Sex Differences in the Bitterness Perception of an Aromatic Myrtle Bitter Liqueur and Bitter Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Participants
2.3. Gustatory Perception Assessment
2.4. Olfactory Function Assessment
2.5. PROP Bitterness Assessment
2.6. Aromatic Myrtle Herbal Liqueur
2.7. Procedures to Assess Odor and Taste Pleasantness, Intensity, and Familiarity of Aromatic Myrtle Herbal Liqueur (Mirtamaro)
2.8. Preparation of n-Hexane Extracts from Aromatic Myrtle Herbal Liqueur (Mirtamaro)
2.9. GC-FID and GC-MS Analysis of n-Hexane Extracts from Aromatic Myrtle Herbal Liqueur (Mirtamaro)
2.10. Statistical Analyses
3. Results
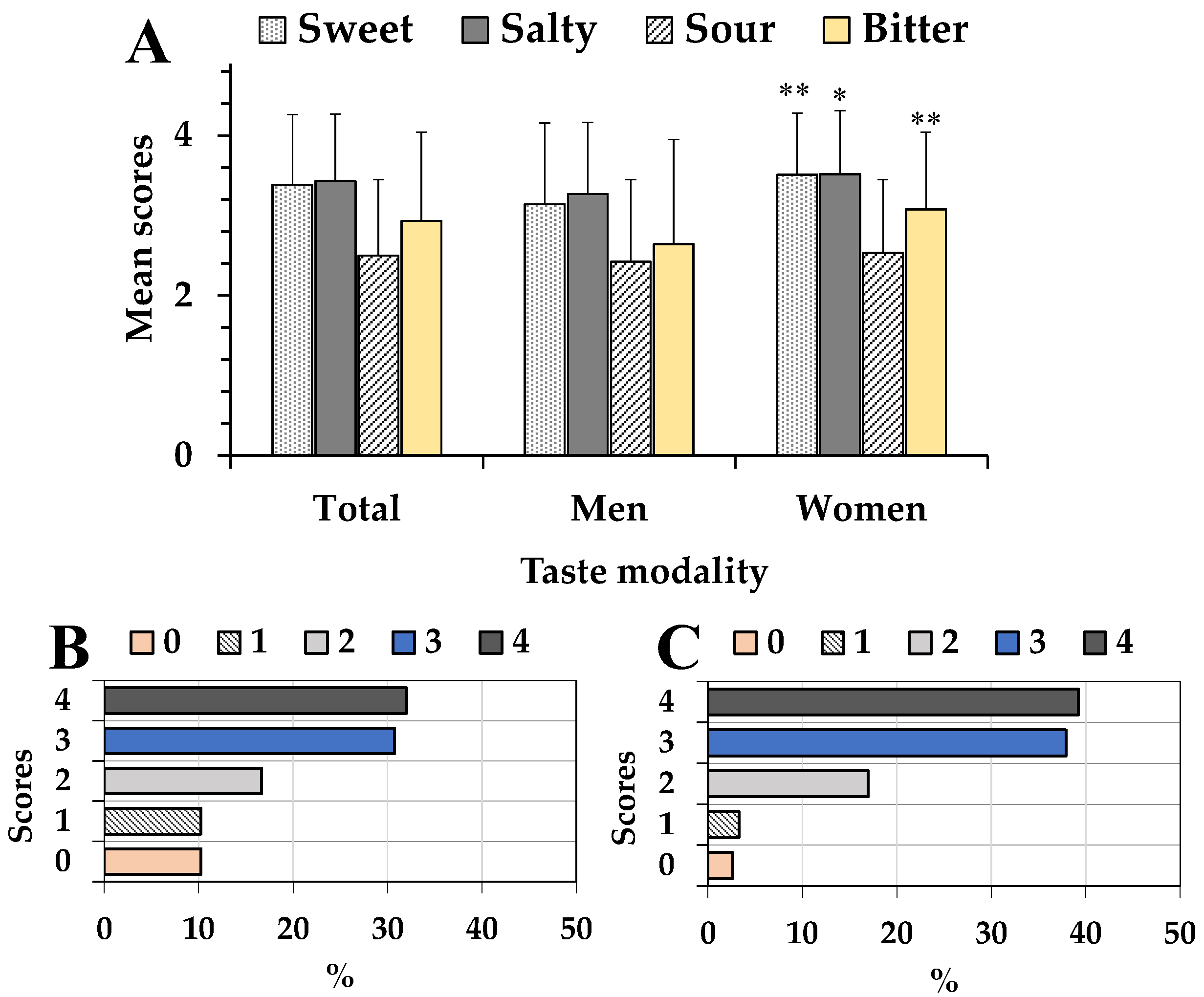
3.1. Determination of Olfactory and Gustatory Perception in Subjects
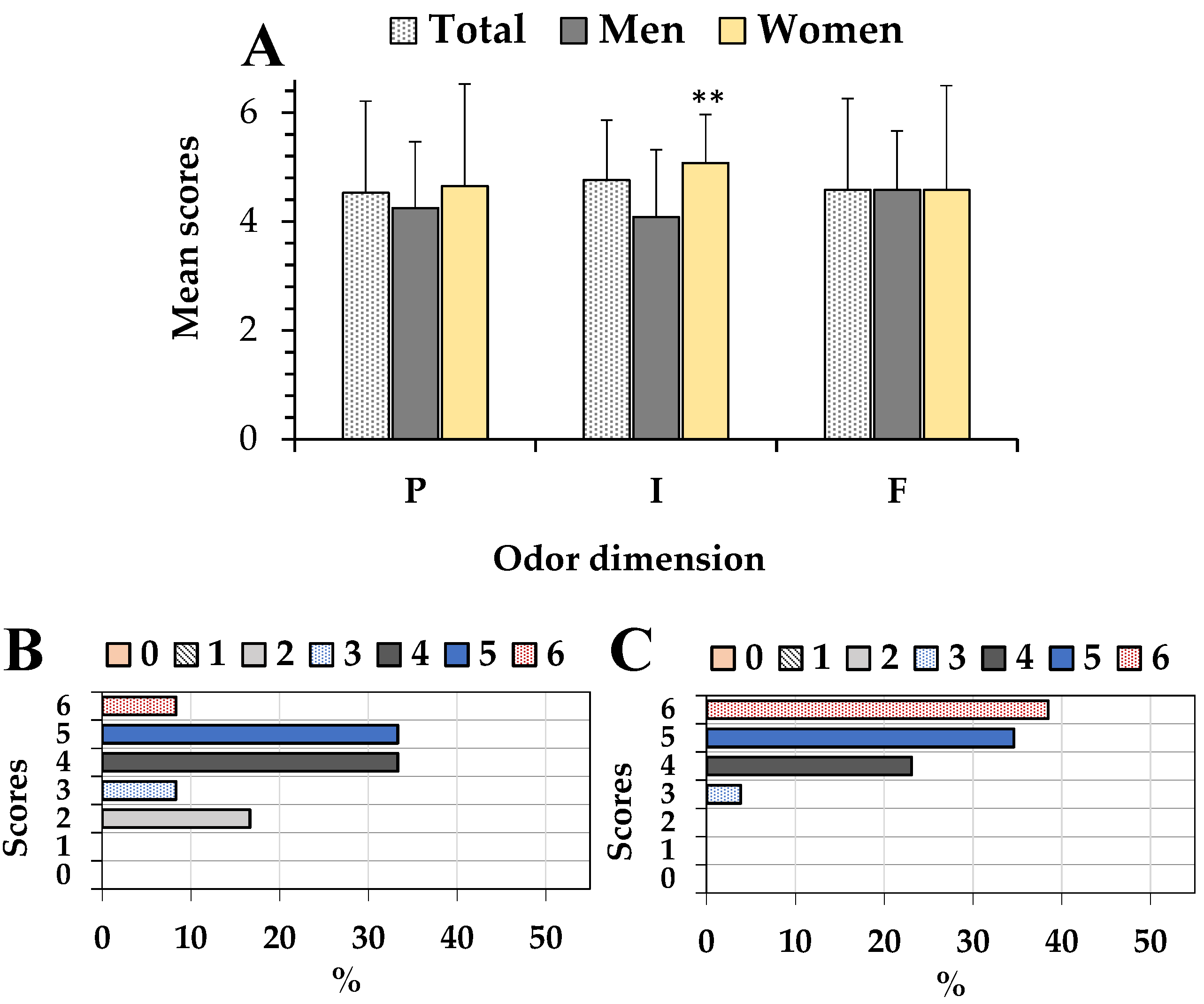
3.2. Ratings of Odor and Taste Pleasantness, Intensity, and Familiarity of the Aromatic Myrtle Herbal Liqueur (Mirtamaro)
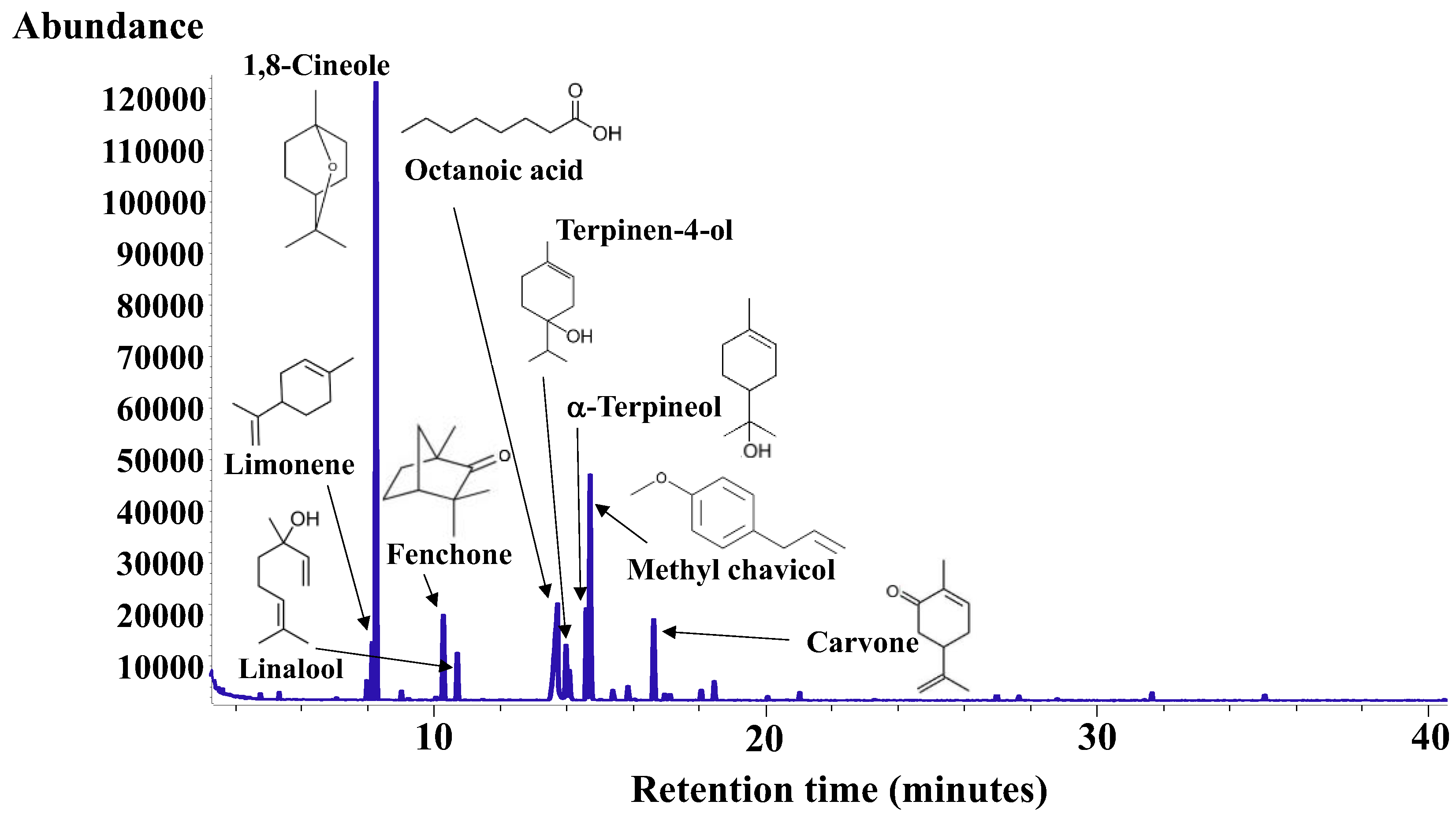
3.3. Main Volatile Compounds in the Aromatic Myrtle Herbal Liqueur (Mirtamaro)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nissim, I.; Dagan-Wiener, A.; Niv, M.Y. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in alcoholic beverages: The profiles of perception, constituents, and contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Behrens, M.; Gu, M.; Fan, S.; Huang, C.; Meyerhof, W. Bitter substances from plants used in traditional Chinese medicine exert biased activation of human bitter taste receptors. Chem. Biol. Drug Des. 2018, 91, 422–433. [Google Scholar] [CrossRef]
- Vecchio, R.; Cavallo, C.; Cicia, G.; Del Giudice, T. Are (All) consumers averse to bitter taste? Nutrients 2019, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Dragoș, D.; Petran, M.; Gradinaru, T.-C.; Gilca, M. Phytochemicals and inflammation: Is bitter better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef]
- Ke, X.; Ma, H.; Yang, J.; Qiu, M.; Wang, J.; Han, L.; Zhang, D. New strategies for identifying and masking the bitter taste in traditional herbal medicines: The example of Huanglian Jiedu Decoction. Front. Pharmacol. 2022, 13, 843821. [Google Scholar] [CrossRef]
- Deng, M.; Hida, N.; Yamazaki, T.; Morishima, R.; Kato, Y.; Fujita, Y.; Nakamura, A.; Harada, T. Comparison of bitterness intensity between prednisolone and quinine in a human sensory test indicated individual differences in bitter-taste perception. Pharmaceutics 2022, 14, 2454. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, C.H.; Lifshitz, L.M.; ZhuGe, R. Extraoral bitter taste receptors in health and disease. J. Gen. Physiol. 2017, 149, 181–197. [Google Scholar] [CrossRef]
- Devillier, P.; Naline, E.; Grassin-Delyle, S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther. 2015, 155, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Vukosavljević, P.; Đurović, S.; Antić, M.; Gorjanović, S. New herbal bitter liqueur with high antioxidant activity and lower sugar content: Innovative approach to liqueurs formulations. J. Food Sci. Technol. 2019, 56, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Bonanomi, G.; de Falco, B. Wild and cultivated plants used in traditional alcoholic beverages in Italy: An ethnobotanical review. Eur. Food Res. Technol. 2022, 248, 1089–1106. [Google Scholar] [CrossRef]
- Alamprese, C.; Pompei, C.; Scaramuzzi, F. Characterization and antioxidant activity of nocino liqueur. Food Chem. 2005, 90, 495–502. [Google Scholar] [CrossRef]
- Johnson, A.J.; Heymann, H.; Ebeler, S.E. Volatile and sensory profiling of cocktail bitters. Food Chem. 2015, 179, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Diéguez, S.; Otero-Cerviño, C.; Rodeiro-Mougán, H.; Feijóo-Mateo, J.A. Quantitative descriptive analysis of traditional herbal and coffee liqueurs made with grape marc spirit (Orujo). Foods 2020, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Schmitz, O.J.; Meckelmann, S.W. Chemical characterization of eight herbal liqueurs by means of liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2020, 1631, 461560. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, V.; Rivera, D.; Obon, C.; Alcaraz, F.; Ríos, S. Medicinal plants in traditional herbal wines and liquors in the east of Spain and the Balearic Islands. Front. Pharmacol. 2021, 12, 713414. [Google Scholar] [CrossRef]
- Shizukuda, S.; Marchini, J.S.; Adell, A.; Santos, M.A.; Cunha Brandao, C.F.; Martires Lima, C.M.; Carvalho Cunha, S.F.; Itikawa, E.N.; Silvah, J.H. Influences of weight, age, gender, genetics, diseases, and ethnicity on bitterness perception: A narrative review of current methodological aspects. Nutrire 2018, 43, 4. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: Sex-specific analysis, modulation by genetic and taste-preference associations in 18 to 80 year-old subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liang, K.L.; Lin, W.J.; Chen, C.Y.; Jiang, R.S. Influence of age and sex on taste function of healthy subjects. PLoS ONE. 2020, 15, e0227014. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Portolés, O.; Sotos-Prieto, M.; Fernández-Carrión, R.; Ramirez-Sabio, J.B.; Zanón-Moreno, V.; Mattei, J.; Sorlí, J.V.; Ordovas, J.M. A Guide to applying the sex-gender perspective to nutritional genomics. Nutrients 2018, 11, 4. [Google Scholar] [CrossRef]
- Rosa, A.; Pinna, I.; Piras, A.; Porcedda, S.; Masala, C. Flavoring of sea salt with Mediterranean aromatic plants affects salty taste perception. J. Sci. Food Agric. 2022, 102, 6005–6013. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent trends in aroma release and perception during food oral processing: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex differences in human olfaction: A meta-analysis. Front. Psychol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Besser, G.; Oeller, F.; Mueller, C.A.; Renner, B. Bitter taste perception of the human tongue mediated by quinine and caffeine impregnated taste strips. Ann. Otol. Rhinol. Laryngol. 2020, 129, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Loy, F.; Pinna, I.; Masala, C. Role of aromatic herbs and spices in salty perception of patients with hyposmia. Nutrients 2022, 14, 4976. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef]
- Rosa, A.; Isola, R.; Nieddu, M.; Masala, C. The Role of lipid composition in the sensory attributes and acceptability of the salted and dried mullet roes (Bottarga): A study in human and animal models. Nutrients 2020, 12, 3454. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Giampieri, F.; Cianciosi, D.; Forbes-Hernández, T.Y. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: New undiscovered sources of natural compounds with promising health benefits. Food Front. 2020, 1, 276–295. [Google Scholar] [CrossRef]
- Masala, C.; Käehling, C.; Fall, F.; Hummel, T. Correlation between olfactory function, trigeminal sensitivity, and nasal anatomy in healthy subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Christensen, C.M.; Cao, J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001, 73, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid extraction of volatile compounds using a new simultaneous microwave distillation: Solvent extraction device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Atzeri, A.; Dessì, M.A.; Falconieri, D.; Porcedda, S. Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco-2 cancer cells. J. Food Sci. 2012, 77, C448–C453. [Google Scholar] [CrossRef] [PubMed]
- NIST/EPA/NIH; Mass Spectral Library. National Institute of Standard and Technology: Gaithersburg, MD, USA, 2005.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- PubChem—NIH. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 27 March 2023).
- Pu, D.; Shan, Y.; Duan, W.; Huang, Y.; Liang, L.; Yan, Y.; Zhang, Y.; Sun, B.; Hu, G.H. Characterization of the key aroma compounds in the fruit of Litsea pungens Hemsl. (LPH) by GC-MS/O, OAV, and sensory techniques. J. Food Qual. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M.S.; Dubovski, N.; Margulis, E.; Niv, M.Y. BitterDB: Taste ligands and receptors database in 2019. Nucleic Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]
- Fischer, M.E.; Cruickshanks, K.J.; Schubert, C.R.; Pinto, A.; Klein, B.E.K.; Klein, R.; Nieto, F.J.; Pankow, J.S.; Huang, G.-H.; Snyder, D.J. Taste intensity in the Beaver Dam Offspring Study. Laryngoscope 2013, 123, 1399–1404. [Google Scholar] [CrossRef]
- Gaudette, N.J.; Pickering, G.J. The efficacy of bitter blockers on health-relevant bitterants. J. Funct. Foods. 2012, 4, 177–184. [Google Scholar] [CrossRef]
- Deepankumar, S.; Karthi, M.; Vasanth, K.; Selvakumar, S. Insights on modulators in perception of taste modalities: A review. Nutr. Res. Rev. 2019, 32, 231–246. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Gibitz-Eisath, N.; Seger, C.; Schwaiger, S.; Sturm, S.; Stuppner, H. Simultaneous Quantitative analysis of the major bioactive compounds in gentianae radix and its beverages by UHPSFC-DAD. J. Agric. Food Chem. 2022, 70, 7586–7593. [Google Scholar] [CrossRef]
- Ponticelli, M.; Lela, L.; Moles, M.; Mangieri, C.; Bisaccia, D.; Faraone, I.; Falabella, R.; Milella, L. The healing bitterness of Gentiana lutea L., phytochemistry and biological activities: A systematic review. Phytochemistry 2023, 206, 113518. [Google Scholar] [CrossRef]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009, 97, 213–228. [Google Scholar] [CrossRef]
- Delplanque, S.; Grandjean, D.; Chrea, C.; Aymard, L.; Cayeux, I.; Le Calvé, B.; Velazco, M.I.; Scherer, K.R.; Sander, D. Emotional Processing of Odors: Evidence for a Nonlinear Relation between Pleasantness and Familiarity Evaluations. Chem. Senses 2008, 33, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Costell, E.; Tárrega, A.; Bayarri, S. Food Acceptance: The Role of Consumer Perception and Attitudes. Chemosens. Percept. 2009, 3, 42–50. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavor perception. Cell 2015, 161, 24–35. [Google Scholar] [CrossRef]
- Spence, C. What is the relationship between the presence of volatile organic compounds in food and drink products and multisensory flavour perception? Foods 2021, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Falconieri, D.; Porcedda, S.; Marongiu, B.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Supercritical CO2 extraction of volatile oils from Sardinian Foeniculum vulgare ssp. vulgare (Apiaceae): Chemical composition and biological activity. Nat. Prod. Res. 2014, 28, 1819–1825. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Ali, N.S.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, A.A.; Sendra, E. Flavors and aromas. In Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; Yahia, E.M., Carrillo-López, A., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 385–404. [Google Scholar]
- Pagliarini, E.; Proserpio, C.; Spinelli, S.; Lavelli, V.; Laureati, M.; Arena, E.; Di Monaco, R.; Menghi, L.; Toschi, T.G.; Braghieri, A.; et al. The role of sour and bitter perception in liking, familiarity and choice for phenol-rich plant-based foods. Food Qual. Prefer. 2021, 93, 104250. [Google Scholar] [CrossRef]
- Ai, Y.; Han, P. Neurocognitive mechanisms of odor-induced taste enhancement: A systematic review. Int. J. Gastron. Food Sci. 2022, 28, 100535. [Google Scholar] [CrossRef]
- Ponticorvo, S.; Prinster, A.; Cantone, E.; Di Salle, F.; Esposito, F.; Canna, A. Sex differences in the taste-evoked functional connectivity network. Chem. Senses 2022, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Total Subjects (n = 231) | Men (n = 78) | Women (n = 153) |
|---|---|---|---|
| Age | 35.8 ± 15.6 | 35.9 ± 15.8 | 35.7 ± 16.0 |
| Weight (kg) | 64.8 ± 15.0 | 75.8 ± 12.6 | 59.2 ± 12.9 *** |
| Height (m) | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| BMI | 24.0 ± 6.4 | 26.3 ± 8.6 | 22.8 ± 4.4 ** |
| OThr | 7.3 ± 4.7 | 7.5 ± 5.0 | 7.1 ± 4.5 |
| ODi | 11.6 ± 2.2 | 11.4 ± 2.3 | 11.7 ± 2.2 |
| OId | 12.9 ± 1.8 | 12.7 ± 1.7 | 13.0 ± 1.9 |
| TDI score | 31.7 ± 6.4 | 31.5 ± 6.6 | 31.8 ± 6.4 |
| Parameters | Bitter Intensity | |||||
|---|---|---|---|---|---|---|
| Total Subjects | Men | Women | ||||
| r | p | r | p | r | p | |
| Sex | −0.187 | p < 0.01 | - | - | - | - |
| Age | 0.003 | p > 0.05 | 0.060 | p > 0.05 | −0.034 | p > 0.05 |
| Weight | −0.164 | p < 0.05 | 0.125 | p > 0.05 | −0.217 | p < 0.01 |
| BMI | −0.063 | p < 0.05 | 0.116 | p > 0.05 | −0.191 | p < 0.05 |
| OTr | −0.029 | p > 0.05 | −0.050 | p > 0.05 | 0.000 | p > 0.05 |
| ODi | −0.034 | p > 0.05 | −0.059 | p > 0.05 | −0.044 | p > 0.05 |
| OId | 0.030 | p > 0.05 | −0.038 | p > 0.05 | 0.047 | p > 0.05 |
| TDI score | −0.024 | p > 0.05 | −0.068 | p > 0.05 | −0.001 | p > 0.05 |
| Sweet | 0.233 | p < 0.01 | 0.283 | p < 0.05 | 0.132 | p > 0.05 |
| Salty | 0.117 | p > 0.05 | 0.028 | p > 0.05 | 0.144 | p > 0.05 |
| Sour | 0.103 | p > 0.05 | 0.173 | p > 0.05 | 0.034 | p > 0.05 |
| Total taste | 0.651 | p < 0.001 | 0.695 | p < 0.001 | 0.585 | p < 0.001 |
| Parameters | Unstandardized Coefficients | Standard Coefficients | |||
|---|---|---|---|---|---|
| B | Std Error | β | t | Significance | |
| Total subjects | |||||
| Age | −0.244 | 0.177 | −0.104 | −1.373 | p > 0.05 |
| Sex | −0.437 | 0.152 | −0.187 | −2.873 | p < 0.01 |
| Sweet | 0.255 | 0.082 | 0.201 | 3.094 | p < 0.01 |
| Weight | −0.006 | 0.006 | 0.281 | 3.094 | p > 0.05 |
| Men | |||||
| Age | 0.004 | 0.009 | 0.074 | 0.661 | p > 0.05 |
| Weight | 0.011 | 0.012 | 0.110 | 0.989 | p > 0.05 |
| Sweet | 0.372 | 0.143 | 0.289 | 2.603 | p < 0.05 |
| Women | |||||
| Age | 0.001 | 0.005 | 0.021 | 0.261 | p > 0.05 |
| Weight | −0.016 | 0.006 | −0.211 | −2.626 | p < 0.01 |
| Sweet | 0.149 | 0.101 | 0.119 | 1.471 | p > 0.05 |
| Sex | Sensory Input | Sensory Perceived Attributes |
|---|---|---|
| Men | Odor | Myrtle; herbs; liqueur; licorice; bitter; natural essences. |
| Taste | Bitter; very bitter, and a bit sour; sweet at the beginning, then bitter; myrtle note. | |
| Women | Odor | Liqueur; bitter liqueur; myrtle; alcohol; bitter; very bitter; chinotto; licorice; juniper; berries; orange; woody; spicy; rum; nicotine; coffee; sambuca; medication; pungent. |
| Taste | Bitter; alcohol; very bitter; myrtle note; Citrus note; pungent; chinotto note; slightly bitter aftertaste; mint aftertaste; sweet at the beginning, then very bitter; woody note; quinine; whiskey; sweet; caramel aftertaste. |
| Data | Quinine | PROP | OP | OI | OF | TP | TI | TF |
|---|---|---|---|---|---|---|---|---|
| Quinine | 1 | |||||||
| PROP | 0.214 | 1 | ||||||
| OP | −0.172 | −0.094 | 1 | |||||
| OI | −0.469 | −0.113 | 0.347 | 1 | ||||
| OF | −0.021 | −0.269 | 0.777 ** | 0.434 | 1 | |||
| TP | −0.158 | −0.237 | −0.138 | 0.093 | −0.012 | 1 | ||
| TI | −0.140 | −0.316 | 0.562 * | 0.687 * | 0.606 * | 0.055 | 1 | |
| TF | −0.064 | −0.625 * | −0.168 | 0.351 | 0.024 | 0.651 * | 0.320 | 1 |
| Quinine | 1 | |||||||
| PROP | −0.305 | 1 | ||||||
| OP | 0.176 | −0.234 | 1 | |||||
| OI | 0.159 | 0.117 | 0.017 | 1 | ||||
| OF | 0.147 | −0.283 | 0.735 *** | 0.160 | 1 | |||
| TP | 0.303 | −0.304 | 0.564 ** | 0.041 | 0.542 ** | 1 | ||
| TI | 0.201 | 0.244 | −0.142 | 0.508 ** | −0.064 | −0.200 | 1 | |
| TF | 0.175 | −0.319 | 0.518 ** | 0.149 | 0.572 ** | 0.659 *** | −0.072 | 1 |
| Parameters | Unstandardized Coefficients | Standard Coefficients | |||
|---|---|---|---|---|---|
| B | Std Error | β | t | Significance | |
| Men | |||||
| OP | −0.029 | −0.038 | 0.972 | −0.113 | p > 0.05 |
| OI | −0.154 | −0.152 | −0.187 | −0.582 | p > 0.05 |
| OF | −0.028 | −0.036 | 0.999 | −0.109 | p > 0.05 |
| TI | −0.171 | −0.036 | 0.898 | −0.654 | p > 0.05 |
| TF | 0.796 | 0.293 | 0.651 | 2.715 | p < 0.05 |
| Women | |||||
| OP | 0.562 | 0.168 | 0.564 | 3.342 | p < 0.01 |
| OI | −0.040 | −0.265 | 0.794 | −0.056 | p > 0.05 |
| OF | 0.278 | 1.125 | 0.272 | 0.228 | p > 0.05 |
| TI | −0.122 | −0.709 | 0.486 | −0.146 | p > 0.05 |
| TF | 0.436 | 0.150 | 0.502 | 2.915 | p < 0.01 |
| RT | RI | RILit | Compound | % w/w | Identification a | Odor Description b |
|---|---|---|---|---|---|---|
| 5.475 | 940 | 930 | alpha-thujene | 0.26 ± 0.02 | RI,MS | Woody, green, herbal [38] |
| 8.120 | 1027 | 1026 | orto-cymene | 1.12 ± 0.23 | RI,MS | - |
| 8.281 | 1032 | 1029 | Limonene | 3.10 ± 0.39 | RI,MS | Citrus-like, orange, fresh, sweet [38,39] |
| 8.393 | 1035 | 1031 | 1,8-Cineole | 35.11 ± 2.81 | RI,MS | Eucalyptus, camphor-like [38,39] |
| 10.429 | 1089 | 1086 | Fenchone | 5.30 ± 0.38 | RI,MS | Camphor-like, fresh, woody [38,39] |
| 10.845 | 1098 | 1096 | Linalool | 2.81 ± 0.03 | RI,MS | Floral, sweet, spicy, woody, green [38,39] |
| 13.803 | 1172 | 1171 | Octanoic acid | 9.72 ± 2.87 | RI,MS | Faint, fruity-acid [38] |
| 14.124 | 1179 | 1177 | Terpinen-4-ol | 3.26 ± 0.19 | RI,MS | Pine [38] |
| 14.733 | 1192 | 1188 | alpha-terpineol | 5.65 ± 0.26 | RI,MS | Floral, lilac [38] |
| 14.853 | 1195 | 1196 | Methyl chavicol | 13.73 ± 1.85 | RI,MS | Spicy, green, herbal, fennel, anise [38,39] |
| 15.158 | 1201 | 1199 | gamma-terpineol | 1.77 ± 0.05 | RI,MS | Lilac [38] |
| 16.769 | 1242 | 1243 | Carvone | 5.16 ± 0.32 | RI,MS | Spicy, mint, green [39] |
| 18.604 | 1283 | 1284 | (E)-Anethole | 0.87 ± 0.34 | RI,MS | Anise, licorice, medicinal [38,39] |
| - | - | - | N.I. | 12.17 ± 2.70 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, A.; Pinna, I.; Piras, A.; Porcedda, S.; Masala, C. Sex Differences in the Bitterness Perception of an Aromatic Myrtle Bitter Liqueur and Bitter Compounds. Nutrients 2023, 15, 2030. https://doi.org/10.3390/nu15092030
Rosa A, Pinna I, Piras A, Porcedda S, Masala C. Sex Differences in the Bitterness Perception of an Aromatic Myrtle Bitter Liqueur and Bitter Compounds. Nutrients. 2023; 15(9):2030. https://doi.org/10.3390/nu15092030
Chicago/Turabian StyleRosa, Antonella, Ilenia Pinna, Alessandra Piras, Silvia Porcedda, and Carla Masala. 2023. "Sex Differences in the Bitterness Perception of an Aromatic Myrtle Bitter Liqueur and Bitter Compounds" Nutrients 15, no. 9: 2030. https://doi.org/10.3390/nu15092030
APA StyleRosa, A., Pinna, I., Piras, A., Porcedda, S., & Masala, C. (2023). Sex Differences in the Bitterness Perception of an Aromatic Myrtle Bitter Liqueur and Bitter Compounds. Nutrients, 15(9), 2030. https://doi.org/10.3390/nu15092030










