzheimer’s Disease Cohorts. J. Mol. Neurosci. 2016, 60, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, D.M. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimer's Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Martinsen, A.; Saleh, R.N.M.; Harden, G.; Fox, C.; Muller, M.; Vauzour, D.; Minihane, A.-M. DHA-Enriched Fish Oil Ameliorates Deficits in Cognition Associated with Menopause and the APOE4 Genotype in Rodents. Nutrients 2022, 14, 1698. [Google Scholar] [CrossRef]
- Jennings, A.; Cunnane, S.C.; Minihane, A.M. Can nutrition support healthy cognitive ageing and reduce dementia risk? BMJ 2020, 369, m2269. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Pontifex, M.; Vauzour, D.; Minihane, A.-M. The effect of APOE genotype on Alzheimer's disease risk is influenced by sex and docosahexaenoic acid status. Neurobiol. Aging 2018, 69, 209–220. [Google Scholar] [CrossRef]
- López-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Titos, E.; Clària, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef]
- Tanaka, K.; Farooqui, A.A.; Siddiqi, N.J.; Alhomida, A.S.; Ong, W.-Y. Effects of Docosahexaenoic Acid on Neurotransmission. Biomol. Ther. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Taha, A.Y.; Cheon, Y.; Ma, K.; Rapoport, S.I.; Rao, J.S. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J. Psychiatr. Res. 2013, 47, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.; Fong, B.; MacGibbon, A.; McJarrow, P. Analysis of Phospholipids in Rat Brain Using Liquid Chromatography—Mass Spectrometry. Lipids 2009, 44, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Shui, G.; Fernandis, A.Z.; Lim, W.L.F.; Berger, T.; Hone, E.; Taddei, K.; Martins, I.J.; Ghiso, J.; Buxbaum, J.D.; et al. Profiling Brain and Plasma Lipids in Human APOE ε2, ε3, and ε4 Knock-in Mice Using Electrospray Ionization Mass Spectrometry. J. Alzheimer's Dis. 2010, 20, 105–111. [Google Scholar] [CrossRef]
- Kariv-Inbal, Z.; Yacobson, S.; Berkecz, R.; Peter, M.; Janaky, T.; Lütjohann, D.; Broersen, L.M.; Hartmann, T.; Michaelson, D.M. The Isoform-Specific Pathological Effects of ApoE4 in vivo are Prevented by a Fish Oil (DHA) Diet and are Modified by Cholesterol. J. Alzheimer's Dis. 2012, 28, 667–683. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Rioux-Perreault, C.; Fortier, M.; Tremblay-Mercier, J.; Zhang, Y.; Lawrence, P.; Vohl, M.C.; Perron, P.; Lorrain, D.; Brenna, J.T.; et al. Disturbance in uniformly13C-labelled DHA metabolism in elderly human subjects carrying the apoE ε4 allele. Br. J. Nutr. 2013, 110, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.N.; Rawat, V.; Mack, W.J.; Quinn, J.F.; Yurko-Mauro, K.; Bailey-Hall, E.; Aisen, P.S.; Chui, H.C.; Schneider, L.S. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimer's Res. Ther. 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Ebright, B.; Assante, I.; Poblete, R.A.; Wang, S.; Duro, M.V.; Bennett, D.A.; Arvanitakis, Z.; Louie, S.G.; Yassine, H.N. Eicosanoid lipidome activation in post-mortem brain tissues of individuals with APOE4 and Alzheimer’s dementia. Alzheimer's Res. Ther. 2022, 14, 152. [Google Scholar] [CrossRef]
- Yassine, H.N.; Braskie, M.N.; Mack, W.J.; Castor, K.J.; Fonteh, A.N.; Schneider, L.S.; Harrington, M.; Chui, H.C. Association of Docosahexaenoic Acid Supplementation With Alzheimer Disease Stage in Apolipoprotein E ε4 Carriers. JAMA Neurol. 2017, 74, 339–347. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Conway, V.; Minihane, A.M.; Jackson, K.G.; Lovegrove, J.A.; Plourde, M. Interaction between BMI and APOE genotype is associated with changes in the plasma long-chain–PUFA response to a fish-oil supplement in healthy participants. Am. J. Clin. Nutr. 2015, 102, 505–513. [Google Scholar] [CrossRef]
- Martinsen, A.; Tejera Hernandez, N.; Vauzour, D.; Harden, G.; Dick, J.; Shinde, S.; Barden, A.; Mori, T.; Minihane, A.M. Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J. 2019, 33, 10315–10326. [Google Scholar] [CrossRef] [PubMed]
- Trebble, T.; Arden, N.K.; Stroud, M.A.; Wootton, S.A.; Burdge, G.C.; Miles, E.A.; Ballinger, A.B.; Thompson, R.L.; Calder, P.C. Inhibition of tumour necrosis factor-α and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br. J. Nutr. 2003, 90, 405–412. [Google Scholar] [CrossRef] [PubMed]
- De Cosmi, V.; Mazzocchi, A.; D’oria, V.; Re, A.; Spolidoro, G.C.I.; Milani, G.P.; Berti, C.; Scaglioni, S.; Giavoli, C.; Bergamaschi, S.; et al. Effect of Vitamin D and Docosahexaenoic Acid Co-Supplementation on Vitamin D Status, Body Composition, and Metabolic Markers in Obese Children: A Randomized, Double Blind, Controlled Study. Nutrients 2022, 14, 1397. [Google Scholar] [CrossRef]
- Song, L.; Zhou, H.; Yu, W.; Ding, X.; Yang, L.; Wu, J.; Song, C. Effects of Phytosterol Ester on the Fatty Acid Profiles in Rats with Nonalcoholic Fatty Liver Disease. J. Med. Food 2020, 23, 161–172. [Google Scholar] [CrossRef]
- van Soest, A.P.M.; van de Rest, O.; Witkamp, R.F.; Cederholm, T.; de Groot, L.C.P.G.M. DHA status influences effects of B-vitamin supplementation on cognitive ageing: A post-hoc analysis of the B-proof trial. Eur. J. Nutr. 2022, 61, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Martinsen, A.; Layé, S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015, 89, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kim, H.S.; Yu, S.T.; Shin, S.R.; Lee, S.H.; Seo, G.S. Synergistic anticancer effect of docosahexaenoic acid and isoliquiritigenin on human colorectal cancer cells through ROS-mediated regulation of the JNK and cytochrome c release. Mol. Biol. Rep. 2021, 48, 1171–1180. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Agarwal, P.; Holland, T.M.; James, B.D.; Cherian, L.J.; Aggarwal, N.T.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A. Pelargonidin and Berry Intake Association with Alzheimer’s Disease Neuropathology: A Community-Based Study. J. Alzheimer's Dis. 2022, 88, 653–661. [Google Scholar] [CrossRef]
- Baker, L.D.; Manson, J.E.; Rapp, S.R.; Sesso, H.D.; Gaussoin, S.A.; Shumaker, S.A.; Espeland, M.A. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimer's Dement. 2022, 19, 1308–1319. [Google Scholar] [CrossRef]
- Sun, C.Q.; Johnson, K.D.; Wong, H.; Foo, L.Y. Biotransformation of Flavonoid Conjugates with Fatty Acids and Evaluations of Their Functionalities. Front. Pharmacol. 2017, 8, 759. [Google Scholar] [CrossRef]
- Zhu, Y.; Nwabuisi-Heath, E.; Dumanis, S.B.; Tai, L.M.; Yu, C.; Rebeck, G.W.; Ladu, M.J. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 2012, 60, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur. J. Clin. Nutr. 2013, 67, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Martinsen, A.; Saleh, R.N.M.; Harden, G.; Tejera, N.; Müller, M.; Fox, C.; Vauzour, D.; Minihane, A. APOE4 genotype exacerbates the impact of menopause on cognition and synaptic plasticity in APOE-TR mice. FASEB J. 2021, 35, e21583. [Google Scholar] [CrossRef]
- Ghioni, C.; Bell, J.; Sargent, J. Polyunsaturated fatty acids in neutral lipids and phospholipids of some freshwater insects. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 114, 161–170. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis, 3rd ed.; The Oily Press: Bridgewater, UK, 2003; pp. 205–224. [Google Scholar]
- Ackman, R.G. Fish lipids. In Advances in Fish Science and Technology; Connell, J.J., Ed.; Fishing News Books Ltd.: Farnham, UK, 1980; pp. 83–103. [Google Scholar]
- Lacombe, R.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Chen, C.T.; Metherel, A.H.; Lacombe, R.S.; Thies, F.; Masoodi, M.; Bazinet, R.P. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1092–1098. [Google Scholar] [CrossRef]
- Jensen, S.K. Improved Bligh and Dyer extraction procedure. Lipid Technol. 2008, 20, 280–281. [Google Scholar] [CrossRef]
- Vandal, M.; Alata, W.; Tremblay, C.; Rioux-Perreault, C.; Salem, N.; Calon, F.; Plourde, M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 2014, 129, 516–526. [Google Scholar] [CrossRef]
- Culliford, A.; Bradbury, J. A cross-sectional survey of the readiness of consumers to adopt an environmentally sustainable diet. Nutr. J. 2020, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Oily Fish and Omega-3s Across the Life Stages: A Focus on Intakes and Future Directions. Front. Nutr. 2019, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Jones, N.S.; Rebeck, G.W. The Synergistic Effects of APOE Genotype and Obesity on Alzheimer’s Disease Risk. Int. J. Mol. Sci. 2018, 20, 63. [Google Scholar] [CrossRef]
- I Kosti, R.; I Kasdagli, M.; Kyrozis, A.; Orsini, N.; Lagiou, P.; Taiganidou, F.; Naska, A. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: A systematic review and a dose–response meta-analysis of observational and experimental studies. Nutr. Rev. 2022, 80, 1445–1458. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, P.; He, W.; Chen, J.N.; Wang, W.Q.; Freedman, N.D.; Abnet, C.; Wang, J.B.; Jiao, J.J. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: Prospective analysis of 421 309 individuals. J. Intern. Med. 2018, 284, 399–417. [Google Scholar] [CrossRef]
- Huang, T.L.; Zandi, P.P.; Tucker, K.L.; Fitzpatrick, A.L.; Kuller, L.H.; Fried, L.P.; Burke, G.L.; Carlson, M.C. Benefits of fatty fish on dementia risk are stronger for those without APOEε4. Neurology 2005, 65, 1409–1414. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Vandal, M.; Léveillé, P.; Pinçon, A.; Calon, F.; Plourde, M. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol. Aging 2017, 57, 28–35. [Google Scholar] [CrossRef]
- Nock, T.G.; Chouinard-Watkins, R.; Plourde, M. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Hou, H.; Zhu, Y.; Salemi, J.; Ruscin, A.; Shytle, R.D.; Tan, J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci. Lett. 2010, 471, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Shirai, N.; Suzuki, H. Effect of Dietary Docosahexaenoic Acid and Catechins on Maze Behavior in Mice. Ann. Nutr. Metab. 2004, 48, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.; Evans, J.E.; Emmerich, T.; Crynen, G.; Shackleton, B.; Keegan, A.P.; Luis, C.; Tai, L.; LaDu, M.J.; Mullan, M.; et al. APOE ε4 specific imbalance of arachidonic acid and docosahexaenoic acid in serum phospholipids identifies individuals with preclinical Mild Cognitive Impairment/Alzheimer’s Disease. Aging 2017, 9, 964–985. [Google Scholar] [CrossRef]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic Acid Signalolipidomics in Nutrition: Significance in Aging, Neuroinflammation, Macular Degeneration, Alzheimer's, and Other Neurodegenerative Diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef]
- Conde, T.A.; Mendes, L.; Gaspar, V.M.; Mano, J.F.; Melo, T.; Domingues, M.R.; Duarte, I.F. Differential Modulation of the Phospholipidome of Proinflammatory Human Macrophages by the Flavonoids Quercetin, Naringin and Naringenin. Molecules 2020, 25, 3460. [Google Scholar] [CrossRef]
- Hamilton, J.; Greiner, R.; Salem, N.; Kim, H.-Y. n−3 Fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids 2000, 35, 863–869. [Google Scholar] [CrossRef]
| APOE3 | APOE4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LF | HF | HF FO | HF FLAV | HF FO FLAV | LF | HF | HF FO | HF FLAV | HF FO FLAV | Diet | Genotype | Interaction | |
| Plasma (nmol/mL) | |||||||||||||
| 20:4n-6 (AA) | 857 ± 188 | 1595 ± 161 | 878 ± 95.7 | 2101 ± 137 | 1133 ± 144 | 850 ± 123 | 2224 ± 253 | 1008 ± 97.5 | 1887 ± 216 | 1268 ± 93.5 | <0.000 | NS | NS |
| 22:6n-3 (DHA) | 109 ± 12.8 | 149 ± 14.1 | 542 ± 43.3 | 183 ± 10 | 596 ± 62.5 | 108 ± 11.9 | 199 ± 27.2 | 582 ± 20 | 208 ± 26.1 | 724 ± 42.3 | <0.000 | 0.025 | NS |
| DHA:AA | 0.14 ± 0.01 | 0.09 ± 00 | 0.64 ± 0.04 | 0.09 ± 00 | 0.54 ± 0.02 | 0.13 ± 0.01 | 0.09 ± 00 | 0.58 ± 0.06 | 0.11 ± 0.01 | 0.57 ± 0.06 | <0.000 | NS | 0.021 |
| Red Blood Cells (%) | |||||||||||||
| 20:4n-6 (AA) | 17 ± 0.74 | 19.1 ± 0.46 | 11.4 ± 0.57 | 15.2 ± 0.26 | 13.3 ± 0.37 | 18.3 ± 0.57 | 18.1 ± 0.65 | 13.7 ± 0.58 | 19.7 ± 0.46 | 11.8 ± 0.58 | <0.000 | 0.001 | <0.000 |
| 22:6n-3 (DHA) | 2.48 ± 0.17 | 2.45 ± 0.12 | 7.74 ± 0.61 | 1.53 ± 0.07 | 8.42 ± 0.45 | 2.84 ± 0.14 | 2.34 ± 0.12 | 9.63 ± 0.2 | 2.84 ± 0.14 | 8.33 ± 0.26 | <0.000 | <0.000 | <0.000 |
| DHA:AA | 0.14 ± 0 | 0.13 ± 0.01 | 0.67 ± 0.04 | 0.10 ± 00 | 0.63 ± 0.02 | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.71 ± 0.04 a | 0.14 ± 0.01 b | 0.72 ± 0.05 c | <0.000 | <0.000 | <0.000 |
| Brain (%) | |||||||||||||
| 20:4n-6 (AA) | 8.48 ± 0.4 | 8.64 ± 0.28 | 7.55 ± 0.32 | 9.68 ± 0.37 | 8.45 ± 0.42 | 9.21 ± 0.32 | 9.53 ± 0.33 | 8.03 ± 0.33 | 8.95 ± 0.72 | 8.41 ± 0.39 | 0.010 | NS | NS |
| 22:6n-3 (DHA) | 10.9 ± 0.33 | 10.0 ± 0.3 | 12.3 ± 0.54 | 11.3 ± 0.33 | 13.0 ± 0.63 | 11.6 ± 0.45 | 11.3 ± 0.43 | 11.9 ± 0.42 | 10.5 ± 1.3 | 13.0 ± 0.53 | 0.001 | NS | NS |
| DHA: AA | 1.3 ± 0.03 | 1.16 ± 0.03 | 1.63 ± 0.06 | 1.18 ± 0.04 | 1.55 ± 0.05 | 1.26 ± 0.03 | 1.19 ± 0.03 | 1.49 ± 0.04 | 1.11 ± 0.13 | 1.56 ± 0.04 | <0.000 | NS | NS |
| Diet Group | LPC | LPE | PC | PE | PI | Total |
|---|---|---|---|---|---|---|
| APOE3 | ||||||
| LF | 3.65 ± 0.66 | 4.55 ± 0.35 | 1049 ± 43.2 | 13,376 ± 802 | 9.66 ± 0.91 | 14,628 ± 802 |
| HF | 3.79 ± 0.23 | 4.35 ± 0.21 | 955 ± 41.2 | 12,355 ± 305 | 6.94 ± 0.97 | 13,519 ± 316 |
| HF FO | 3.53 ± 0.57 | 4.88 ± 0.34 | 1151 ± 29.8 ** | 14,732 ± 469 | 9.43 ± 1.04 | 16,142 ± 461 ** |
| HF FLAV | 2.50 ± 0.64 | 4.32 ± 0.30 | 932 ± 43.2 | 13,169 ± 451 | 7.71 ± 1.76 | 14,305 ± 473 |
| HF FO FLAV | 3.96 ± 0.42 | 5.29 ± 0.62 | 1089 ± 50.0 | 14,495 ± 455 | 8.57 ± 1.30 | 15,851 ± 448 * |
| APOE4 | ||||||
| LF | 2.06 ± 0.53 | 4.59 ± 0.35 | 927 ± 32.4 | 13,090 ± 186 | 6.10 ± 1.29 | 14,199 ± 186 |
| HF | 2.43 ± 0.41 | 4.70 ± 0.37 | 941 ± 28.1 | 13,025 ± 295 | 7.10 ± 0.99 | 14,157 ± 310 |
| HF FO | 4.12 ± 0.71 | 5.09 ± 0.36 | 1102 ± 52.9 * | 14,295 ± 508 | 7.82 ± 1.52 | 15,643 ± 468 |
| HF FLAV | 1.82 ± 0.50 | 4.32 ± 0.41 | 810 ± 106 | 11,801 ± 1536 | 5.11 ± 1.84 | 12,799 ± 1631 |
| HF FO FLAV | 3.47 ± 0.56 | 5.42 ± 0.77 | 1129 ± 34.2 ** | 14,896 ± 418 * | 5.22 ± 0.97 | 16,269 ± 386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinsen, A.; Saleh, R.N.M.; Chouinard-Watkins, R.; Bazinet, R.; Harden, G.; Dick, J.; Tejera, N.; Pontifex, M.G.; Vauzour, D.; Minihane, A.-M. The Influence of APOE Genotype, DHA, and Flavanol Intervention on Brain DHA and Lipidomics Profile in Aged Transgenic Mice. Nutrients 2023, 15, 2032. https://doi.org/10.3390/nu15092032
Martinsen A, Saleh RNM, Chouinard-Watkins R, Bazinet R, Harden G, Dick J, Tejera N, Pontifex MG, Vauzour D, Minihane A-M. The Influence of APOE Genotype, DHA, and Flavanol Intervention on Brain DHA and Lipidomics Profile in Aged Transgenic Mice. Nutrients. 2023; 15(9):2032. https://doi.org/10.3390/nu15092032
Chicago/Turabian StyleMartinsen, Anneloes, Rasha N. M. Saleh, Raphael Chouinard-Watkins, Richard Bazinet, Glenn Harden, James Dick, Noemi Tejera, Matthew G. Pontifex, David Vauzour, and Anne-Marie Minihane. 2023. "The Influence of APOE Genotype, DHA, and Flavanol Intervention on Brain DHA and Lipidomics Profile in Aged Transgenic Mice" Nutrients 15, no. 9: 2032. https://doi.org/10.3390/nu15092032
APA StyleMartinsen, A., Saleh, R. N. M., Chouinard-Watkins, R., Bazinet, R., Harden, G., Dick, J., Tejera, N., Pontifex, M. G., Vauzour, D., & Minihane, A.-M. (2023). The Influence of APOE Genotype, DHA, and Flavanol Intervention on Brain DHA and Lipidomics Profile in Aged Transgenic Mice. Nutrients, 15(9), 2032. https://doi.org/10.3390/nu15092032










